Continuing Education Activity
Hepatocellular adenoma, also called "hepatic adenoma," is a rare, benign epithelial liver tumor frequently associated with oral contraceptive pill intake. Conditions like anabolic steroid abuse, Fanconi anemia, and aplastic anemia have also been implicated in the development of this lesion. Hepatic adenomas are benign but carry a higher hemorrhage and malignant transformation risk.
Elective resection is recommended in all men with adenomas of any size and women with tumors larger than 5 cm. Rupture may complicate untreated lesions, manifesting with hemorrhage, severe abdominal pain, and hypotension, and may lead to hypovolemic shock and death. Women on oral contraceptives are most susceptible to liver adenoma rupture, which requires immediate recognition due to its potentially catastrophic consequences.
This activity for healthcare professionals is designed to improve learners' competence in evaluating and managing hepatocellular adenoma. Participants gain profound insights into the condition's pathophysiology, symptomatology, and evidence-based diagnostic and treatment practices. Learners become prepared to collaborate within an interprofessional team caring for patients with or at risk for hepatic adenoma.
Objectives:
Identify the historical and physical examination features suggestive of a hepatocellular adenoma.
Create a clinically guided diagnostic strategy for a person with suspected hepatic adenoma.
Implement current evidence-based treatment options for an individual diagnosed with hepatocellular adenoma.
Collaborate with the interprofessional team to educate, treat, and monitor individuals with or at risk for hepatocellular adenoma.
Introduction
Hepatocellular adenoma (HCA), otherwise known as hepatic adenoma, is a rare, benign epithelial hepatic neoplasm often linked to exogenous estrogen intake, usually in the form of oral contraceptive pills. The lesion is also associated with steroid abuse, Fanconi anemia, aplastic anemia, metabolic syndrome, and glycogen storage disease (GSD). HCAs are benign but are at high risk of hemorrhage and malignant transformation.[1]
Although typically solitary, multiple adenomas can occur. Hepatic adenomatosis refers to the presence of 10 or more tumors. Advances in radiologic diagnosis and subtype classifications based on molecular behavior have emerged, which provide a more systematic approach to treating patients with hepatic adenomas. Elective resection is recommended in men with adenomas regardless of size and in women with adenomas greater than 5 cm.[2]
Liver Anatomy and Histology
The liver is located inferior to the diaphragm and occupies the abdominal right upper quadrant (RUQ). This organ has a complex anatomical configuration crucial for its multifunctional roles. The liver is encased in the visceral peritoneum and extends from the midclavicular line to the right costal margin at the level of the 5th intercostal space. The organ's superoposterior aspect houses the bare area where the diaphragm and inferior vena cava (IVC) converge.[3]
The Couinaud classification subdivides the liver into 8 functionally independent segments based on vascularization, bile duct distribution, and lymphatic drainage. These wedge-shaped segments have apices directed toward the hepatic hilum and receive a single branch each of the bile duct, portal vein, and hepatic artery.
Hepatic veins run between adjacent segments, ultimately draining into the IVC, while the middle hepatic vein delineates the liver's right and left lobes. The right hepatic vein further partitions the right lobe into posterior and anterior segments, while the falciform ligament separates the left lobe into medial and lateral segments. The portal vein horizontally divides segments into superior and inferior sections. Segment I is the caudate lobe, distinguished from other lobes by its unique characteristics, such as a dual blood supply and direct drainage into the IVC. Segments II, III, and IV constitute the left hepatic lobe sections. Segments V, VI, VII, and VIII constitute the right lobe hepatic sections.
The liver's blood supply, primarily from the portal vein, enhances liver imaging during the portal venous phase—a property crucial for diagnostic purposes. Tumors supplied by the hepatic artery exhibit enhanced imaging during arterial phases, a principle utilized in therapeutic interventions like transarterial chemoembolization. Hepatic veins appear as anechoic tubes on ultrasound and drain into the IVC. The portal triad—consisting of portal veins, hepatic arteries, and bile ducts—manifests as echogenic foci within the liver parenchyma.[4]
The hepatic lobule serves as the primary functional unit, comprising hexagonal arrays of hepatocytes surrounding central veins. Hepatocytes organize into cords within lobules, enveloping sinusoids housing Kupffer and stellate cells. The lobule's organization into portal triads ensures efficient blood flow across zones delineated by their proximity to portal tracts or central veins. This alternative organization, known as the portal acinus, delineates functional zones. Zone 1 surrounds portal tracts involved in oxidative metabolism. Zone 3 envelopes central veins primarily engaged in drug biotransformation. Zone 2 exhibits mixed functionality.
