Continuing Education Activity
Posterior urethral valves are a rare congenital disorder affecting the male urinary tract, characterized by abnormal membranous folds in the posterior urethra. This condition can lead to urinary tract obstruction, resulting in a spectrum of clinical manifestations ranging from delayed voiding to severe urinary retention. This activity delves into the intricate anatomy, embryology, and pathophysiology of posterior urethral valves, providing insights into the underlying mechanisms of this complex disorder. Furthermore, it outlines the current classification systems and discusses the evolving understanding of posterior urethral valves based on ongoing research and clinical observations.
Clinicians managing patients with posterior urethral valves face unique challenges due to the rarity of the condition and its diverse clinical presentations. This activity comprehensively describes the diagnostic workup and management strategies tailored to address the specific needs of affected individuals. Moreover, this activity underscores the importance of a collaborative interprofessional approach in providing comprehensive care to patients, emphasizing the pivotal role of transitioning from pediatric to adult healthcare services to ensure continuity of care and optimize long-term outcomes.
Objectives:
Identify posterior urethral valves promptly based on clinical presentations.
Implement timely and appropriate management strategies for posterior urethral valves.
Apply evidence-based guidelines and recommendations to optimize patient outcomes in managing posterior urethral valves.
Collaborate with a multidisciplinary team to provide comprehensive care for patients with posterior urethral valves.
Introduction
Posterior urethral valves are the most common cause of urinary tract obstruction and chronic kidney disease resulting from obstructive uropathy in the pediatric population.[1][2][3][4] The valves are actually obstructing membranous folds situated within the posterior urethral lumen attached to the verumontanum. These folds are found exclusively in male patients.
Posterior urethral valves were first described by Morgagni in 1717. Langenbeck further observed valve-like folds in dissected cadavers in 1802, and Hugh Hampton Young reaffirmed their presence in 1919.[5]
Posterior urethral valves can lead to a broad range of pathological conditions, spanning from asymptomatic cases to incompatible with sustaining life. Complications may encompass acute and chronic urinary retention, renal failure, bladder outlet obstruction, hydroureteronephrosis, vesicoureteral reflux, voiding dysfunction, and, in severe cases, pulmonary hypoplasia secondary to decreased amniotic fluid levels.
Posterior urethral valves are classically split into 3 subtypes based on Young's criteria, which delineate the orientation of the valves within the urethra.[6]
- Type I (95%): Posterior urethral folds (plicae colliculi) originate from the caudal verumontanum along the lateral margins of the urethra. These folds fuse anteriorly, causing an obstruction. They represent remnants of the Wolffian duct.
- Type II: Bicuspid leaflets or membranes are attached to the bladder neck proximally, originating from the verumontanum. These are now considered hypertrophic plicae colliculi, not true obstructive posterior urethral valves.
- Type III (5%): A round membrane situated at the caudal verumontanum, featuring a hole in the middle that is either above the verumontanum (type IIIa) or below (type IIIb). Neither subtype's hole directly communicates with the verumontanum.
Nevertheless, this classification has faced challenges. Dewan suggested that types I and III, as Young described, may represent the same structure. They posit that these structures appear distinct only because a central defect ruptures antenatally either naturally or due to iatrogenic instrumentation before birth.[7] In place of the term posterior urethral valves, Dewan introduced the concepts of a congenital obstructive posterior urethral membrane and "Cobb's collar." [8][9]
- Congenital obstructive posterior urethral membrane: An alternative term for the classic type of posterior urethra valves and is always associated with the verumontanum.[8][9] This congenital lesion manifests as a potentially obstructive proximal membrane featuring paramedian folds or leaflets extending along the posterior urethral wall to the verumontanum.[8][9]
- Cobb collar: Not a valve or a classical posterior urethral valve but a congenital bulbar urethral stricture or obstructive membrane unassociated with the verumontanum.[8] Located distally to the verumontanum in the bulbar urethra, it remains separate from the verumontanum and the external urinary sphincter.[8] This anomaly is believed to represent a persistent remnant of the urogenital membrane.[8]
Etiology
Posterior urethral valves are recognized as a congenital malformation, yet their exact embryological origin continues to be debated. A consensus has yet to be reached regarding this matter. Early theories proposed that posterior urethral valves could stem from persistent attachment of the verumontanum to the urethral roof or from a persistent urogenital membrane.
