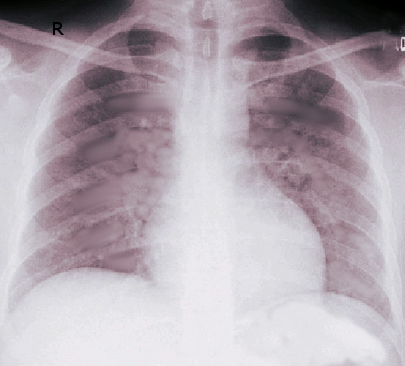
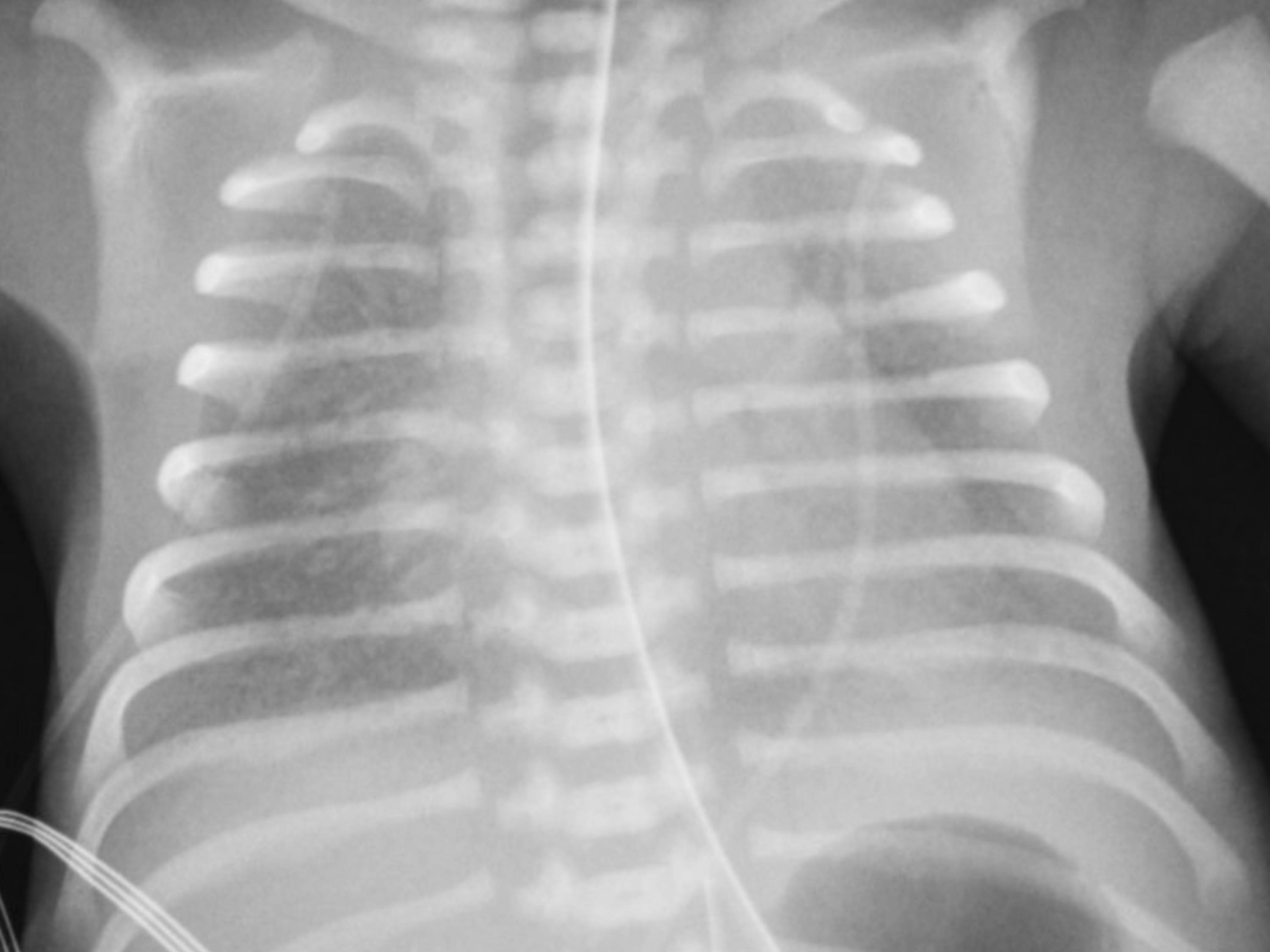
[1]
Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. The New England journal of medicine. 2017 Aug 10:377(6):562-572. doi: 10.1056/NEJMra1608077. Epub
[PubMed PMID: 28792873]
[2]
Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018 Feb 20:319(7):698-710. doi: 10.1001/jama.2017.21907. Epub
[PubMed PMID: 29466596]
Level 3 (low-level) evidence
[3]
Meyer NJ, Christie JD. Genetic heterogeneity and risk of acute respiratory distress syndrome. Seminars in respiratory and critical care medicine. 2013 Aug:34(4):459-74. doi: 10.1055/s-0033-1351121. Epub 2013 Aug 11
[PubMed PMID: 23934715]
[4]
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, LUNG SAFE Investigators, ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23:315(8):788-800. doi: 10.1001/jama.2016.0291. Epub
[PubMed PMID: 26903337]
[5]
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005 Oct 20:353(16):1685-93
[PubMed PMID: 16236739]
[6]
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012 Jun 20:307(23):2526-33. doi: 10.1001/jama.2012.5669. Epub
[PubMed PMID: 22797452]
[7]
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ, American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. American journal of respiratory and critical care medicine. 2017 May 1:195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST. Epub
[PubMed PMID: 28459336]
Level 1 (high-level) evidence
[8]
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England journal of medicine. 2000 May 4:342(18):1301-8
[PubMed PMID: 10793162]
[9]
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006 Jun 15:354(24):2564-75
[PubMed PMID: 16714767]
[10]
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A, ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. The New England journal of medicine. 2010 Sep 16:363(12):1107-16. doi: 10.1056/NEJMoa1005372. Epub
[PubMed PMID: 20843245]