Continuing Education Activity
Optic ischemia is a severe condition threatening vision integrity by causing ischemic damage along the optic nerve's intricate pathway. The condition arises from compromised blood flow to the optic nerve, leading to inadequate oxygen and nutrient delivery. This vascular insufficiency can result from various etiologies, including arteriosclerosis, embolic phenomena, vasculitis, or compression of the vascular supply, culminating in ischemic damage to the optic nerve and subsequent vision impairment. Risk factors include hypertension, diabetes mellitus, and smoking.
The diagnosis of optic ischemia entails a multifaceted approach combining clinical evaluation, imaging, and ancillary tests to determine the underlying cause and severity. Early detection is pivotal for preventing irreversible vision loss and deciding effective management strategies. Treatment of optic ischemia aims to address the underlying cause while optimizing ocular perfusion and preserving vision. Interventions may include managing systemic vascular risk factors, antiplatelet therapy, vasodilators, corticosteroids (in cases of vasculitis), and surgical interventions to improve blood flow.
This activity for healthcare workers is designed to enhance learners' competence in evaluating and managing optic ischemia. Participants gain a deeper grasp of the optic nerve's anatomy, vascular supply, and susceptibility to ischemic insults. Learners also enhance their understanding of optic ischemia's pathophysiology, symptomatology, complications, and treatment urgency. Participants become prepared to collaborate effectively within an interprofessional team to enhance outcomes for patients with this disorder.
Objectives:
Differentiate the various etiologies of optic ischemia.
Create a clinically guided diagnostic strategy for a patient with suspected optic ischemia.
Implement comprehensive treatment plans for optic ischemia, utilizing evidence-based interventions to target the underlying etiology, mitigate further ischemic damage, and preserve remaining vision.
Apply effective strategies to improve care coordination among interprofessional team members to facilitate positive outcomes for patients with optic ischemia.
Introduction
Optic Ischemia Overview
Optic ischemia, otherwise known as ischemic optic neuropathy, encompasses vascular diseases affecting the optic nerve. The condition is categorized into anterior and posterior types. Anterior ischemic optic neuropathies often present with optic disc edema, while posterior ones do not.
Nonarteritic acute anterior ischemic optic neuropathy (NAION) is the most common form of optic ischemia, typically occurring in individuals with a small, crowded optic nerve. However, the exact cause of this disorder remains unknown, and curative or preventive treatment is not available. Posterior ischemic optic neuropathy is rare and typically associated with cardiovascular risk factors or perioperative conditions. This subset also lacks specific treatment.
Urgent evaluation for arteritic causes, notably giant-cell arteritis (GCA), is essential, with immediate intravenous methylprednisolone administration indicated to limit vision loss in the affected eye and prevent involvement of the other eye.[1]
Optic Nerve Anatomy
The optic nerve spans from the eyeball to the chiasm and has 4 segments: intraocular (1 mm), intra-orbital (30 mm), intracanalicular (6-10 mm), and intracranial (10-16 mm). Nerve fibers arise from retinal ganglion cells and converge at the optic disc, commonly known as "the blind spot," before traveling approximately 1 mm within the globe and penetrating the sclera through a sieve-like structure called "lamina cribrosa." Encased by the optic nerve sheath, the optic nerve also sits amid extraocular muscles and fat tissue.