A study by Stephens in 1955 using voiding cystourethrography alongside microscopic and macroscopic analysis proposed a hypothesis regarding the formation of posterior urethral valves. According to this hypothesis, the valves arise due to the failure of Wolffian ducts to adequately integrate into the urethra, ultimately leading to an obstructing membrane.[10]
Dewan's recent research in the 1990s has contributed to resolving some disparities between the congenital obstructive posterior urethral membrane and Cobbs collar theories. Their findings indicated that the Cobbs' collar malformation arises from the persistence of the urogenital membrane. In contrast, the congenital obstructive posterior urethral membrane is attributed to the attachment of the verumontanum to the urethral roof.[8][9]
In normal embryological development, the distal portion of the Wolffian duct becomes absorbed and part of the primitive cloaca.[11] This occurs at the site of the future verumontanum at about 4 weeks of gestational age, which explains why all true posterior urethral valves have some attachment to the verumontanum.[11] Usually, this process leaves only small remnant urethral folds called the plicae colliculi.
Posterior urethral valves develop when there is an aberrant insertion or absorption of the caudal Wolffian ducts or if there is persistent embryological tissue between the verumontanum and the anterior wall of the prostatic urethra.[9][11]
Epidemiology
Worldwide, the incidence of posterior urethral valves varies, with approximately 1:5,000 live male births commonly cited and about 50% of affected individuals progressing to end-stage renal disease (ESRD) within 10 years.[12]
In the United States, posterior urethral valves are estimated to occur in approximately 1 in 4,000 to 8,000 live births or roughly 500 new cases yearly.[1][2][3][11] However, worldwide incidence varies. For instance, in Australia, a multicenter study revealed a rate of 1 in 7,800 live births in a 5-year retrospective analysis.[1] A higher incidence of 1 in 3,800 is reported in the United Kingdom (UK) and Ireland.[2]
About a third of children born with posterior urethral valves will progress to ESRD. Additionally, between 10% and 15% of all children who undergo kidney transplantation will have a history of posterior urethral valves.
In the UK, 35% of cases of posterior urethral valves were detected antenatally, while 42% were diagnosed during the neonatal period or infancy. Approximately 23% of cases were identified later, at 5 years or older.[2]
Pathophysiology
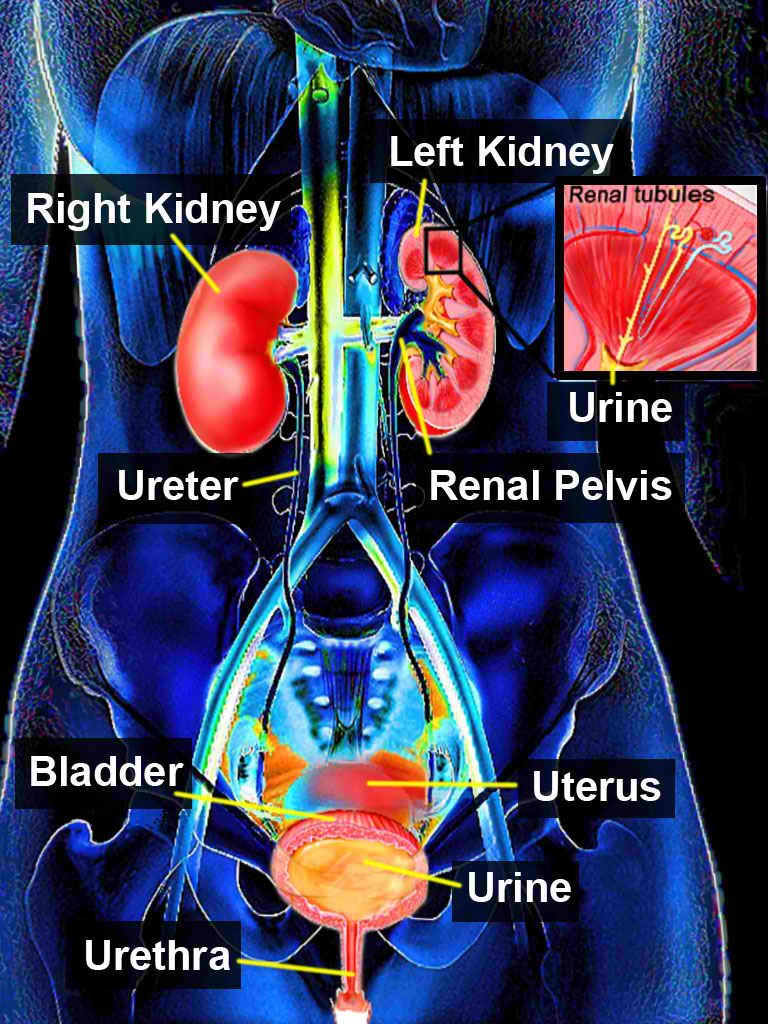
Posterior urethral valves can cause a lower urinary tract obstruction (see Image. Urinary System). The severity of obstruction determines whether the condition is diagnosed antenatally or present after birth. It often manifests with various voiding symptoms, such as acute urinary retention. Timely diagnosis and appropriate management are crucial as they can significantly impact the lifelong function of the urinary tract.
