Introduction
Traumatic Brain Injury (TBI) may result from anywhere between a simple blow to the head to a penetrating injury to the brain. In the United States, around 1.7 million people suffer TBI with older adolescents (ages 15 to 19 years) and older adults (ages 65 years and older) among the most likely to sustain a TBI. The frontal and temporal areas of the brain are the main areas involved. Mild TBI (mTBI), also known as brain concussion, initially considered as a benign event, has galvanized tremendous attention for some of its adverse neuropsychological outcomes in civilians (e.g., athletes who play contact sports) as well as military personnel. Moderate to severe TBI is a primary cause of injury-induced death and disability. In the United States, It has an annual incidence of approximately 500 in 100,000. However, around 80% of all TBI cases are categorized as mild head injuries.[1][2][3][4]
Etiology
Mild TBI or brain concussion usually results from closed brain injuries, the incident when the head is being struck by an object, such as a bat or a fist during a fight or when the head is affected by a nearby blast or explosion. Such injuries have shown to affect the structural integrity of the neurons.
Epidemiology
The male to female ratio is 2:1. A private study of 1084 individuals with traumatic brain injuries revealed that TBI was a high-risk factor not only for post-traumatic stress disorder (PTSD) but also for other psychiatric disorders. Statistically, the Center for Disease Control and Prevention (CDC) has estimated that annually, about 1.5 million Americans survive a traumatic brain injury (TBI). Among these, approximately 230,000 are hospitalized. In 2000, there were 10,958 TBI diagnoses. In 2015, this number jumped to 344,030. Mortality across all TBI severities is approximately 3%, yet morbidity is more difficult to estimate.
Pathophysiology
The Monro-Kellie hypothesis states that the total intracranial volume (composed of brain tissue, cerebrospinal fluid, venous blood, and arterial blood) should always remain a constant since the cranium is a rigid and non-expansile container. When an additional compartment is introduced (like a hematoma), there must be a compensatory reduction in another compartment in order to prevent intracranial hypertension. Cerebral perfusion pressure (CPP) is defined as mean arterial pressure (MAP) – intracranial pressure (ICP). When the ICP increases, the CPP will be reduced and can lead to secondary cerebral ischemia and infarct. The goal of TBI management is to prevent this secondary insult.[5]
The following are the different types of TBI commonly encountered[6]:
Concussion
This is usually a mild TBI without any gross structural damage and occurs secondary to a nonpenetrating TBI. It usually results from acceleration/deceleration forces occurring secondary to a direct blow to the head. It causes a transient altered mental status, which can range from confusion to loss of consciousness. This cannot be diagnosed with a routine computed tomogram (CT) scan or magnetic resonance imaging (MRI) scan. Special sequence MRI like diffusion tensor imaging and functional MRI may result in earlier diagnosis of concussion.
Second impact syndrome: The initial event is often a concussion, but if the patient (often an athlete) starts to play without fully recovering from this and sustains another injury, there can be a rapid evolution of malignant cerebral edema, ensuing over a short-time course of time.
Chronic Traumatic Encephalopathy (CTE): This is usually a delayed manifestation of repetitive mild TBI. This is common in athletes and can lead to psychiatric disturbances and suicidal behavior, attention deficits, and derangements in memory and executive functions.
Extra-axial Hematoma
Extra-axial hematomas include both epidural hematomas (EDH) and subdural hematomas (SDH). EDH usually results from bleeding from the middle meningeal artery and its branches or a fracture and is usually acute.[7] SDH can result from the bleeding of a bridging vein and can be acute or chronic.
Contusion
Contusions (bruising of the brain) can be a coup or contrecoup type. Coup contusions occur at the site of impact, whereas contrecoup injuries typically take place on the contralateral side of impact, usually the basi-frontal lobe and anterior temporal lobe.
Traumatic Subarachnoid Hemorrhage (SAH)
Subarachnoid hemorrhage is most commonly caused by trauma and results from the tearing of small capillaries with blood subsequently entering into the subarachnoid space. It commonly occurs over the convexity, whereas SAH secondary to aneurysmal rupture occurs in the basal cisterns.