The optic nerve has a diameter of about 3 mm, while the optic nerve sheath measures approximately 1 mm thick. The sheath consists of the pia mater, subarachnoid space, arachnoid mater, and dura mater. This covering varies in thickness from 0.09 to 0.15 mm for the pia and arachnoid mater, to 0.1 to 0.29 mm for the subarachnoid space, and 0.3 to 0.5 mm for the dura. The subarachnoid space features a complex structure of trabeculae, septa, and pillars immersed in cerebral spinal fluid, with variations along the nerve's course.[2]
The optic nerve is divided into anterior and posterior portions and may be damaged by ischemia anywhere along its path. The optic nerve area between the retina and sclera is supplied by a network of 6 to 12 short posterior ciliary arteries at the back of the globe. These vessels form a circumferential network called the "circle of Zinn-Haller." The optic nerve head is perfused in a centripetal and segmental fashion. Branches from the pial plexus supply the optic nerve's retrolaminar portion.[3]
The anterior part contains trabeculae of 5 to 7 µm diameter, while the midsection exhibits septa and pillars dividing the space into communicating chambers, with pillars ranging from 10 to 30 µm in diameter. Both pillars and trabeculae are present in the posterior part, where the sheath traverses the optic canal. The right and left optic nerves cross each other within the cranium, forming the optic chiasm and connecting with the visual cortex within the occipital lobe.[4]
Etiology
Anterior ischemic optic neuropathy (AION) is far more common than posterior ischemic optic neuropathy (PION). AION is further divided into arteritic and nonarteritic forms.
Arteritic Anterior Ischemic Optic Neuropathy
Arteritic AION (AAION) occurs in patients older than 70. The condition arises from inflammatory and thrombotic occlusion of short posterior ciliary arteries. AAION is associated with systemic vasculitis, GCA being its most common cause.[5]
Nonarteritic Anterior Ischemic Optic Neuropathy
The cause of nonarteritic AION (NAION) is uncertain. However, anatomical structure variation, ie, structural crowding of the optic disc, is a major contributing factor to this condition's development. This optic disc condition is commonly called a "disc at risk." The prevailing theory is that autoregulation in the presence of any stress factor leads to capillary filling pressure falling below a critical level and damage. The associated risk factors include:
- Systemic hypertension [6]
- Diabetes mellitus [7]
- Hyperlipidemia [8]
- Hypercoagulable disorders [9]
- Sleep apnea [10]
- Nocturnal hypotension [11]
- Phosphodiesterase inhibitors (sildenafil) [12]
- Anemia [13]
- Smoking [14]
- Migraine
- Optic nerve head drusen [15]
Posterior Ischemic Optic Neuropathy
PION is notably less prevalent than AION, mainly occurring under 3 circumstances. First, PION may arise in perioperative settings, particularly during spinal, cardiac, head, and neck surgeries. Factors such as significant blood loss, prolonged anesthesia, and hypotension during this period can compromise the optic nerve's blood supply. This etiology is most common in this group.[16] Second, PION may occur in arteritic cases, with GCA being the most common culprit.[17] Third, nonarteritic PION may develop from conditions similar to NAION.
Epidemiology
NAION comprises most AION cases (nearly 85%), with an estimated 6000 new cases annually in the United States. NAION more commonly affects men and white individuals. The condition's annual incidence rate is 10.3 per 1000,000 people, with the median age being 72 at diagnosis.[18] AAION is much less common, with an annual incidence of 0.36 cases per 100,000 people.[19]
History and Physical
Individuals with optic ischemia typically present with sudden, painless loss of vision. Other signs and symptoms depend on the type involved, as explained below. Patients should be asked about the onset and duration of visual symptoms. Associated symptoms like headache, jaw pain, or scalp tenderness may indicate underlying conditions like GCA. Other information that must be elicited includes systemic vascular risk factors, current medications, surgical history (especially head, neck, or cardiovascular procedures), and family history of ocular or vascular diseases.
The physical examination findings vary depending on the nerve part and pathophysiologic mechanism involved. The patient's visual acuity must be assessed using standard charts. A formal visual field test and funduscopy are recommended. Pupillary responses, intraocular pressure, and extraocular movements must be evaluated. A comprehensive systemic examination should be performed, including vital signs, cardiovascular evaluation, and signs of systemic vasculitis, which may also guide diagnosis and treatment.