Obstruction caused by posterior urethral valves can lead to detrusor muscle and bladder neck hypertrophy, bladder stretching accompanied by increased collagen deposition, and thickening of the bladder wall. These changes can diminish bladder compliance and increase detrusor voiding pressure, potentially leading to complications such as vesicoureteral reflux, hydronephrosis, and progressive renal damage.
The stretching of the bladder increases detrusor fibrous tissue, which reduces bladder compliance and leads to sustained high bladder pressures. Over time, these elevated pressures are transmitted to the kidneys, causing renal tubular damage and subsequent polyuria. This polyuria exacerbates detrusor damage and promotes further bladder stretching. Other symptoms may include difficulty voiding (straining), urinary tract infections (UTIs), incomplete emptying, urinary frequency, and incontinence.
In severe cases, inadequate urine output can lead to antenatal oligohydramnios and pulmonary hypoplasia.[13] Normal amniotic fluid levels are required between 16 and 28 weeks of gestational age for normal canalicular phase lung development. To provide context, kidney formation is complete by the 12th week of gestation, and any renal damage becomes permanent by the 20th week.[14]
Severe bladder outlet obstruction leading to reduced urine production results in oligohydramnios, which leads to lung hypoplasia.[13] When severe posterior urethral valves are associated with this condition, outcomes typically become very poor.[13]
In older children, typically around age 11, bladder capacity is often increased, accompanied by detrusor decompensation. Renal tubular dysfunction may lead to nephrogenic diabetes insipidus, resulting in polyuria. This polyuria places additional stress on voiding function and further increases bladder volume.
During puberty, bladder function may transition to chronic urinary retention characterized by high bladder voiding pressure, leading to lifelong voiding issues.[15] Bladder dysfunction often persists into adulthood in up to 50% of patients with a history of posterior urethral valves, and as many as 15% of adults who had posterior urethral valves as children will continue to experience urinary incontinence.[16]
History and Physical
The history and physical examination play crucial roles in assessing posterior urethral valves. A thorough history should include prenatal ultrasonographic findings, neonatal presentation, voiding patterns, and associated symptoms. Common neonatal symptoms include urinary retention, distended bladder, and respiratory distress.
During the physical examination, attention should be paid to signs of renal dysfunction, such as hypertension or growth failure, as well as signs of bladder dysfunction, including abdominal distension or palpable bladder. Urological anomalies, such as undescended testicles or hypospadias, should also be evaluated.
Antenatal Presentation
In current practice, most cases are identified through antenatal ultrasound screenings.[17] About 60% of infants diagnosed prenatally with posterior urethral valves will also exhibit renal dysplasia.[18]
An antenatal diagnosis of posterior urethral valves typically indicates a poorer overall prognosis, often associated with higher baseline creatinine levels and a greater incidence of hydronephrosis and renal dysplasia than those identified later in infancy.[2] However, antenatal detection can theoretically facilitate early neonatal surgical intervention to ablate the posterior urethral valves, thereby optimizing bladder and renal function.[19]
On antenatal imaging, notable findings may include a large bladder diverticulum, patent urachus, or a urinoma from urinary extravasation. In cases of posterior urethral valves diagnosed antenatally, prompt management strategies can mitigate potential renal complications and optimize long-term outcomes for affected infants.
Postnatal Presentation
In severe cases, neonates may present with respiratory distress attributed to pulmonary hypoplasia, necessitating immediate medical attention.[6] This condition is associated with prenatal oligohydramnios and may require ventilatory support. More severe cases may also present with urinary retention, suprapubic distension, and inability to void.