Diffuse Axonal Injury (DAI)
This can underlie mild to moderate TBI and potentially results from any shearing, stretching, or twisting injuries to the neuronal axons. This phenomenon is mainly seen at the junction of the gray and white matter where neuronal axons are entering a more dense, fatty (myelinated), and less fluid-filled white matter. Such shearing forces cause the neuronal axon to be stretched, and the subsequent damage to the cytoskeleton may lead to axonal swelling, increased permeability, calcium influx, detachment, and axonal death. Diffuse laminar necrosis is typically seen on autopsy.[8][9]
Histopathology
The events of posttraumatic accumulation of fluid (cerebral edema), the disruption of the blood-brain barrier (BBB), and histopathological changes were studied in mice model studies. Researchers have discovered significant neuronal cell death in some areas of the left hippocampus following a closed-head injury. Immunohistochemistry using multiple antibodies to the amyloid precursor protein and/or amyloid precursor protein-like proteins showed a novel axonal degeneration in the striatum, corpus callosum, and injured cortex. Histological evaluation of injured brains illustrated an expansion of the cortical cavity, enlargement of the lateral ventricles, deformation of the hippocampus, and thalamic calcifications.
Toxicokinetics
In addition to the biomarkers released secondary to the neuronal injury (proteins that leak from astrocytes and neurons when are damaged), researchers have studied the role of cJun N-terminal kinase (JNK) that mediates neuronal death in response to stress and injury in the CNS and peripheral nervous system. Some of the other variables in settings of TBI include interleukin-1beta concentration, aspartate, glutamate in the CSF, and microdialysate lactate, glucose, pyruvate, and glycerol, along with brain tissue partial oxygen pressure and intracranial pressure. Most of these were measured while studying different modalities of therapy like hyperbaric oxygen treatment and hypothermia in TBI.
History and Physical
Unfortunately, many incidents of mild-moderate traumatic brain injuries in our everyday life do not even present to the emergency room or other healthcare settings, especially when they are associated with sports-related or recreation-related settings. Per the Journal of Pediatrics, cases of TBI in children of age 18 and under were believed to be caused mainly by sports and recreation-related concussions. However, the vast majority of service members and veterans experience a TBI in a non-deployed setting (diagnosed in up to 80%) due to the nature of their training or participation in sports and leisure activities.[10][11][12][13]
The American Journal of Psychiatry published a study in 2015 that showed the prevalence of comorbidity of disorders among soldiers classified at T2 (3 months post-deployment) as experiencing a major depressive episode, PTSD, generalized anxiety disorder, or suicidality in the past 30 days went up from 12.9% to 16.8% at T3 (9 months post-deployment).
Apart from the obvious physical complaints, neuropsychiatric symptoms noticeably vary out of proportion with the severity of the correspondent TBI. The patients who experience post-concussion syndrome may have somatic complaints like a headache, dizziness, cognitive impairment, and neuropsychiatric symptoms like anxiety, irritability, depression, and sleep disorders. They may have frontal lobe syndrome with behaviors like labile affect, poor social judgment, and lewd behavior with loss of social graces, aggressiveness, and perseveration. These are common in up to 23% of adult patients with TBI.
Working memory is largely affected among patients with mTBI. They may also abuse drugs and experience PTSD symptoms more than the general population (up to 18% and 22%, respectively).
Psychotic disorder following traumatic brain injury (PDFTBI) occurs in 0.7% to 8.9% of persons who sustain TBI. Psychosis following TBI causes patients to have deficits: brain-injured (primarily executive), and psychotic (executive and semantic). Although infrequent, auditory hallucinations are a debilitating complication of TBI that can manifest itself 4 to 5 years after the occurrence of the TBI. The latency between traumatic brain injury (TBI) and the onset of psychotic symptoms is highly variable but typically characterized by paranoid delusions and auditory hallucinations, with visual hallucinations and negative symptoms being less common. Studies showed that PDFTBI has an additive effect on executive dysfunction among TBI patients. Significant executive dysfunction was evident in patients with PFTBI on all measures.