Arteritic Anterior Ischemic Optic Neuropathy
AAION usually occurs in older patients (mean age of 70 years). Most cases are linked to vasculitis, with GCA being the most common. Vision loss is severe, at less than 20/200 in 70% of cases, while 20% of patients have no light perception.[20][21]
Inquiring about GCA symptoms is crucial due to the high risk of vision loss in the fellow eye without prompt treatment, often occurring rapidly. GCA is also associated with other potentially life-threatening complications, such as stroke, aortic dissection, and myocardial infarction, which are preventable with timely intervention.
Systemic GCA symptoms include temporal headache, scalp tenderness, and jaw claudication due to masseter muscle ischemia. Patients may also experience nonspecific manifestations such as weight loss, fever, night sweats, malaise, and depression. Polymyalgia rheumatica commonly coexists with GCA. The condition is characterized by pain and stiffness in proximal muscle groups like shoulders and thighs.
The 2022 American College of Rheumatology Criteria for Giant Cell Arteritis has classification guidelines for diagnosing GCA, particularly useful when confirming medium or large vessel vasculitis. The diagnosis requires a total score of 6 or more points based on the criteria and their respective point values. Table 1 shows the criteria and point values (see Table. 2022 American College of Rheumatology Criteria for Giant Cell Arteritis).[22]
Table. 2022 American College of Rheumatology Criteria for Giant Cell Arteritis
|
Absolute criterion: Onset at age 50 or older
|
| Additional Criteria |
Point Values |
| Temporal artery biopsy positive for vasculitis, with a predominance of mononuclear-cell infiltration or granulomatous inflammation, usually with multinucleated giant cells |
+5 |
| Erythrocyte sedimentation rate (ESR) > 50 mm/h using the Westergren method or C-reactive protein (CRP) > 10 mg/liter² |
+3 |
| Sudden vision loss |
+3 |
| Axillary involvement bilaterally |
+2 |
| 18-fluoro-deoxyglucose positron emission tomography (FDG-PET) activity throughout the aorta |
+2 [23] |
| Scalp tenderness |
+2 |