Infants who fail to void within the first 24 hours after birth should be promptly evaluated for possible posterior urethral valves as well as other underlying disorders.[20] Typically, ultrasound is the preferred initial imaging modality for evaluation. Normal newborns void about 23 mL every hour.[20]
Neonates with undiagnosed posterior urethral valves can present in various ways, with the most common presentation being a UTI found in about 50% of such patients.[1] Other possible urinary tract pathologies and symptoms may include delayed voiding, weak stream, grunting or discomfort during voiding, urosepsis, or a palpable bladder.[21][22] The presentation may also manifest more indolently, with symptoms such as lethargy, poor feeding, or failure to thrive.[21][22]
Infants and older boys may present with similar complaints to neonates, including specific urinary symptoms such as urinary retention, UTI, or secondary symptoms related to sepsis or azotemia (elevated levels of nitrogen-containing compounds in the blood). In rare cases, urinary ascites or a urinoma can develop following bladder perforation or forniceal rupture.[23][24][25] These abnormalities help preserve kidney function by reducing hydrostatic pressure in the renal pelvis.[23][24][25]
In older males (>5 years) with previously undiagnosed posterior urethral valves, diurnal enuresis often emerges as the most common initial symptom, followed by UTIs, voiding pain, weak stream, hematuria, proteinuria, and renal failure.[26] Hydronephrosis and vesicoureteral reflux are frequently observed, and posterior urethral valve patients with these associated disorders are at greater risk for progression to ESRD.[26]
Posterior urethral valves may also be incidentally identified during the evaluation of renal failure, proteinuria, or hydronephrosis. The most common symptoms in a child with posterior urethral valves include:[27][28][29][30]
- Abdominal or lower back pain
- Bladder diverticula
- Developmental delays
- Difficult or painful urination (dribbling, frequency, weak stream, incomplete emptying, incontinence, straining, or urinary retention)
- Distended bladder
- Failure to thrive [21][22]
- Frequent UTIs
- Hematuria
- Hydronephrosis (usually bilateral but may be unilateral) [26]
- Lower abdominal swelling
- Patent urachus
- Poor weight gain
- Unable to void
- Urinoma due to a bladder perforation or a forniceal rupture
Physical Examination
Many of the clinical signs observed in neonates with posterior urethral valves are commonly encountered in other conditions associated with oligohydramnios resulting from fetal compression within the uterus. These signs may include indentation of the knees and elbows, skin dimpling, and Potter facies.
Associated urological abnormalities may encompass a palpable or distended bladder, urethral stenosis, posterior urethral diverticula, urethral duplication anomalies, megaureters, undescended testicles, and hypospadias.[6][31]
Nonurological anomalies with a higher incidence in patients with posterior urethral valves may include anomalous venous drainage patterns, club feet and other lower extremity deformities, Down's syndrome, hypertension, lethargy, imperforate anus, inguinal hernias, mitral valve stenosis, patent ductus arteriosus, poor growth, pulmonary hypoplasia, and tracheal hypoplasia.[18][31][32] Additionally, careful evaluation of these associated anomalies is crucial for comprehensive management and improved outcomes in patients with posterior urethral valves.
Evaluation
Antenatally, maternal and fetal ultrasonography serves as the primary methods of evaluation. Findings such as a distended bladder with thickened walls and bilateral hydronephrosis suggest posterior urethral valves when detected antenatally in male infants. However, this distinction may not apply to cases diagnosed after birth.[33] Notably, approximately one-third of all cases of posterior urethral valves are now diagnosed by antenatal ultrasonography.[34]
A "keyhole" sign detected on ultrasound indicates obstructive uropathy, with posterior urethral valves being the most common cause.[35] However, studies have shown that this sign is more commonly associated with urethral atresia than with posterior urethral valves, and its predictive value for posterior urethral valves is not significant.[36]
Serial scans are essential not only for antenatal diagnosis but also for identifying poor prognostic markers, such as low levels of amniotic fluid and evidence of renal dysplasia. Repeat ultrasound examinations after the first week following birth may be necessary as oliguria can be a physiological occurrence during this period. Additionally, ultrasound performed through the perineal approach may reveal dilated posterior urethra and valve leaflets.[6]
Fetal urinary electrolytes and β-2 microglobulin serve as the most accurate measures of fetal renal function. In physiologic conditions, fetal urine should exhibit hypotonicity with an osmolality of less than 210 mEq/L. Elevated osmolality and a β-2 microglobulin level exceeding 4 mg/L indicate irreversible renal dysfunction.[14]
Postnatally, investigations can be categorized into blood tests, imaging modalities, and urodynamic studies. Within the first 48 hours following birth, the neonate's serum biochemistry is primarily influenced by the mother's levels. Therefore, blood for fetal serum testing must be collected after this period to obtain accurate results.
Testing serum creatinine, BUN, and potassium levels is critical for assessing the degree of neonatal renal dysfunction and screening for hyperkalemia. Blood gas testing is valuable, mainly if the child is unwell, as it helps to determine if metabolic acidosis is present.[14]
Respiratory function assessment is also essential, as some neonates may experience pulmonary hypoplasia due to oligohydramnios secondary to posterior urethral valves. This condition can significantly impact respiratory outcomes and may require appropriate interventions to support lung development and function.