Etiology
Chen and colleagues enhanced our understanding of hepatocellular adenoma formation when their 2002 study identified the role of β-catenin in the Wnt signaling pathway.[5] Subsequently, Bioulac-Sage and associates developed a phenotypic-genotypic classification system for hepatocellular adenomas based on molecular behavior patterns.[6][7] Other groups have since validated this classification scheme, which categorizes hepatocellular adenomas into 4 main groups:
Hepatocyte Nuclear Factor -1α Inactivated Mutations
Patients with hepatocyte nuclear factor-1α (HNF-1α) inactivated mutations comprise 35% to 40% of cases. The condition involves biallelic mutations of the T-cell factor-1 gene that encodes the transcription factor HNF-1α. This protein is involved in hepatocyte differentiation, liver development, and glucose and lipid metabolism. HCA lesions in this group are noticeably more steatotic on biopsy. Women are mostly affected. The mutation is likewise often associated with mature-onset diabetes of the young (MODY3). About 90% of the mutations are somatic, although MODY3 association can involve the germline and commonly manifests with adenomatosis. Lesion size smaller than 5 cm reduces complication risk.
β-Catenin Activated Mutations
Individuals with β-catenin activated mutations constitute 15% to 20% of liver adenoma cases. The condition is frequently linked to androgen exposure, glycogenesis, and familial adenomatous polyposis. Mild hepatic cytological or architectural abnormalities lead to an acinar, pseudoglandular pattern. The mutation is rarely detected in liver steatosis and inflammatory cases. Adenomas in this group have an increased malignant transformation risk. The lesions typically exhibit positivity for glutamine synthetase and abnormal β-catenin expression on immunohistochemical staining.
Inflammatory Hepatocellular Adenomas
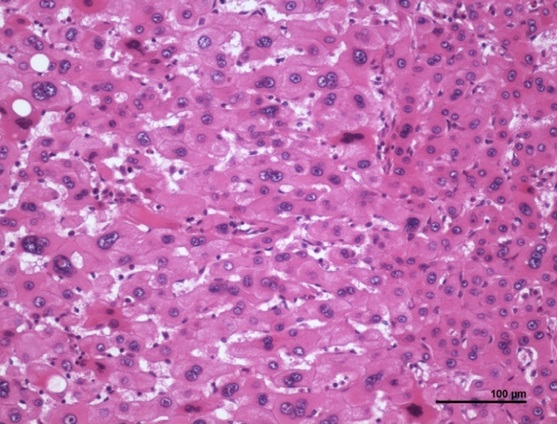
Inflammatory HCA (IHCA) is seen in 40% to 50% of patients with HCA (see Image. Inflammatory Hepatocellular Adenoma Histopathology). Risk factors include female sex, high body mass index (BMI), excessive alcohol consumption, and systemic inflammatory syndrome. The lesions lack both HNF-1α and β-catenin mutations. IL-6 inflammatory pathway activation is implicated in IHCA development, manifesting with dystrophic vessels and telangiectasia. Specifically, the arteries have thick walls, and the sinusoids are dilated, a condition called "peliosis." Adenomas in this category were formerly called “telangiectatic focal nodular hyperplasia.” The tumors exhibit positivity for serum amyloid A (SAA) and C-reactive protein (CRP) and demonstrate inflammatory infiltrates and ductular reactions.
Unclassified Type
Patients with unclassified HCA comprise 10% of cases. The lesions are negative for CRP, SAA, β-catenin, and glutamine synthetase but exhibit typical liver fatty acid-binding protein (LFABP) staining.[8][9]
Epidemiology
HCA's annual incidence among patients taking oral contraceptive pills is 30 to 40 cases per million, compared to 1 case per million in people who do not take these medications. A higher risk is seen after more than 2 years of use.[10] A study found a 25-fold relative risk increase in women using oral contraceptive pills for over 109 months compared to those using them for less than 12 months. Discontinuation of oral contraceptives often results in spontaneous tumor regression, supporting the link to sex hormones. HCA is more common in women than men, with a ratio of 4 to 1, although this ratio is changing due to sports-related anabolic drug use.