| Temporal artery examination abnormalities, eg, temporal artery tenderness or decreased pulsation to palpation |
+2 |
| Shoulder or neck morning stiffness |
+2 |
| Tongue or jaw claudication |
+2 |
| New headache in the temporal region |
+2 |
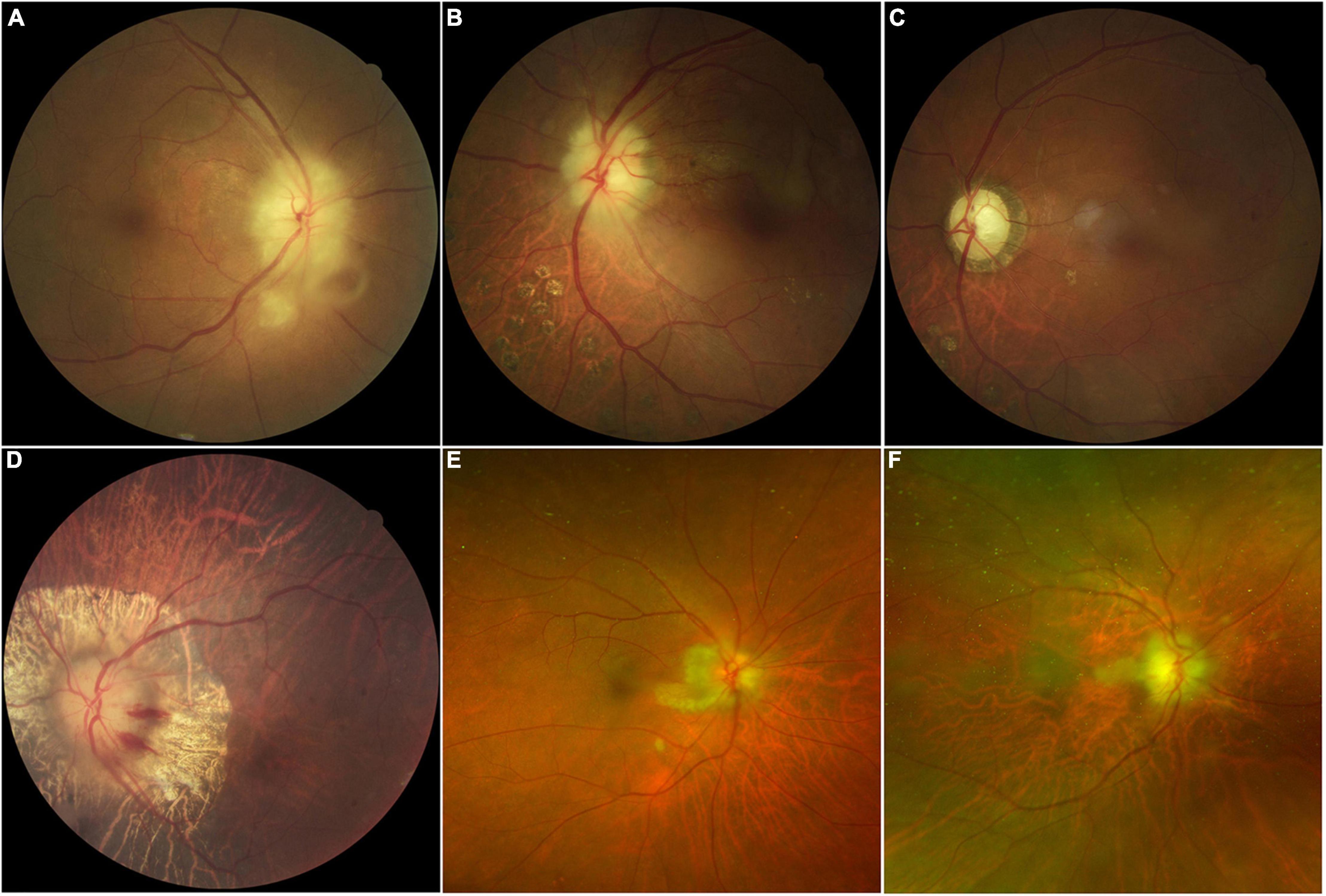
Pupil reaction is sluggish on examination, with a relative afferent pupillary deficit unless both eyes are examined simultaneously. The fundus examination may show chalky white optic disc edema, indicative of swelling at the optic nerve head. Cotton wool spots are areas of retinal ischemia resulting from impaired blood flow to the nerve fiber layer. These spots may appear distant from the optic disc edema but are important signs of ischemia within the retinal layers (see Image. Giant Cell Arteritis Funduscopic Findings).
Nonarteritic Anterior Ischemic Optic Neuropathy
NAION vision loss may be acute, subacute, or stepwise and is not usually associated with pain. Vision loss is less severe than in AAION, as about half of patients have a 20/60 visual acuity examination result or better.[24][25] Patients usually report vision loss after awakening in the morning, which may progress over weeks to months before the final stabilization of vision.
Fundus examination in the acute stage often exhibits diffuse or segmental optic disc edema on the affected side, predominantly in the superior or inferior regions. This finding is accompanied by heightened perfusion, characterized by hyperemia. Small hemorrhages may also be observable near the optic nerve head. The fellow eye usually shows a small or absent cup, with a cup-to-disc ratio of 0.2 or less, indicative of a disc at risk.
Optic edema gradually resolves, leading to decreased nerve fiber crowding and subsequent optic cup enlargement as atrophy develops. The second eye becomes involved gradually, resembling pseudo-Foster-Kennedy syndrome, where one eye shows atrophy while the other exhibits hyperemia and swelling.[26] True Foster-Kennedy syndrome exhibits optic disc atrophy in one eye due to optic nerve compression by an intracranial mass, while the contralateral eye presents with optic disc edema due to elevated intracranial pressure.
