Introduction
Lacunar syndromes are clinical manifestations of lacunar infarctions. The affected arteries arise at sharp angles from major vessels and are prone to constriction and occlusion. These penetrating arteries do not have any collaterals and vary in size between 400 μ to 900 μ in diameter.[1]
The term “lacune” was first described in the late 19th and early 20th century and usually designates a small, chronic cavity that represents the healed phase of lacunar infarction.[2] However, some reported cases reveal that a lacune results from an extensive infarct or intracerebral hemorrhage.
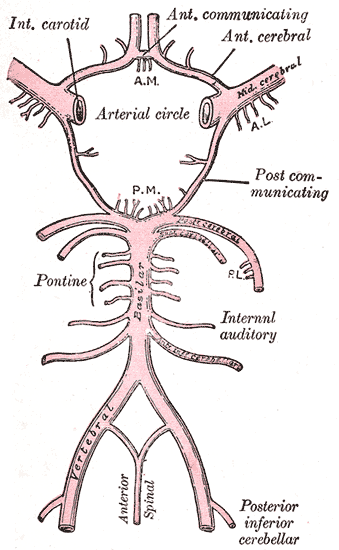
The anatomic distribution of lacunar syndromes and infarctions is commonly the basal ganglia (globus pallidus, putamen, thalamus, and caudate), the pons, and the subcortical white matter structures (internal capsule and corona radiata). These anatomical sites correspond to lesions at the lenticulostriate arteries, the anterior choroidal artery, thalamoperforating arteries, paramedian branches of the basilar artery, and the recurrent artery of Heubner from the anterior cerebral artery.[3]
Lesions at these specific sites account for many of the symptoms of lacunar syndromes. Over 20 lacunar syndromes have been described, but the most common are pure motor hemiparesis, pure sensory stroke, ataxic hemiparesis, sensorimotor stroke, and dysarthria-clumsy hand syndrome.
Etiology
Multiple mechanisms have been proposed as the etiology of lacunar infarctions. The usual etiology of small lacunar infarctions (between 3 mm and 7 mm) is lipohyalinosis of the small perforating arteries feeding deep subcortical structures. Another mechanism is micro-atheroma formation at the origin of penetrating arteries from major cerebral arteries like the middle cerebral artery, Circle of Willis, or the distal basilar artery. These first 2 mechanisms are pathological and likely due to chronic hypertension resulting in small vessel disease.[4]
If the lacune is larger than 5 mm to 7 mm, it is caused by an atherothrombotic lesion involving the mainstem middle cerebral artery, not occlusion of 1 or 2 lenticulostriate arterial branches. These infarcts are named striatocapsular infarcts by Bladin and Berkovic.[5]
Other proposed mechanisms that are not pathological include tiny emboli causing obstruction and cerebral arteriolar and capillary endothelial dysfunction leading to small vessel disease due to the extravasation of blood products.
Out of all the risk factors of lacunar strokes, hypertension is the most common modifiable risk factor. For every 10 mm Hg decrease in blood pressure, there is a one-third lower risk of stroke due to primary prevention. As a consequence, blood pressure control is effective in preventing future strokes. Cigarette smoking doubles stroke risk. The other risk factors include older age, diabetes, obesity, and hyperlipidemia.[6]
Epidemiology
One community-based study in Rochester, MN, estimated that around 16% of first ischemic strokes in the United States are lacunar strokes.[7] In a similar community-based study of African-Americans in Cincinnati, lacunar infarctions accounted for 22% of first-time ischemic stroke events.[8] Other studies suggest they comprise 20% to 25% of all strokes.[1]
Data comparing the frequency of lacunar strokes worldwide are not readily accessible. One study in Japan does state that the frequency of lacunar infarcts has decreased since the 1960s due to more aggressive control of risk factors, primarily hypertension.[9]
Pathophysiology
The pathophysiology of lacunar syndromes is inherently linked to 2 vascular pathologies of the penetrating arteries from major intracranial and extracranial arteries: (1) thickening of the media resulting in decreased arterial diameter and ultimately occlusion, (2) obstruction of the origins by microatheroma formation.