Evaluation
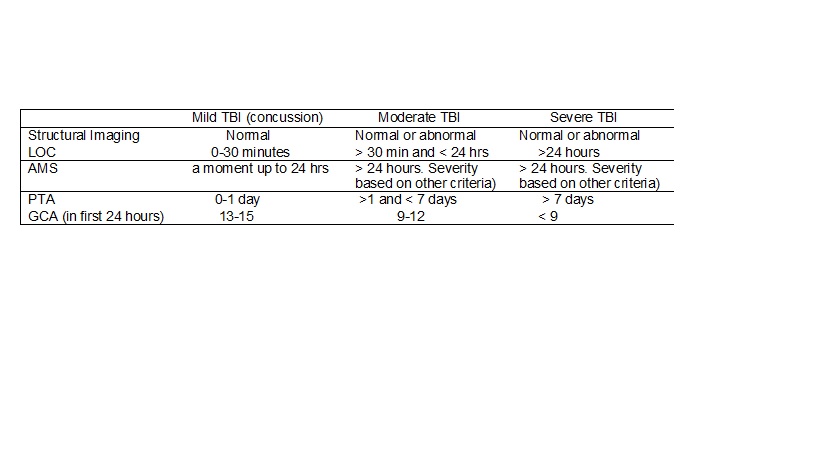
Level of consciousness (LOC), altered mental status (AMS), post-traumatic amnesia (PTA), and Glasgow coma scale (GCS) are used in the evaluation of the severity of the TBI (see table 1). Moreover, recent studies recommend the implementation of the portable cognitive assessment tools at the time of the incidence of the TBI as a potential indicator for the long-term effects. One of the scales used in measuring the severity of deficit in cognitive functioning is the Ranchos Los Amigos Scale, as below:
- Level I = No response
- Level II = Generalized response
- Level III = Localized response
- Level IV = Confused-agitated
- Level V = Confused-inappropriate
- Level VI = Confused-appropriate
- Level VII = Automatic-appropriate
- Level VIII = Purposeful-appropriate
A comprehensive evaluation should include the utilization of the following tools:
- Clinical history/presentation with thorough neurological (Glasgow Coma Scale score)[14] and psychiatric evaluation including cognitive assessment
- Labs: Serum levels of two biomarkers: Glial fibrillary acidic protein (GFAP) and Ubiquitin C-terminal hydrolase (UCH-L1) correlated with the degree of brain injury, with GFAP being the more reliable of the two up to 7 days after impact. They are proteins that leak from astrocytes and neurons when are damaged.
- Neuroimaging
CT head and MRI are used to measure changes in anatomical or physiological parameters of TBI. These include hemorrhage, edema, vascular injury, and intracranial pressure. However, for most cases of mild TBI, CT and MRI often show no abnormalities.
Diffusion tensor imaging (DTI) is used to detect axonal injury for mild to moderate TBI.
Functional MRI (fMRI) is often used to differentiate TBI from control groups and has been used to study activation patterns in patients with TBI.
Brain Perfusion Single Photon Emission Computed Tomography (SPECT) is used to measure cerebral blood flow and activity patterns. It is indicated for the evaluation of TBI in the absence of anatomical findings. Some authors suggest that SPECT should be part of the clinical evaluation in the diagnosis and management of TBI. A recent meta-analysis showed that PTSD patients have significant activation of the mid-line retrosplenial cortex and precuneus when presented with trauma-related stimuli. The preliminary data suggest it has a potential role in distinguishing PTSD from TBI. When compared to subjects with TBI, relative increases in perfusion were observed in PTSD in the limbic regions, cingulum, basal ganglia, insula, thalamus, prefrontal cortex, and temporal lobes. These results suggest that TBI is associated with hypoperfusion while PTSD is associated with regional hyperperfusion, providing important insights regarding pathophysiological differences between these disorders. Recent studies are also highly promising in differentiating PTSD from TBI using both region of interest (ROI) and visual readings (VR) analysis (The study was published on July 1, 2015, in PLOS One).