Imaging Modalities
Ultrasonography of the kidneys and bladder is typically the preferred initial imaging test for its lack of ionizing radiation exposure to the infant, ready availability, and quick performance. It is an efficient modality to identify various abnormalities, including hydronephrosis, hydroureteronephrosis, bladder distension, bladder wall thickening, urinary retention, and a dilated posterior urethra ("keyhole" sign).[33]
Renal ultrasonography in male newborns can confirm antenatal findings of hydroureteronephrosis, characterized by a trabeculated, thick-walled bladder and a dilated posterior urethra. It can also measure renal cortical thickness and identify corticomedullary differentiation. In symptomatic older boys, ultrasonography is a useful tool for screening for similar findings associated with posterior urethral valves.
Common ultrasound findings associated with posterior urethral valves include: [14][17][37]
- Bilateral hydronephrosis or hydroureteronephrosis
- Dilated prostatic urethra ("keyhole" sign)
- Distended bladder with a thickened wall (>3 mm) with poor emptying over 30 minutes. (This may also be a standard variant, so repeated scans are recommended.)
- Fetal ascites
- Focal renal parenchymal cysts (demonstrating renal dysplasia) [37]
- Oligohydramnios
- Urinoma
Contrast-enhanced serial voiding ultrasonography has emerged as a promising alternative to repeated voiding cystourethrograms for monitoring patients with posterior urethral valves.[38][39][40] This technique offers several advantages, including avoidance of ionizing radiation, high sensitivity, and real-time imaging capabilities.[38][39][40]
Voiding cystourethrography: Remains the "gold standard" imaging modality for diagnosing posterior urethral valves. However, even if properly conducted, negative voiding cystourethrography (VCUG) cannot definitively rule out the diagnosis.[41][42][43]
Suggestive signs of posterior urethral valves include bladder neck hypertrophy, a trabeculated bladder with thickened walls, and sonographic findings suggestive of possible posterior urethral valves. Additionally, unexplained and refractory symptoms of bladder overactivity may raise suspicion.[41] In such cases, a cystoscopy is recommended even if the VCUG results are negative.[14]
Typical findings on cystoscopy in patients with posterior urethral valves include a thickened bladder wall with prominent trabeculations. Vesicoureteral reflux (unilateral or bilateral) and the presence of diverticula may also be observed.[6][44] A hypertrophic bladder neck, visualized as a radiolucent ring, may be present.[6][44] Notably, the obstructing membranous valve, represented by a linear radiolucent band, may or may not be visualized during cystoscopy.[6][44]
During cystoscopy, the prostatic urethra may appear elongated, widened, or dilated, corresponding to the "keyhole" ultrasonographic sign, sometimes described as a "spinning top."[6][44] Additionally, an abrupt or sudden narrowing between the dilated prostatic (posterior) urethra and the narrowed bulbous urethra would indicate the location of the blockage and the obstructive membrane.[6][44]
VCUG can miss late cases of posterior urethral valves, and improper technique can hinder correct interpretation and diagnosis.[45][41][46]
Vesicoureteral reflux is present in up to half of all patients with posterior urethral valves, stemming from the persistently high intravesical pressure caused by the outlet obstruction.[26][32] Following surgical intervention to open and relieve the outlet obstruction, reflux resolution occurs in one-third or more cases, typically within 2 years.[32][47]
Bilateral vesicoureteral reflux is a significant risk factor for ESRD and chronic kidney failure in patients with posterior urethral valves. Approximately 25% of individuals with posterior urethral valves and bilateral reflux develop ESRD before the age of 16, contrasting with only 4% for those without reflux.[32][47]
While the VCUG remains the "gold standard" for the diagnosis of posterior urethral valves and vesicoureteral reflux, it can be challenging to perform correctly. Additionally, it can be uncomfortable for the patient, embarrassing for older individuals, and terrifying for younger boys. Moreover, the procedure exposes the pelvis and genitals of young children to ionizing radiation, poses a risk of injury or infection from catheterization, and may occasionally fail to detect important pathology.[48][49][50][51]
The technique for voiding cystourethrography typically involves several steps:
- Considering prophylactic antibiotics starting 1 to 2 days before the VCUG and continuing for 2 to 3 days afterward.
- Suggesting the use of xylocaine jelly, either injected into the urethra or coating the catheter.
- Gently catheterizing the patient with a soft, small-caliber urethral catheter.
- Dripping or gently injecting a diluted (50:50) contrast agent into the bladder. In newborns, the typical bladder capacity is roughly 20 to 30 mL.
- Placing the patient in a lateral or oblique position to better visualize the posterior urethra.