Chronic hypertension, diabetes, and other genetic factors cause medial thickening by lipohyalinosis or arteriosclerosis.[10] As a result, an occlusive disease in these penetrating arteries causes a small infarct in the territory that the small vessel supplies. Since collateral circulation in these distant pontine and subcortical areas is absent or extremely limited, and multiple penetrating vessels are likely affected in these patients, areas of infarct coalesce to form lake-like areas of infarcted or edematous brain tissue. Healing of this tissue ultimately forms "lacunes."[11]
Each lacunar syndrome usually represents an infarct affecting a specific anatomical area supplied by a penetrating artery. The most common lacunar syndromes are:
Pure Motor Hemiparesis: the infarct site is most commonly the internal capsule caused by occlusion of a lenticulostriate artery, or less commonly, the ventral pons caused by occlusion of a basilar paramedian branch.
Pure Sensory Stroke or Hemianesthesia: is the result of an infarct affecting the lateral thalamus due to occlusion of one of the thalamic perforators from the posterior cerebral artery.
Ataxic-Hemiparesis: is caused by an infarct of the internal capsule or ventral pons, similar to pure motor hemiparesis.
Clumsy-Hand Dysarthria Syndrome: sites of the infarcts are similar to pure motor hemiparesis.
Internuclear Ophthalmoplegia: is caused by a small infarct affecting the medial longitudinal fasciculus in the dorsomedial pons due to occlusion of a paramedian basilar arterial branch.
Histopathology
Dr Miller Fisher first described arterial pathology under lacunes in the mid-1900s. These vessels contained focal enlargements and small hemorrhagic extravasations through the endothelium of the arteries. Fibrinoid tissue replaces subintimal foam cells that obliterate the vascular lumens. This process was described as fibrinoid degeneration and lipohyalinosis.[11] Small vessel arteriolosclerosis represents another pathological finding.
History and Physical
Most commonly, lacunar syndromes affect the elderly with long-standing hypertension with acute or sudden onset. Younger patients with lacunar syndromes may be diagnosed with rare genetic conditions, such as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy).[12] The presenting complaints usually do not include cortical signs or symptoms such as agnosia, aphasia, neglect, apraxia, or hemianopsia. These lacunar infarcts usually cause symptoms acutely over minutes to hours but may progress with a stuttering course.
The clinical features and physical exam findings of lacunar syndromes are characteristic of the type of lacunar syndrome. Generally, the wider the area of involvement, the more likely a true lacunar infarct causes the syndrome. In contrast, a limited area of involvement, such as the face or arm only, will make a lacunar infarct unlikely.[13]
Pure Motor Hemiparesis: The patient presents with weakness on one side of the body (face, arm, and leg) without cortical signs and sensory symptoms.
Pure Sensory Stroke: The patient presents with unilateral numbness of the face, arm, and leg without cortical signs or motor deficits. All sensory modalities are impaired.
Ataxic Hemiparesis: The patient presents unilateral limb ataxia and weakness that exceeds the strength or motor deficit. The weakness and ataxia affect the same extremities and contralateral to the infarct site.
Sensorimotor Stroke: Patients present with weakness and numbness of the face, arm, and leg without cortical signs. Cortical function testing should distinguish between a frontoparietal lobe (MCA) stroke and a subcortical stroke (posterior thalamus and internal capsule).
Dysarthria-Clumsy Hand Syndrome: This is a less common form of the lacunar syndrome. Patients present with facial weakness, dysarthria, dysphagia, and dysmetria or clumsiness of one upper extremity.
