Introduction
Belonging to the family Taeniidae, the tapeworm Echinococcus is responsible for causing echinococcosis in humans. There are four species of Echinococcus known to produce infections in humans. Among these, E. granulosus and E. multilocularis are the most common ones responsible for causing cystic echinococcosis (CE) and alveolar echinococcosis (AE), respectively. On the other hand, E. vogeli and E. oligarthrus cause polycystic echinococcosis, but the incidence in humans is quite rare.
Etiology
Echinococcosis is caused by infection from the parasite Echinococcus (a tapeworm). The life cycle of Echinococcus includes a definitive host (usually dogs) and an intermediate host (like sheep, goats, or swine). Humans are accidental hosts, and they do not play a part in the transmission. Adult tapeworm resides in the small intestine of the definitive host, where it lays eggs containing embryos (oncospheres). These eggs expelled via feces get ingested by the intermediate or incidental host. After being hatched from eggs, oncospheres migrate via blood or lymphatics after piercing the intestinal mucosa to the liver, lungs, or other visceral organs and form cysts. Protoscoleces develop within these cysts. The life cycle gets completed when the definitive host consumes the infected organs of the intermediate host.[1]
Epidemiology
The epidemiology of echinococcal disease varies from species to species. Although E. granulosus is almost prevalent throughout the world, higher incidence rates are found in South America, the former Soviet Union, the Middle East and the eastern Mediterranean, few sub-Saharan African countries, and western China. In particular, the areas where dogs can consume organs of infected animals, such as the rural and the grazing ones, possess higher infection rates.
E. multilocularis is mostly limited to the Northern Hemisphere in areas such as the northern parts of Europe, Asia, and North America, as well as Central Europe. On the other hand, the remaining two Echinococcus species, E. vogeli and E. oligarthrus, are only limited to South and Central America.[1]
The prevalence of echinococcal disease increases with age. Older and immunocompromised people are more vulnerable to develop such infections. There is no sex predilection for hydatid cysts. While cystic Echinococcus is the disease of adulthood (average age of 30 to 40 years), alveolar Echinococcus tends to occur in old age (older than 50 years of age).
Pathophysiology
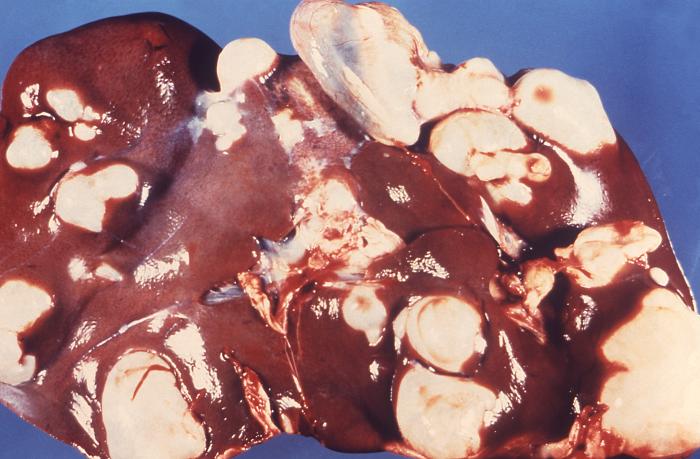
Once the eggs of the tapeworm have been ingested by a human, oncosphere larvae are released from the egg, which can pierce the lamina propria of the intestine. As it penetrates, it enters the blood or lymphatics and gets transported to the liver, lungs, or other internal organs, where it develops into hydatid cysts (metacestode larvae). These cysts possess an inner germinal layer and an outer laminated layer surrounded by a fibrous capsule derived from the host. The inner cellular layer gives rise to smaller "daughter" cysts. In humans, cysts grow slowly and can be up to multiple liters in volume, and contain thousands of protoscolices. Over time, septations and daughter cysts disrupt the typical unilocular pattern of echinococcal cysts.[2]
History and Physical
Symptoms of E. granulosus infection depend on two factors: the size and the site of the cysts. While the small and/or calcified cysts tend to remain asymptomatic indefinitely, larger ones can produce pressure or mass effects on the surrounding structures or can rupture, leading to anaphylaxis or acquire a secondary bacterial infection.[3]
The clinical picture of liver cysts ranges from asymptomatic to hepatomegaly associated with right upper quadrant (RUQ) pain, nausea, vomiting, biliary colic, obstructive jaundice, and pancreatitis (when a cyst ruptures into the biliary tree). Pressure effects can lead to hepatic veins, portal veins, and IVC obstruction, leading to venous obstruction, portal hypertension, and Budd Chiari syndrome. Peritonitis can also occur if the cyst ruptures into the peritoneum.[4]
The involvement of the lungs can give rise to cough, chest pain, dyspnea, hemoptysis, and less frequently nausea, vomiting, and malaise.