Other populations at risk for developing HCA include individuals with GSD types I and III, iron-overload conditions like β-thalassemia and hemochromatosis, and states of endogenous sex hormonal imbalance such as Klinefelter and polycystic ovarian syndromes. Except for polycystic ovarian syndrome, these other conditions predominantly affect men and are often diagnosed during childhood.[11][12]
Pathophysiology
Genetic mutations offer new insights into the behavior of these tumors. However, traditional knowledge highlights the strong association between sex hormones, notably oral contraceptive pills and anabolic steroids, and HCA development. Other drugs that have been implicated include clomiphene, recombinant human growth hormones, and barbiturates.[13]
Histopathology
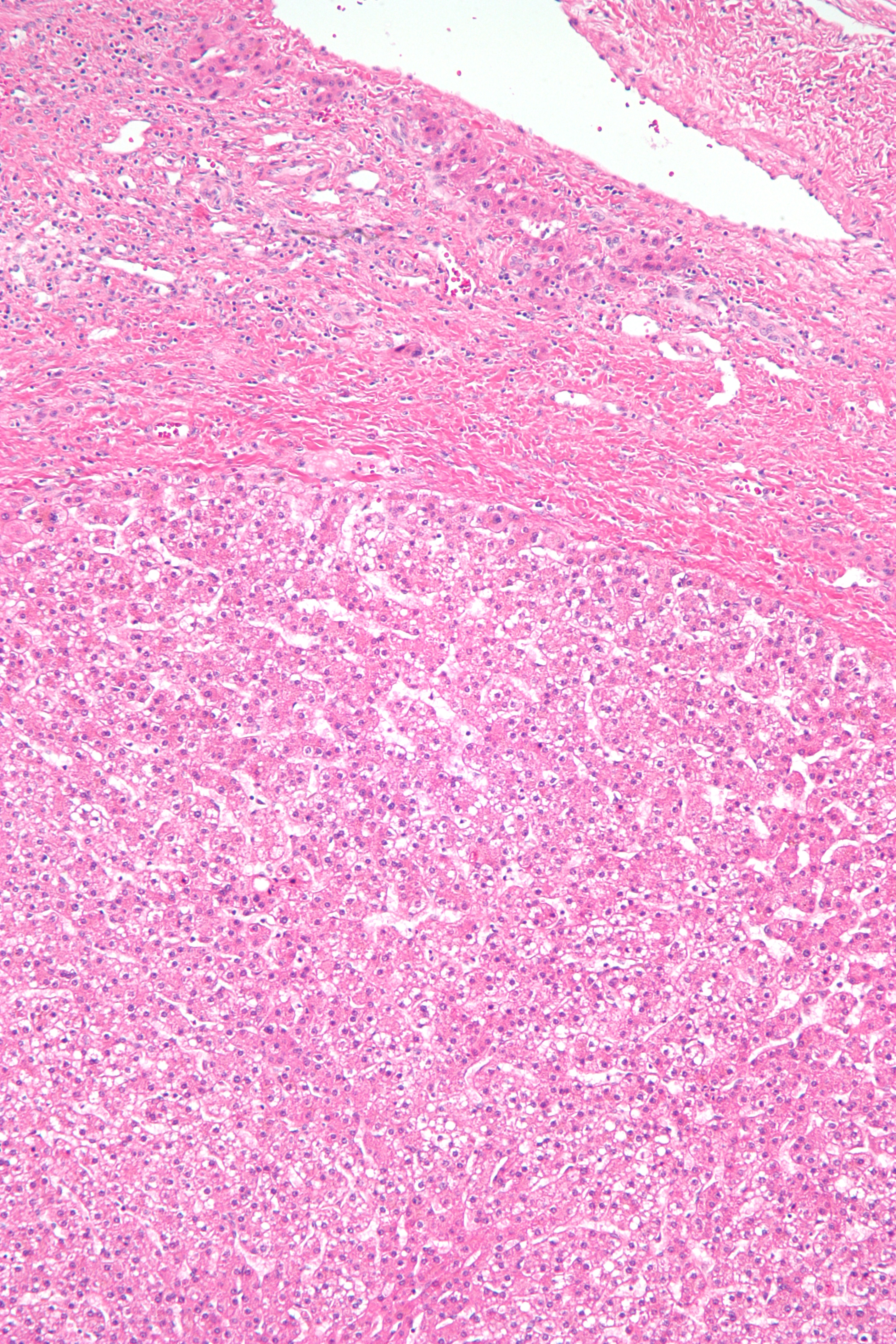
Gross examination of HCAs reveals yellow or light brown well-circumscribed lesions with a soft consistency. The lesions are usually solitary, with sizes ranging from 2 to 15 cm. HCAs tend to be larger in women on oral contraceptives. Most are located in the right lobe and are subscapular. Microscopic examination shows a lack of malignancy features and abundant glycogen or fat. Liver architecture is absent, with central veins, bile ducts, and portal tracts often not visualized. However, HCAs are well-vascularized with small, thin-walled arterioles.