Evaluation
Initial evaluation of a suspected lacunar stroke involves brain imaging with CT and MRI. Since small perforating arteries are hard to visualize with CTA and MRA, the diagnosis is made by matching a patient’s clinical features with a small, noncortical infarct seen on CT or MRI. The initial CT or MRI is also useful in ruling out life-threatening conditions such as intracerebral hemorrhage or herniation.
MRIs have a higher sensitivity and specificity than CT.[14] Diffusion-weighted imaging (DWI) is considered because it has higher sensitivity for acute infarcts when compared to T2-weighted MRI or FLAIR sequences.
In most cases, mapping a patient’s history of hypertension or diabetes and his or her clinical features with findings of acute ischemia on brain imaging is the minimum criteria for the diagnosis of lacunar infarcts. However, if the patient is young and has no cerebral risk factors, further investigation may be required to determine if there is an embolic source. Vascular imaging and transcranial Doppler are warranted after initial CT or MRI to rule out large vessel ischemia.[15] An identified large vessel stenosis, such as the extracranial internal carotid artery on the same side of the lacunar infarct, may still cause the lacunar infarct secondary to artery-to-artery microembolism.
Treatment / Management
The acute treatment of lacunar infarctions is similar to that of acute ischemic strokes. "Time is brain," and within 4.5 hours of symptom onset, patients should receive intravenous (IV) alteplase therapy. The contraindications to IV thrombolysis are the same as those for other types of acute ischemic strokes, which include ischemic stroke or head trauma in the past 3 months, previous intracranial hemorrhage, gastrointestinal (GI) malignancy or hemorrhage in the past 21 days, intracranial or spinal surgery in the past 3 months, and intra-axial intracranial neoplasm. Aspirin therapy is recommended for patients not eligible for IV alteplase therapy. Otherwise, the acute treatment involves stabilization and early involvement of rehabilitation with speech and physical therapy. Endovascular therapy with thrombectomy generally does not apply to the acute treatment of lacunar strokes.
The most important aspect of treatment for these patients is secondary prevention. Aggressive blood pressure control, early high-dose statin therapy, and antiplatelet therapy are crucial. Antiplatelet therapy with aspirin, clopidogrel, and fixed-dose aspirin with dipyramidal are acceptable medications. With the results of the CHANCE trial and the POINT trial,[16] dual antiplatelet therapy for 3 weeks, followed by single antiplatelet, provides the best therapy. More prolonged dual antiplatelet therapy (clopidogrel + aspirin) has not been shown to decrease the risk of recurrent strokes and resulted in an increased risk of bleeding and death.[17]
The present-day recommendation with antiplatelet treatment, aggressive blood pressure control with a target of 120/80 mm Hg, high dose statin, reasonable blood sugar control, cessation of smoking, sodium reduction, weight control, and lifestyle modification should be able to prevent at least 80% of strokes.[6] The LDL should be lowered to less than 70 mg/dL with a high-intensity statin. Usually, a high-intensity statin decreases LDL by 50% on average. Strategies to reduce HbA1C by less than 7% are also fundamental.
Differential Diagnosis
The primary differential diagnoses should include acute intracerebral hemorrhage, large vessel ischemic strokes (most commonly in the middle cerebral artery territory), other intracranial hemorrhages (subarachnoid bleeds, subdural bleeds), seizures, and complicated migraine.
As described earlier, large vessel ischemic strokes and intracranial hemorrhages are ruled out by brain imaging with CT and MRI. A large or giant lacune is pathophysiologically not a lacuna but rather a striatocapsular infarct, resulting from atherothrombosis of the middle cerebral artery main stem.[18] If an acute lacunar infarction is not visible on initial DWI, seizures and complicated migraines should be included in the differential.
Seizures are present with cortical signs in addition to focal neurologic deficits. Usually, neurologic deficits will also improve 24 to 48 hours after the seizure event and post-ictal phase. Lastly, an EEG may be used to further rule out a seizure as the cause of the patient’s symptoms.