Posterior Ischemic Optic Neuropathy
PION's vision loss may be unilateral or bilateral. Optic disc edema is absent in the acute stages, though optic atrophy typically occurs after a few months. PION is a rare disease and is mainly considered a diagnosis of exclusion. The condition is associated with radical neck dissection or spine surgery.
Evaluation
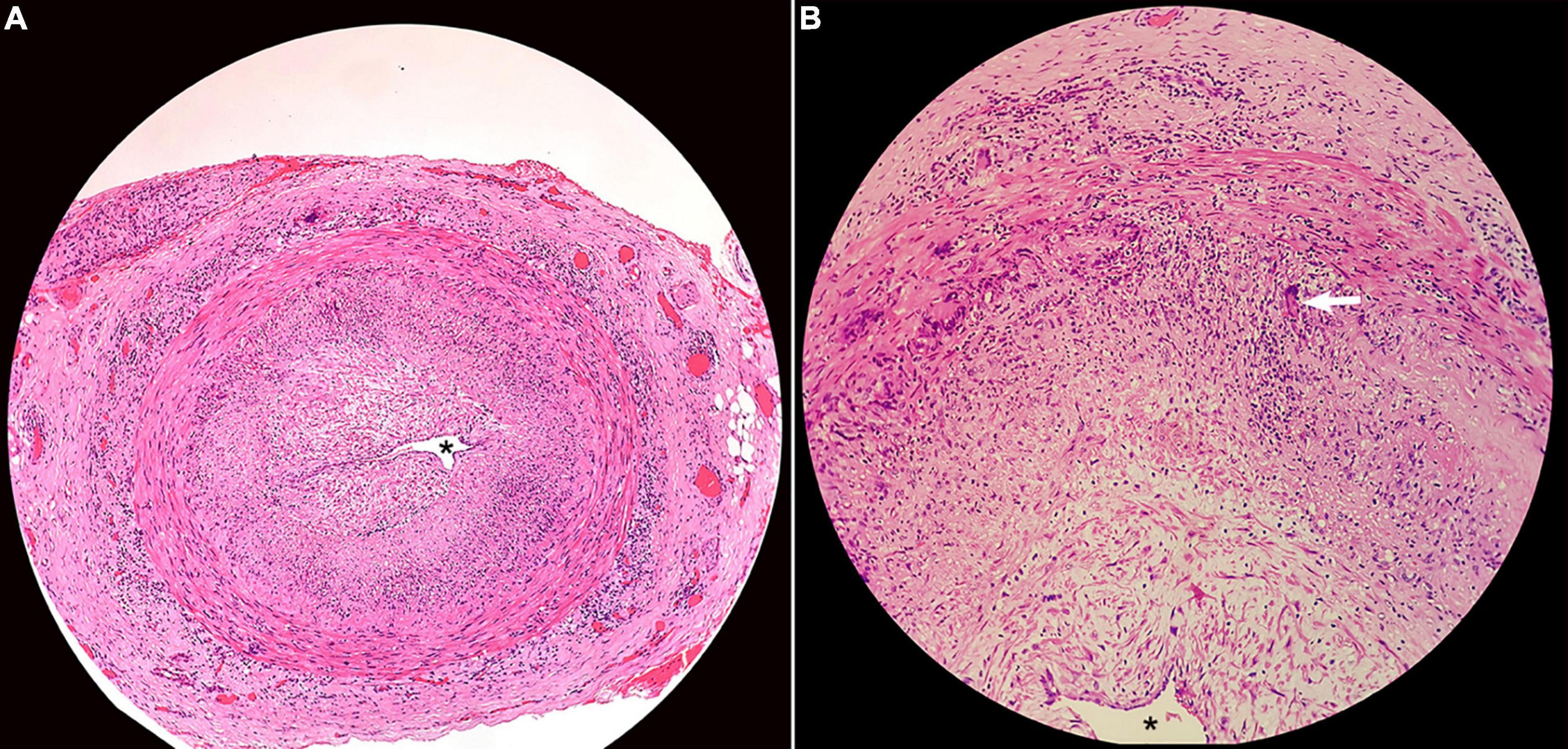
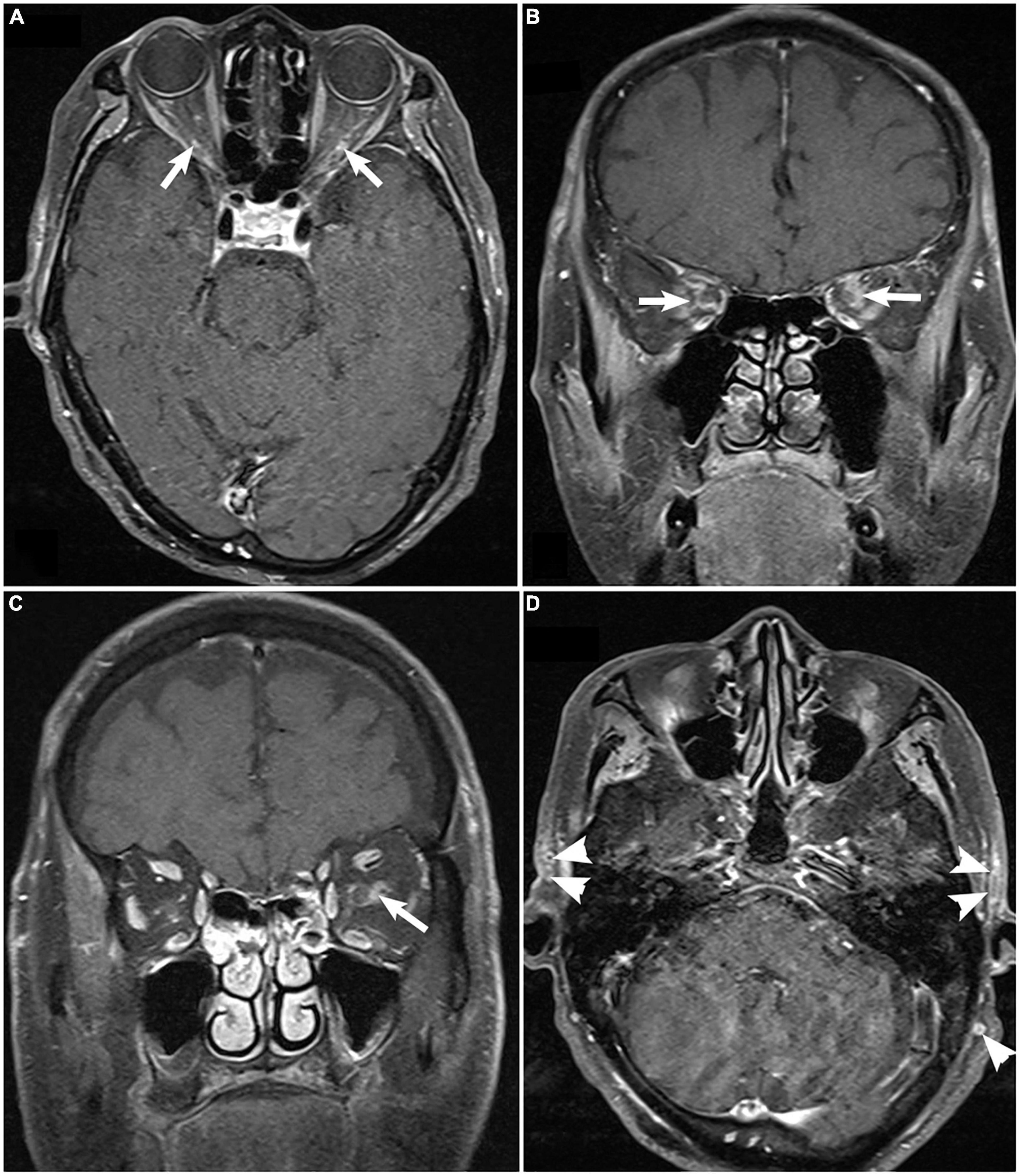
GCA should always be ruled out in individuals with AAION. Elevated ESR and CRP are sensitive but not specific to GCA. Temporal artery biopsy may help rule out GCA. A minimum arterial specimen length of 2 cm is required.[27] Biopsy findings often reveal granulomatous inflammation involving the internal elastic lamina (see Image. Superficial Temporal Artery Histopathology). However, biopsies may occasionally yield false-negative results, prompting the use of noninvasive techniques such as magnetic resonance imaging (MRI) and Doppler ultrasound (see Image. Giant Cell Arteritis on Magnetic Resonance Imaging.).[28][29][30]
Vascular risk factors must be assessed, including blood pressure, lipid profile, blood glucose, and hemoglobin A1c levels, as patients may be unaware of them. Patients must be asked sleep apnea screening questions. The patient's medications must be reviewed, assessing for phosphodiesterase type 5 inhibitor intake to rule out nocturnal hypotension. Testing for hyperhomocysteinemia is recommended in individuals younger than 50.