- Removing the catheter before the patient voiding for optimal results.
- Utilizing video fluoroscopy with static films to observe and record reflux and any abnormal urethral anatomy during voiding.
- Observing techniques to minimize radiation exposure to the child.[14]
Renal radionuclide scans: Used to identify renal parenchyma abnormalities and detect functional differences between the kidneys. These studies may be either functional, providing dynamic information about kidney function over time, or static, offering a snapshot of kidney function at a specific moment.
Static radionuclide renal imaging, using radiotracers such as dimercaptosuccinic acid (DMSA), involves the extraction of these tracers by the proximal renal tubules, where they are retained with minimal urinary excretion.[6][52] This imaging modality is valuable for demonstrating differential renal function, identifying scarring, and detecting focal parenchymal defects.[6][52] Additionally, it is recommended in selected cases of significant vesicoureteral reflux to evaluate the extent of renal scarring.[14]
Functional tests, such as diethylenetriamine pentaacetate (DTPA) and mercaptuacetyltriglycine (MAG-3), involve injecting a radiotracer that undergoes glomerular filtration by the proximal renal tubules into the renal pelvis and bladder. As a result, these tests necessitate a sufficient level of renal function to properly excrete the radiotracer.
MAG-3 is generally preferred for evaluating possible urinary tract obstruction in neonates due to its superior excretion rate and renal imaging capabilities, particularly in cases of hydronephrosis. In the classic diuretic (furosemide) renal scan, MAG-3 studies can provide insights into differential renal function.[53] However, due to their ongoing renal development, functional radionuclide studies are usually not performed on neonates. A baseline scan to evaluate renal function has been recommended at 1 month of age.[14] In patients with hydronephrosis, the presence of a catheter should be confirmed to ensure adequate bladder drainage.[14]
Nuclear medicine studies of the kidneys are limited in evaluating posterior urethral valves as they do not give detailed anatomical information about the lower urinary tract. However, they can help assess comparative renal function, detect renal scarring, and identify other abnormalities. Ureterovesical junction obstruction, neurogenic bladder, and ureteroceles may mimic the findings of posterior urethral valves in nuclear medicine studies.
Computed tomography scans: These are seldom necessary in neonates and are not routinely recommended due to their limited ability to directly visualize posterior urethral valves. Instead, they typically reveal secondary signs of obstruction, such as hydronephrosis, bladder distension with thickening and trabeculation of the bladder wall, urinary extravasation, and diverticula. However, identifying a dilated posterior urethra or visualizing valve leaflets is challenging with computed tomography (CT) scans.
A VCUG and ultrasonography are preferred for more accurate information about posterior urethral valves. This is especially true in cases where intravenous contrast is contraindicated based on the serum creatinine level.
Urodynamic studies: Play a crucial role in assessing dysfunctional bladder disorders, especially after ablative surgical treatment for obstructive posterior urethral valves.[54] Periodic repeat urodynamic studies are recommended to track alterations in bladder functionality over time.
Bladder changes linked to posterior urethral valves include uninhibited bladder contractions, diminished bladder compliance, decreased maximum volume capacity, and elevated filling pressure. Additionally, bladders may become weakened from prolonged overdistension, characterized by low detrusor muscle activity and increased post-void residual volumes.[32] Bladder-neck hypertrophy is observed in over 50% of patients, often accompanied by high post-void residual urine volumes.[14]
Detrusor overactivity and reduced bladder compliance are the predominant urodynamic findings in patients with posterior urethral valves.[55] Decreased detrusor compliance is associated with persistent hydronephrosis.[6]
Pressure flow studies are valuable in assessing patients for postoperative bladder outlet obstruction, aiding in identifying individuals who may require additional evaluation and treatment.[56][57] Indications for urodynamic studies in patients with treated posterior urethral valves include: [14][58]
- Before renal transplantation
- Daytime incontinence persisting after age 5
- Persistent vesicoureteral reflux in patients on anticholinergics
- Persistent voiding dysfunction or treatment failure
- Persistently high unexplained postvoid residual urine volumes
- Renal function deterioration with no apparent cause
- Suspected detrusor-sphincter dyssynergia
Treatment / Management
Management typically involves a multidisciplinary approach encompassing neonatology, pediatric urology, and nephrology. Early diagnosis through prenatal screening and prompt intervention are crucial for optimizing outcomes. Treatment strategies include surgical ablation of the obstructing valves, urinary diversion procedures, and ongoing monitoring to address potential complications and ensure optimal bladder and renal function.