Toxicity and Adverse Effect Management
The main adverse event is a hemorrhagic transformation or adverse bleeding events after IV alteplase therapy. Close monitoring with frequent neurologic exams and a high degree of suspicion can prevent these devastating complications.
Preventative treatment is generally well tolerated. However, patients should be notified that there is an increased risk of bleeding on long-term antiplatelet therapy. Side effects of antihypertensive therapy, such as orthostatic hypotension and an increased risk of falls, should be explained to the patient. No data exists that lacunar infarct patients are at increased risk of statin myopathy over the general population. These medications should be prescribed at the lowest therapeutic dose.
Prognosis
Lacunar infarcts are small infarcts. The prognosis should understandably be better compared with cortical infarcts. The prognosis for lacunar infarctions is better than other types of strokes. Multiple population-based epidemiological studies on lacunar infarcts have shown significantly better survival among patients who suffered from lacunar infarctions than those who suffered from non-lacunar infarcts.[7][19] These studies showed a case fatality of 0% to 3% within the first month and 3% to 9% within the first year, compared with 14% and 28%, respectively.
Though the short-term prognosis for patients with lacunar syndromes is better, there is not as stark a difference in the long-term prognosis compared to non-lacunar events. Multiple studies have shown that the stroke recurrence rate and the risk of death between lacunar infarcts and non-lacunar infarcts are similar after 5 years. The main reason for the better 5-year survival rate in lacunar syndrome patients is the lower mortality within the first year of the ischemic event.[7][20]
Complications
The complications of lacunar syndromes are similar to other acute stroke syndromes and also similar to other non-ambulatory patients.
These include:
- Aspiration with pneumonia
- Deep vein thrombosis with pulmonary embolism
- Decubitus ulcers
- Mood disorders, including depression. This is not as common as cortical infarcts[21]
- Recurrence
Postoperative and Rehabilitation Care
Rehabilitation is always necessary with any kind of stroke, including lacunar syndromes. Early rehabilitation is essential to improve the outcome. Early physical medicine, physical therapy, behavioral intervention, and occupational therapies are helpful in improving the final prognosis.
Consultations
After initial stabilization and neurology evaluation, many consultations are not required in the acute phase setting. Physical therapy (PT), speech therapy (ST), occupational therapy (OT), and rehabilitation services led by a Physical Medicine and Rehabilitation (PM and R) clinician are recommended. Lacunar syndrome patients should be evaluated in the hospital and followed closely in the outpatient setting to ensure that the patient recovers quickly.
Furthermore, communication between the patient’s neurologist, primary care clinician, physical medicine and rehabilitation clinician, and therapists after hospital discharge prevents recurrent lacunar or ischemic strokes.
Deterrence and Patient Education
Patients must be educated on the importance of compliance with preventative medical therapy after they suffer from lacunar syndrome. The pathophysiology of their syndrome or infarct should be explained, emphasizing that the most common cause of lacunar infarctions is chronic, uncontrolled hypertension.
This includes antihypertensive, statin, and diabetic medication compliance, following up regularly with their primary care clinician, neurologist, and physical therapist, and regular exercises through physical therapy to regain neurologic function and improve quality of life.
Finally, public education and awareness are important so people may recognize early stroke signs and symptoms, especially the FAST (face, arm, speech, and time)-centered programs.[22]
Enhancing Healthcare Team Outcomes
As mentioned, for comprehensive care, patients with lacunar infarctions must be managed and seen by a neurologist, a PM and R clinician, and physical, occupational, and social therapists. Neurologists may treat deficits like ataxic and motor hemiparesis in the outpatient setting with muscle relaxants like baclofen, tizanidine, or botox.
More importantly, preventative measures with intense antihypertensive therapy, high-dose statin therapy, and strict control of blood sugars should be initiated after a lacunar ischemic event. Outpatient rehabilitation is recommended until neurological function returns to a level similar to baseline.