Other organs involved include the heart (pericardial tamponade), the brain (seizures and signs of raised ICP), the spinal cord (spinal cord compression), the kidneys (flank pain and hematuria), and the bones (pathologic fractures).
E. multilocularis infection clinical features tend to be nonspecific. The most common complaints include malaise, weight loss, RUQ pain owing to hepatomegaly, and obstructive jaundice. It may also mimic hepatocellular carcinoma.
When obtaining medical history, risk factors of echinococcosis, especially contact with canines and cattle, should be assessed.
Evaluation
The diagnosis of echinococcal infection is made based on imaging and serology.
- Blood tests may reveal leukopenia, thrombocytopenia, mild eosinophilia, and abnormal liver function tests, but these are nonspecific and not diagnostic.[4]
- Imaging: Ultrasonography (USG), CT, and MRI are the modalities that can help to diagnose the echinococcal infection. While the USG is convenient and comparatively inexpensive, CT and MRI have the upside of being more precise in terms of specifying the number and the location of cysts, the presence or absence of daughter cysts, and the ability to rule out any complication, thus help in guiding the clinical management.
Ultrasonography is 90% to 95% sensitive for E.granulosus. Based on ultrasonographic images, echinococcal cysts have been categorized by WHO to help in guiding the correct treatment. This categorization is as follows[5]:
- CE1: Unilocular anechoic cystic lesion with double line sign (active stage)
- CE2: Multiseptated, "rosette-like", "honeycomb" cyst (active stage)
- CE3a: Cyst with detached membranes (water-lily sign) (transitional stage)
- CE3b: Cyst with daughter cysts in the solid matrix (transitional stage)
- CE4: Cyst with heterogenous hypoechoic/hyperechoic contents; no daughter cysts (inactive stage)
- CE5: Solid plus calcified wall (inactive stage)
Radiography can detect calcifications in up to 30% of the cases. These calcifications are generally ring-like and can progress throughout all the stages of the disease.[6]
CT is superior to USG in terms of higher sensitivity (95 to 100%), determining the size, site, and location of the cysts (extrahepatic as well), and assessing for complications.[7]
MRI offers no major advantage over CT except in determining intra- and extrahepatic venous changes.
Serology helps in the primary diagnosis of echinococcosis as well as in the follow-up after treatment. There are numerous diagnostic serologic techniques, and among these, ELISA is the most specific and sensitive one. Few limitations to serological testing involve nonviable and calcified cysts. Also, liver cysts tend to be more seropositive than lung cysts, and serology is more specific for E. multilocularis than for E. granulosus.
Percutaneous aspiration or biopsy is preferred when other modes have proven to be inconclusive. However, because of the potential anaphylaxis and secondary spread of infection, aspiration, if required, should always be performed under the guidance of either USG or CT. If the disease involves the biliary tree, then endoscopic retrograde cholangiopancreatography (ERCP) can be used for diagnostic and therapeutic purposes.[8]
Treatment / Management
There are four modalities of treatment for echinococcal disease.[8] These include surgery, percutaneous management, drug therapy, and observation. The WHO classification helps to choose the modality of choice for the treatment of echinococcal cysts.
For WHO stage 1 and 3a (these cysts have a single compartment), cysts having a size less than 5 cm are treated with albendazole or PAIR (Puncture, Aspiration, Instillation, and Re-aspiration). In contrast, cysts larger than 5 cm are treated with PAIR along with the adjunctive treatment with albendazole.
For WHO stage 2 and 3b, in which cysts have multiple compartments rendering PAIR ineffective, these are managed with modified catheterization technique (Non-PAIR percutaneous therapy) or surgery with adjunctive albendazole.
WHO Stage 4 and 5 require only observation as they are inactive.
- Surgery: Surgical options include liver resection, local excision of the cyst, and deroofing with the evacuation of the contents. During surgery, contamination of surgical sites with active daughter cysts is a major concern, and this can be avoided by adjunctive therapy with albendazole and perioperative addition of praziquantel. Also, the peritoneum should be packed with 20% hypertonic saline packs, and the same mixture should be instilled into the cyst before opening.