HCAs are typically solitary tumors with monoclonal cell lines. These tumors lack a true capsule, though hepatocyte sheets in the outer boundaries form their "pseudo-capsule" and are surrounded by otherwise normal liver tissue. The lack of biliary ducts distinguishes the lesion from normal liver tissue (see Image. Hepatocellular Adenoma Histopathology).
History and Physical
History
About half of patients with HCAs are asymptomatic, with the tumor discovered incidentally on imaging. The condition presents variably in symptomatic individuals, ranging from mild, ill-defined epigastric pain with bloating to severe, acute RUQ pain.[14] Adenoma rupture may be accompanied by dizziness, intractable vomiting, and lethargy.[15] The pain may radiate to the right flank in some cases, resembling cholecystitis or a urinary tract pathology. Rupture occurs in up to 27% of HCAs, resulting in a 5% to 10% mortality rate.[16]
Individuals with suspected HCA should be asked about the symptoms, especially abdominal pain and its characteristics. Risk factors must be assessed, including prolonged sex hormone use and underlying conditions like GSD and obesity. The family history may reveal liver diseases or malignancies and other genetic conditions predisposing to HCA evolution.
Physical Examination
A complete physical examination helps determine the diagnosis and the patient's hemodynamic status. Individuals with abdominal pain due to gastrointestinal pathology often lie still, in contrast to patients with renal colic. Tachycardia and hypotension are possible indicators of shock. Fever may manifest due to inflammation and is a physical feature shared with many other conditions, including hepatitis.
Some patients may have dry oral mucosa and poor skin turgor from dehydration. Pallor and poor pulses may be appreciated due to hemorrhage. Normal chest auscultation findings may rule out a cardiac or pulmonary cause of referred abdominal pain unless such illnesses coexist. The abdominal examination may reveal hepatomegaly with tenderness. Abdominal rigidity may be a sign of peritoneal irritation from hemoperitoneum due to tumor rupture.
Evaluation
Laboratory tests are generally unhelpful in diagnosing hepatocellular adenomas, though they can help rule out other disorders and determine hematologic and metabolic status. α-fetoprotein (AFP) levels are typically normal but can rise if malignant transformation to hepatocellular carcinoma (HCC) occurs. Hepatitis tests should be conducted to rule out these infections. Alkaline phosphatase and γ-glutamyl transferase levels may increase two- to threefold, especially in IHCAs. White blood cell count, fibrinogen, and CRP may also be elevated.[17][18][19]
Core needle biopsy has limited diagnostic value in HCA. However, immunohistochemical markers may be helpful in expert centers. Biopsy should be limited to rare cases where imaging is equivocal, and the diagnosis may alter management strategy.
Ultrasonography often fails to distinguish between benign and malignant tumors. Doppler ultrasonography may demonstrate arterial hypervascularity with vessels running along the lesion's border in a "basket" pattern. Some tumors may appear hyperechoic due to the hepatocytes' steatotic content.