Antenatal Intervention
There are currently no validated criteria for antenatal intervention in posterior urethral valve cases, and its use is considered investigational in the US due to associated high morbidity. However, it has been proposed for consideration in cases diagnosed by the second trimester accompanied by severe oligohydramnios.[6][14] The threshold for intervention regarding renal function varies internationally, but evidence of preserved renal function would generally be a prerequisite.
In the United Kingdom, indications for antenatal intervention would include favorable renal function parameters, such as fetal urinary chemistry showing sodium (Na) levels less than 100 mEq/L, chloride (Cl) levels less than 90 mEq/L, osmolality less than 210 mOsm/L, and β2 microglobulin levels less than 6 g/L.[59][60][61] Obtaining fetal urine for analysis typically involves tapping the bladder or performing fetal vesicocentesis.
Antenatal interventions for posterior urethral valves include a range of procedures to address the obstruction and mitigate associated complications. These interventions may consist of vesicoamniotic shunt placement, vesicostomy creation, fetal cystoscopy, and endoscopic valve ablation. Each approach carries its considerations and potential benefits in managing the condition and optimizing outcomes for the affected fetus.
- In a single-center case series involving 40 patients, the majority (36) underwent antenatal intervention. Unfortunately, 5 deaths occurred among prematurely born infants, primarily due to respiratory failure or shunt failure complications (in 1 infant). Of the 8 surviving patients followed up over an average of 11.6 years, a significant portion experienced chronic kidney disease (5 individuals), with 3 requiring renal transplantation.[62]
- In another randomized trial, the PLUTO study, the efficacy of percutaneous vesicoamniotic shunting was compared to conservative management for fetal lower urinary tract obstruction.[63] The trial included 31 women from the UK, Ireland, and the Netherlands. Of these, 16 were allocated to the vesicoamniotic shunt group and 15 to conservative treatment. The results were as follows:
- 1 intrauterine death in each group, 3 terminations in the treatment arm, and 2 in the conservative arm.
- 8 neonates from the treatment arm survived 28 days compared to 4 from the conservative management arm, with a relative risk (RR) of 1.88 (p=0.27).
- The trial was stopped prematurely due to recruitment challenges. Although survival appeared better in the treatment arm, statistical significance was not reached due to the small sample size.
- More recent studies indicate some improvement in outcomes. In one case series, 17 out of 30 patients underwent fetal cystoscopy and survived to 1 year of life, with 13 of them exhibiting normal renal function at that time.[63]
The most recent meta-analysis revealed no disparity in survival between comparable cases treated with vesicoamniotic shunts compared to conservative management at 6, 12, or 24 months. Additionally, there was no variance in postnatal renal function, although perinatal survival was superior in the surgically-treated group.[64]
Antenatal intervention carries substantial risks and should be reserved for clinical trials and specialized centers with expertise in such intricate and risky procedures. A potential candidate might be a fetus diagnosed with significant posterior urethral valves accompanied by severe oligohydramnios in the mid-trimester, exhibiting good renal function and a normal karyotype.
Further, well-designed studies are necessary to determine the appropriate role of antenatal surgery in cases of severe posterior urethral valves, identify the optimal indications for such procedures, and determine which surgical approach yields the most favorable outcomes.[14][64] These studies should encompass long-term follow-up to assess survival rates, renal function, and quality of life outcomes.
Postnatal Intervention
The treatment approach should be tailored to address immediate concerns such as electrolyte imbalances, hydration status, and respiratory support. A multidisciplinary team, including neonatologists, urologists, and nephrologists, should collaborate to ensure comprehensive care. Once stabilized, definitive management options, such as surgical intervention to address the posterior urethral valves, should be carefully considered based on the neonate's clinical status and the severity of the condition.
In the event of acute retention or inability to pass urine, the bladder will likely require drainage.[14] The choice of catheter or soft feeding tube size depends on the neonate's age and size (5 or 8 French is often used in neonates), with smaller sizes typically preferred for neonates to minimize discomfort and the risk of urethral trauma.