Normal choroidal filling on fluorescein angiography occurs 3 to 5 seconds faster than the retinal arteries. However, AAION exhibits delayed choroidal filling. Visual fields are often abnormal on confrontation tests like finger counting and consistently show abnormalities with automated perimetry, frequently presenting as an altitudinal defect, commonly inferiorly.
Treatment / Management
Arteritic Anterior Ischemic Optic Neuropathy
High-dose corticosteroids should be started immediately in cases of high suspicion after drawing blood samples for CBC, ESR, and CRP. The lab results should not delay corticosteroid administration. Immediate treatment prevents vision loss in the fellow eye, with second eye involvement likely within days to weeks of therapeutic delay. Temporal artery biopsy may be postponed for 7 to 10 days if nearby facilities are unavailable. Temporal artery pathological changes require weeks to months to alter.
High-dose intravenous methylprednisolone 1 g/day for 3 to 5 days is preferred. Studies indicate varying durations. A 3-day course of high-dose intravenous steroids shows potential benefits for visual recovery in the affected eye and prevention of disease in the fellow eye.[31][32] The consensus approach involves initiating moderate-to-high dose oral prednisone (1 mg/kg/day) following intravenous therapy, with a gradual taper over 3 to 12 months based on response. ESR and CRP levels should be closely monitored throughout the course.