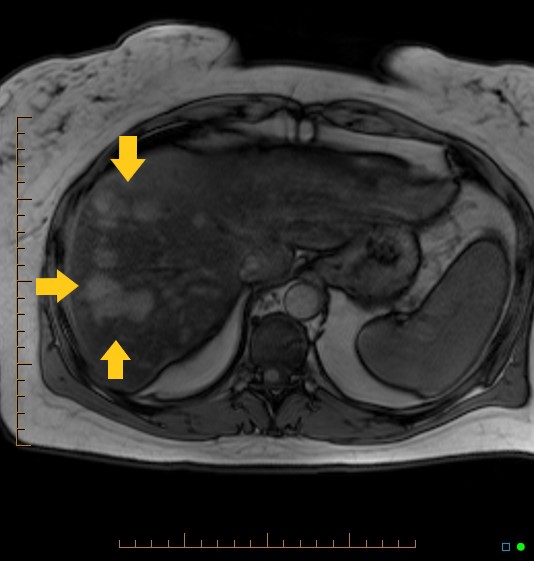
Dynamic magnetic resonance imaging (MRI) with a hepatocyte-specific contrast agent like gadobenate dimeglumine is the modality of choice for diagnosing HCAs. This method can distinguish between hepatocellular adenomas and other benign and malignant liver tumors (see Image. Hepatocellular Adenoma on Magnetic Resonance Imaging). The lesion may exhibit a clearly defined central margin with nearly parallel vessels entering from the periphery, giving the appearance of a spoked wheel. In some cases, dynamic MRI may demonstrate a tortuosity of peripheral vessels with central necrosis.
A dynamic computed tomography (CT) scan is also potentially useful in evaluating HCAs. Dynamic CT may show peripheral enhancement of the tumors during the early phase, transitioning to centripetal flow in the later portal venous phase.
Treatment / Management
HCAs less than 5 cm in size and linked to oral contraceptive pills are initially approached conservatively. Oral contraceptive withdrawal with regular imaging surveillance has demonstrated significant tumor regression in numerous cases. Resuming oral contraceptive intake requires careful radiologic monitoring. The optimal follow-up duration remains unestablished, with some authors suggesting surveillance until menopause. Refractory tumors often correlate with obesity.
Most hepatic adenomas remain stable during pregnancy. Tertiary centers often monitor adenomas smaller than 5 cm serially in pregnant women every 3 months and during the postpartum period. Patients with small adenomas are not discouraged from getting pregnant.
Surgical resection is recommended for all male patients regardless of the tumor size and for women with tumors larger than 5 cm. The technique does not require a wide margin or regional lymphadenectomy. Emergent surgery for a ruptured hepatic adenoma with intraperitoneal bleeding has a mortality rate of 5% to 10%. In contrast, elective resection's mortality rate is less than 1%.
Transarterial embolization (TAE) is recommended for hepatic adenomas complicated by hemorrhage. Patients with intratumoral hemorrhage rarely present with hemodynamic instability. In such cases, TAE may be followed by elective surgical resection of the adenoma. TAE is indicated within 2 to 3 days of tumoral hemorrhage.
Radiofrequency ablation is appropriate only for a very select patient population. The modality is not appropriate for surgical candidates and individuals with hormone-sensitive tumors, underlying liver disease, or a desire for pregnancy. Radiofrequency ablation should be used to treat patients with adenomas less than 4 cm in size.
Obesity, nonalcoholic steatohepatitis, and metabolic syndromes increase the risk of developing hepatic adenomatosis. Complications like hemorrhage or malignant transformation correlate less with adenomatosis than the tumors' molecular signatures. Moreover, most liver adenomatoses are typically associated with HNF-1α mutations, which have a low risk for malignant transformations. Thus, a liver transplant is generally not indicated for nonresectable hepatic adenomas. Exceptions involve men with intrahepatic portosystemic venous shunts with nonresectable hepatic adenomas. Genetic counseling is recommended for patients with liver adenomatosis, especially if associated with familial adenomatous polyposis or MODY3.
About 4% to 5% of hepatic adenomas are at risk for malignant transformation to HCC, with the risk increased by Fanconi anemia and androgen treatments. The Wnt and β-catenin pathways are particularly associated with malignant transformation. Surgical resection is typically recommended for this subtype.[20]
Differential Diagnosis
The differential diagnosis of liver lesions suspicious for HCA includes hemangioma, focal nodular hyperplasia (FNH), HCC, and metastatic tumors. Dynamic contrast-enhanced MRI can differentiate between HCA and hemangiomas, which generally exhibit peripheral enhancement and a centripetal fill-in pattern.[21] Gadolinium-based contrast MRI often shows FNH taking up more contrast in the hepatobiliary phase than HCA, along with a central scar and lobulated appearance.[22]
The clinical scenario is crucial in distinguishing between HCA and HCC or metastatic disease. Individuals with HCC typically have underlying chronic liver disease with or without cirrhosis. People with metastatic disease typically have a known extrahepatic primary malignancy and associated risk factors.