- Percutaneous Therapy: It includes PAIR (for cysts without the daughter cysts) and modified catheter technique (for cysts that may contain daughter cysts). In the former technique, an initial course of albendazole is given, followed by a puncture of the cyst under imaging guidance, Aspiration of its contents, instillation of hypertonic saline into the cyst cavity, and re-aspiration. In the presence of communication with the biliary tree, PAIR should not be attempted. In the latter, a wide bore catheter is introduced into the cyst cavity to evacuate all of its contents.
- Medical Management: In definitive treatment, albendazole (15 mg/kg/day divided into two doses or 400 mg twice daily with food in an adult) or mebendazole is given continuously for 1 to 3 months or even 6 months depending on clinical factors. It should be known that medical therapy is futile in cases of cysts larger than 5 cm or having daughter cysts. In adjunctive therapy to surgery and percutaneous management, albendazole or mebendazole is given 4 to 30 days preoperatively and should be given for 1 month in case of albendazole and 3 months in case of mebendazole after surgery. Adjunctive therapy reduces recurrence by inactivating the protoscolices and renders the cyst wall soft, thus facilitating its removal.
- Observation: This is for uncomplicated stage 4 and 5 cysts.
- For alveolar Echinococcus, surgery is the main treatment of choice with two years of adjunctive drug therapy.
- Follow-up by ultrasonography and serology is done for 3 to 5 years.
Differential Diagnosis
Differential diagnosis of cystic echinococcus includes:
- Simple benign cyst: It produces the same symptoms of nausea, malaise, and RUQ discomfort but is easily differentiated by USG.
- Hemangioma: An incidental finding on imaging or laparotomy and presents with abdominal pain and fullness.
- HCC: It has characteristic features on imaging.
- Abscess: It can radiographically and clinically resemble echinococcal disease.
- Tuberculosis: It is differentiated by the presence of acid-fast bacilli on smear or culture.
Prognosis
In cystic echinococcosis, the prognosis is generally good compared to alveolar echinococcosis, where the prognosis is much worse, and the cure is dependent on early detection and complete surgical excision. Prognosis is poor when sites where surgical excision of the cyst poses a difficulty, especially when the heart and spinal cord are involved.[9][10][11][12]
Complications
- Complications related to parasite: recurrence, metastasis, infection, and anaphylactic shock resulting from spillage of cyst contents.[1][13]
- Complications related to drug therapy: hepatic toxicity, anemia, thrombocytopenia, alopecia, and teratogenicity.
- Complications related to PAIR and surgery: hemorrhage, infection, trauma to the surrounding structures, and anaphylaxis.
Deterrence and Patient Education
In general, the public of endemic areas must be made aware of the transmission of Echinococcus and that they should avoid contact with dogs. Once infected, the patient should receive education regarding the complications of echinococcal cysts. All the treatment options should be discussed thoroughly with the patient as to why one is superior to others. The clinician ought to inform the patient that these cysts tend to recur despite proper medical or surgical management. Finally, if cysts are amenable to drug therapy, strict compliance is key to eradicating the parasitic infection.
Endemic areas can benefit from mass screening using ultrasound as it will limit the disease in such areas.[14]
Enhancing Healthcare Team Outcomes
The echinococcal disease is a prevalent parasitic infection, particularly among farmers and shepherds, often seen by the primary care provider, nurse practitioner, or emergency department physician. These clinicians should always consult with the hepatobiliary surgeon and radiologist before starting any treatment. The disease needs to be dealt with in time to avoid morbidity and mortality. Coordination among healthcare professionals is vital for better patient outcomes.
The management and the evaluation of echinococcal disease requires an interprofessional approach as cysts resulting from this condition tend to form in different viscera of the body, thus requiring multiple consultant approaches accordingly. For instance, brain cysts require opinions from a neurologist as well as a neurosurgeon. Likewise, liver and lung cysts require consultant opinions from hepatologists and pulmonologists, respectively, other than the general surgeon. The role of radiologists is evident as imaging helps make the primary diagnosis and guides the treatment of choice. The nursing staff is also a vital segment of the interprofessional group as they assist in educating the patient and family members about the disease. Finally, pharmacists' roles are imperative, too, particularly when drug therapy is initiated and monitored. This type of interprofessional collaboration is the key to achieving optimal patient outcomes in the cases of echinococcosis. [Level 5]