Nonarteritic Anterior Ischemic Optic Neuropathy
Therapeutic efficacy for NAION remains debated, with corticosteroids, optic nerve sheath fenestration surgery, and levodopa showing partial effectiveness.[33][34][35] Ongoing research explores stem cell therapy, demonstrating promising results.[36] Priority lies in preventing vision loss in the fellow eye. Effective secondary prevention strategies for NAION include controlling diabetes mellitus, hypertension, hyperlipidemia, smoking, and obstructive sleep apnea.[37]
Differential Diagnosis
Optic ischemia, especially NAION, can mimic optic neuritis, as it features sudden vision loss, relative afferent pupillary defect, and optic disc swelling with associated hyperemia. Meanwhile, optic neuritis' clinical characteristics include the following:
- Patient age 40 years old and younger
- Painful eye movements
- Central visual field loss
- No delayed disc filling on fluorescein angiography
Contrast-enhanced MRI may help differentiate these 2 conditions. The affected optic nerve appears normal in NAION, but gadolinium contrast enhances the optic nerve in optic neuritis.[38]
Optic nerve compression can mimic AION. Compression can occur from optic nerve tumors, orbital inflammatory disease, or an ophthalmic artery aneurysm. Contrast imaging of the optic nerve's full length is appropriate to differentiate other causes from optic nerve ischemia.