- Sleep studies: Some power-spectral analyses revealed patients in the mTBI group showed lower delta power and higher alpha power in the first NREM period and higher beta power in the first and second NREM periods. REM-period findings included lower beta in the third REM period and higher delta in the first REM period. Recent data suggested that sleep tests may be a sensitive measure of brain injury after mTBI and, theoretically, could be used to determine the anatomy of brain injury.
Treatment / Management
Treatment of psychiatric symptoms following concussion/mTBI should be based on individual factors and the nature and severity of symptom presentation. It may include physiotherapy, psychotherapeutic, and pharmacological treatment modalities.[15][16][17]
Physical Rehabilitation
TBI may result in a decrease in short and long-term global health (physical and behavioral) and put them at an elevated risk for disability, pain, and handicap (i.e., difficulty with a return to work, maintaining peer networks.) Rehabilitation therapies like physical therapy, occupational therapy, speech-language therapy, and assistive devices and technologies may help to strengthen patients to perform their activities of daily living.
Psychotherapy
- Initial education, long-term support groups (symptom-focused and process groups), family education, and social issues like financial, legal and transportation.
- Virtual reality and videogaming-based therapy in treating balance, coordination, and cognitive issues like attention and concentration data are under larger scale clinical trials to prove efficacy.
Medications
- Depakote, NSAIDs, and triptans: May be considered for headaches which are the single most common symptom associated with concussion/mTBI
- SSRIs: Citalopram 10 mg daily for 1 week, then 20 mg daily if tolerated (up to 80 mg daily if needed). Sertraline 25 mg daily increasing weekly in 25 mg increments to a maximum dose of 200 mg/day for depression
- Anticonvulsants: mood stabilization and seizure prevention
- Atypical antipsychotics: for agitation and irritability with beta-blockers in severe cases
- Dopaminergic agents: for concentration and focus
- Cholinesterase inhibitors/cognitive enhancers for memory
- Atypical agents: Buspar for emotional stabilization and Modafinil for focus.
General Guidelines for Using Medications
- Start low, go slow, whenever medications are required
- Rule out social factors first, such as abuse, neglect, caregiver conflict, and environmental issues
- No large quantities of lethal medications, high suicide rate due to disinhibition
- Full therapeutic trials, since under treatment is common
- Minimize benzodiazepines (impairs cognition), anticholinergics (induces sedation), seizure-inducing (impedes neuronal recovery), and antidopaminergic agents
- No caffeine (due to agitation and insomnia), no diet, herbal, or energy drinks (may precipitate aggression).
Other Considerations in Treating PTSD in Patients with mTBI
- Present information at a slower rate
- Use a structured intervention approach with agenda, outline, or handouts
- In groups, ask “PTSD” to respond first, then ask others to respond
- Allow free contribution, use refocus/redirection with a clear transition between topics
- The therapist should avoid frustrating mTBI patients by forcing them to recall incidents that are only partially encoded.
Management of Sleep Dysfunction
Immediately following TBI, the difficulty in falling asleep and frequent waking is common; whereas, after several years excessive somnolence is more typical.
- Acute Phase less than 3 months: Provide education about concussion about changes in sleep quality and duration sometimes associated with concussion. Provide information on good sleep habits with specific suggestions to improve the quality and duration of sleep (regularly scheduled bedtime). Sleep medications may be helpful in the short-term. Zolpidem 5 mg at night, if poor results after 3 nights of therapy, increase to 10 mg nightly. Also, prazosin, with 1 mg at bedtime for 3 days, may increase to 2 mg at bedtime through day 7.
- Chronic phase: more than 3 months: Review current medications and other current health conditions for factors that might contribute to chronic sleep disturbances, including chronic pain or co-morbid psychiatric conditions. Consider sleep study to provide objective evidence of sleep disturbance and to rule out coexisting sleep apnea or other sleep disorders. Consider a course of cognitive-behavioral therapy (CBT) focused on sleep.