Prognosis
The prognosis for patients with hepatocellular adenoma remains poorly established. Oral contraceptive discontinuation may result in the regression or resolution of some lesions. As previously stated, about 27% of HCAs may rupture, which has a 5% to 10% mortality risk. The risk of HCA's malignant transformation is 4.2%.[23] The risk of malignancy generally persists even after discontinuing oral contraceptives.
Complete HCA resolution is unlikely in most patients. About 25% of women will continue to have RUQ pain, with hemorrhage potentially occurring in up to 30% to 45% of women. Bleeding may occur within the lesion or peritoneum. The larger the lesion, the higher the risk of hemorrhage. Pregnancy has been associated with HCA enlargement and increased rupture risk.
Complications
HCA's complications include tumor rupture and hemorrhage, which may result in hemorrhagic shock and death. Tumors larger than 5 cm have an increased rupture risk. The malignant transformation of HCAs is greater in men than women with tumors larger than 5 cm.
Consultations
HCA management requires interprofessional care coordination involving gastroenterologists, radiologists, and surgeons. The interprofessional approach enhances diagnostic accuracy and therapeutic or surveillance efficacy.
Deterrence and Patient Education
Primary prevention of HCAs involves minimizing exposure to known risk factors, such as oral contraceptives and anabolic steroids. Education about the risks associated with these medications and their potential to induce HCA development is crucial. Making healthy lifestyle choices, including weight management and avoiding excessive alcohol consumption, may also help decrease the risk of developing HCAs.
Meanwhile, patients with the condition or genetic risks for its development must be counseled about potential complications, eg, rupture and HCC, and their likelihood if no treatment is taken. Female patients should be advised that pregnancy is not contraindicated, especially with lesions of 5 cm or less. However, pregnant patients with HCA must receive interprofessional care and meticulous tumor monitoring. If surgery is warranted during pregnancy, resections should ideally occur during the 2nd trimester when risks to both mother and fetus are at their lowest. Patients must be fully informed of their condition and any action's options, risks, and benefits.
Pearls and Other Issues
HCAs are rare liver tumors often linked to oral contraceptive intake in reproductive-age women. Obesity, metabolic dysfunction associated with steatohepatitis, and metabolic syndromes are associated with hepatocellular adenomatosis. The most dreaded complications of this condition include rupture and HCC.
The phenotypic-genotypic classification system for this condition is validated and valuable in understanding molecular behavior patterns. The HNF-1α subtype, associated with hepatocyte differentiation, is prevalent in women and individuals with MODY3. β-catenin-activated mutations, found in 15% to 20% of HCAs, are often linked to male hormone exposure and carry a greater risk of malignant transformation. Surgical resection is recommended for this subtype. IHCAs, prevalent in women with higher BMI and alcohol intake, stain positively for SAA and CRP. Unclassified types lack the features exhibited by other types and comprise 10% of HCA cases.
Laboratory diagnosis is often unhelpful diagnostically. However, MRI can help reliably distinguish HCAs from other liver conditions. Imaging complements clinical examination during the diagnostic process. Discontinuation of oral contraceptives can induce regression in adenomas less than 5 cm, warranting continued imaging surveillance. Surgery is generally recommended for tumors greater than 5 cm in size but does not require wide margins or regional lymphadenectomy.
Enhancing Healthcare Team Outcomes
Prevention rather than treatment is the main focus of HCA management. Patient education involves the primary care physician, pharmacist, and nurse, contributing to better outcomes through interprofessional management. Once detected, the gastroenterologist and surgeon's roles—primarily surveillance and possibly treatment—become invaluable in minimizing morbidity and mortality. Pregnant women must be under the care of a high-risk obstetric care specialist, who may coordinate with the interprofessional team.
All women prescribed oral contraceptives should be warned about the risk of developing this lesion, with lower doses potentially reducing the risk. Patients without other contraception options should be educated on HCA symptoms and when to seek help. Pregnancy increases HCA's hemorrhage risk and maternal mortality. Women with large or symptomatic tumors who desire pregnancy should be counseled about considering HCA removal before conceiving. Ongoing imaging surveillance, possibly annually, is recommended, especially for lesions larger than 5 cm.[24][25]