Sometimes, optic disc drusen can mimic optic ischemia. The optic disc appears elevated and small in this condition. Disc blurring occurs due to axoplasmic flow stasis in axons. Most patients with this condition lack visual symptoms, though a few experience transient visual obscurations.
Prognosis
The prognosis is very poor for any type of ischemic optic neuropathy, with visual loss usually becoming permanent. A study revealed that the mean visual acuity following NAION was 20/200.[18] Another study found that following NAION, visual acuity of 20/40 or better was achieved in 83 out of 184 eyes, but only 8 out of 45 eyes achieved that visual acuity level in AAION.[39]
Complications
Optic atrophy is the most serious and inevitable complication of neglected optic ischemia, developing over a few months after the ischemic event.[18] Optic nerve atrophy results from the death of optic nerve tissue, leading to complete vision loss in the corresponding visual field area, as the visual signal fails to transmit from the retina to the brain.
Deterrence and Patient Education
Primary prevention of optic ischemia involves managing modifiable risk factors like hypertension, hyperlipidemia, obstructive sleep apnea, smoking, and diabetes mellitus through lifestyle modifications and pharmacological interventions. Regularly monitoring systemic vascular health and early detection of conditions predisposing to optic ischemia, such as giant cell arteritis, are essential. Educating patients regarding the importance of taking preventive measures and seeking timely medical evaluation for risk factor control can significantly reduce the likelihood of developing optic ischemia.
Patients with optic ischemia on one eye should be educated about immediate medical consultation if they experience sudden, severe vision loss on the other. The cruciality of maintaining routine health checkups in preventing optic ischemia in the second eye to control diseases like hypertension, diabetes, and obstructive sleep apnea should also be emphasized.
AAION is highly associated with GCA. Thus, individuals at risk should be informed of the importance of seeking an immediate investigation for symptoms of fever, unintended weight loss, scalp tenderness, and jaw claudication. Physician referral should be sought for comprehensive care and prevention of long-term complications associated with systemic steroid therapy, considering the extended treatment duration required for AAION.
Pearls and Other Issues
Optic ischemia presents diagnostic and management challenges. AAION often manifests with sudden vision loss. PION may develop more insidiously, complicating diagnosis. Careful history-taking to identify risk factors like hypertension, diabetes, and smoking history guides diagnostic evaluation. Clinical evaluation must include visual acuity assessment, visual field testing, and optic nerve examination.
Recognizing the urgency of early intervention to prevent irreversible vision loss is paramount. Differentiating between arteritic and nonarteritic causes is crucial, with GCA requiring immediate treatment to avert bilateral vision impairment. Prompt initiation of high-dose corticosteroids without waiting for laboratory results in suspected GCA cases is imperative to prevent irreversible vision loss. Imaging tests aid in assessing optic nerve perfusion and structural changes. A biopsy may be performed to rule out GCA but may be postponed in favor of immediate corticosteroid administration.
Long-term management focuses on systemic risk factor control, regularly monitoring inflammatory markers and vascular health to prevent recurrent ischemic events. Collaborative efforts between clinicians, ophthalmologists, and rheumatologists ensure comprehensive care, emphasizing the importance of managing systemic vascular risk factors to mitigate optic ischemia's impact.
Enhancing Healthcare Team Outcomes
Patients with optic nerve ischemia often present initially to non-eye care providers, such as primary care and emergency physicians or specialists in various medical fields. These clinicians must understand the disease's potential complications if not immediately treated. Prompt ophthalmology referral is essential for optimal management. An interprofessional team comprising primary care physicians, neurologists, optometrists, ophthalmologists, radiologists, pathologists, and rheumatologists is ideal for comprehensive management. Close follow-up with this team is vital for treating the affected eye and preventing disease progression in the fellow eye.[40]