Hyperbaric Oxygen Therapy (HBO2)
Some researchers discussed the role of oxygen delivered at supraphysiological amounts in the treatment of TBI. A study published in 2010 included closed-head trauma victims with GCS scores of 3 to 8 after resuscitation, without effects from paralytics, sedation, alcohol, and/or street drugs. HBO2 treatment began within 24 hrs post-injury admission to hospital with a mild or moderate TBI compared the effect of HBO2 to normobaric oxygen. They found a significant post-treatment effect of HBO2 on cerebral oxidative metabolism due to its ability to produce a brain tissue PO2 greater than or equal to 200 mmHg (higher cerebral blood flow lead to higher PO2, lower levels of lactate by 13% compared to control group, and lower intracranial pressure). However, in severe TBI, it is not an all or nothing phenomenon but represents a graduated effect. Some controversy still surrounds the use of HBO2 due to the limitations of studies such as the lack of blinding to the intervention, cost, time-consuming practice, and the validity of the actual diagnoses of the patients with reported TBI and PTSD who had a subsequent improvement.
Hypothermia
Studies have shown some controversy in the practicality of this practice depending on the patient’s characteristics (age, the initial GCS, the presence or absence of pupillary abnormalities, and CT-based classification of the severity of the injury). In general, there has been an increased belief that cooling the body to systemic temperatures around 34 C to 35 C, helps reduce secondary injury and improve behavioral outcomes. Studies have suggested that this occurs because of the ability of hypothermia to suppress the post-traumatic inflammatory response, in turn, preserving the blood-brain barrier and reducing the number of cytokines released as well as glutamate.
Medical Measures to Reduce Intracranial Pressure
Head end of bed elevation to 30 degrees, transient hyperventilation, hyperosmolar therapy, therapeutic cooling, and medically induced comatose state are some measures to reduce intracranial pressure. Some patients will need monitoring of the intracranial pressure.[18]
Surgical Measures to Reduce Intracranial Pressure
This involves the evacuation of intracranial hematoma or decompressive craniectomy.[19][20]
Differential Diagnosis
- Stroke
- Spontaneous acute subdural hematoma
- Alzheimer disease
- Anterior circulation stroke
- Brain metastasis
- Cerebral aneurysms
- Frontal lobe syndrome
- Hydrocephalus
Prognosis
Each year in the US, nearly 52,000 deaths occur as a result of TBI. Individuals who arrive with low GCS have the worst outcomes. Even those who survive have prolonged recovery and some are still left with residual neurological deficits. Complete recovery can take months or even years.
Complications
- Post-traumatic seizures
- Deep vein thrombosis
- Hydrocephalus
- Spasticity
- Mood and behavior changes
- Gait abnormalities
- Cognitive decline
- PTSD
- Post-traumatic headache
- Insomnia
Enhancing Healthcare Team Outcomes
The management of TBI is interprofessional because many organ systems can be affected by brain injury.
TBI in general and mTBI, in particular, are heterogeneous pathological processes. Although post-concussion syndrome and personality changes are frequently presenting symptoms, research remains to be done on the unremarkable neuroimaging results in TBI, the life experiences of young TBI patients with co-morbid symptoms that persist well into adulthood, and the characteristics of families that contribute to function or dysfunction following a TBI. Many therapeutic trials were questioned, including hormonal therapy with progesterone in the first few hours that aimed to augment myelin regeneration and neural recovery, but results were not encouraging. Interestingly enough, there are still many questions that demand answers. These are prognosis, permanency of symptoms, best acute and long-term treatment strategies, and whether literature in sports-related TBI can be used as a guide. Imaging and therapeutic intervention research are still underway in hopes of delivering better outcomes.
Treatment of psychiatric symptoms following concussion/mTBI should be based on individual factors and the nature and severity of symptom presentation. It may include physiotherapy, psychotherapeutic, and pharmacological treatment modalities. Gait problems may require physical therapy, and loss of fine motor skills may require occupational therapy. A mental health nurse should follow the patients as many develop mood and behavior changes. Caregiver education is vital and one should not set unrealistic expectations.
The prognosis of patients with TBI depends on the initial GCS and neurological deficits at presentation. Those patients with a GCS less than 12 usually have a long recovery, and many continue to have residual neuro-psychiatric deficits.[21][22](Level V)