In challenging cases of catheterization due to coiling, utilizing ultrasonic or fluoroscopic guidance can enhance accuracy and reduce complications. If standard catheterization methods fail, alternative options such as suprapubic tube insertion, vesicostomy creation, or other advanced techniques may be necessary to ensure prompt urinary drainage and prevent further urinary tract injury, preserving renal function.[14]
The catheter may be left in place for up to 4 weeks. If the urethra remains dilated, cystoscopic intervention may be considered;[14] otherwise, a vesicostomy can serve as an alternative for urinary diversion. A vesicostomy is preferred if catheterization fails in neonates with posterior urethral valves and urinary retention, as long-term outcomes between the 2 approaches are comparable.[14][65][66]
Postobstructive diuresis: Characterized by polyuria, may occur immediately following bladder drainage in many newborns with posterior urethral valves.[14][67][68][69] This condition is defined as urinary output exceeding 6 mL/Kg/hour during the first 24 hours after birth.[68]
Risk factors for postobstructive diuresis in newborns with posterior urethral valves include prematurity, oligohydramnios, and elevated creatinine levels.[68] Consequently, careful fluid and electrolyte monitoring and appropriate replacement are essential until the period of polyuria subsides.[14] Some patients may also exhibit type 4 renal tubular acidosis, necessitating bicarbonate therapy for management.[70][71][72][73]
The timing of definitive corrective surgery for posterior urethral valves is determined by various factors, including the patient's overall health, the severity of the condition, urethra size, and anesthesia considerations. Typically, surgery is conducted at the earliest feasible time. In the interim, bladder drainage via catheter or vesicostomy is used to preserve bladder and kidney function until the definitive procedure can be safely undertaken.
Cystoscopic ablation: Presently the preferred initial definitive treatment for posterior urethral valves.[18][21][74] This procedure typically resolves the obstruction; however, approximately a third of cases may experience vesicoureteral reflux.[75]
Cystoscopy is a definitive diagnostic tool, enabling direct visualization of the obstructing membranes and confirming the diagnosis.[14] Simultaneously treating the obstruction during cystoscopy is considered a reasonable, safe, and efficient approach, yielding favorable outcomes. This method is generally recommended when technically feasible.[18][21][74]
Initiating cystoscopic ablative therapy early to address the obstructing valves minimizes potential urethral trauma by eliminating the need for additional procedures. Additionally, this approach aids in preserving bladder function.[66][76][77]
Various cystoscopic instruments and accessories are employed for the actual ablative treatment. These include the following:
- Ball-tip, sharp-tipped, or blunt-angled Bugbee electrode
- Cold knife (straight or serrated blade, crescent, or the more popular sickle shape) urethrotomes
- Cutting loop or Collins knife
- Nd:YAG, Holmium:YAG, or thulium laser [14][78]
The sickle-shaped cold knife urethrotome is a popular option for ablation, while laser therapy provides the smallest diameter fiber for passage through even very small cystoscopes.[79][80][81] However, no evidence currently suggests the superiority of any specific ablative methods over others.
The technique generally involves making incisions in the obstructing membrane at 5 and 7 o'clock. An additional incision at 12 o'clock is optional.
Successful cystoscopy with ablative therapy relies on the urethra being large enough to accommodate a neonatal cystoscope, typically a minimum of 8-French. If the urethra is too narrow, options include leaving the catheter or feeding tube in place for passive urethral dilation, replacing it serially for progressive urethral dilation, or opting for a vesicostomy, particularly in premature infants.[14]
In patients with very small urethras, progressive serial urethral dilation is useful. This involves periodically changing the catheter or feeding tube to a larger size until the urethral diameter is sufficient to accommodate a cystoscope easily.[82][83][84] Results are comparable to those in older children who underwent immediate cystoscopic ablative therapy.[82] If an 8-French catheter can be placed, cystoscopy with valve ablation can generally be performed.[82]
For patients with vesicoureteric reflux following cystoscopic valve ablation or bladder neck incision, treatment with prophylactic antibiotics allowed this to resolve spontaneously in 66%. Up to 94% experienced resolution after ureteroneocystostomy.[74]
About a third of patients treated with ablative therapy will require a second procedure. Additionally, a posterior urethra/anterior urethra ratio exceeding 3.1 suggests residual obstruction, indicating the need for further surgery.[85] A standard ratio would be about 2.3 or less.
A common complication is a urethral stricture, often resulting from trauma associated with instrumentation during the procedure.[86][87] Strictures may necessitate further intervention, such as urethral dilation or surgical repair, to restore normal urinary flow.
Circumcision is often advised as it can help minimize the risk of UTIs, common in this vulnerable patient population. Additionally, it may facilitate hygiene and urinary drainage.
Vesicostomy: Typically preferred when cystoscopic access to the posterior urethra is challenging, particularly in extremely premature newborns.[65] These procedures are simple to perform, ensure effective bladder drainage, fully alleviate urinary obstruction caused by posterior urethral valves, and can easily be reversed.[65] Using a stoma size of 18 or 20 French is sufficient and minimizes the risk of prolapse.
A vesicostomy is often favored over more invasive upper urinary tract procedures, such as ureterostomies or nephrostomies, as these interventions have not demonstrated superior long-term outcomes. Additionally, they disrupt the bladder's natural filling and emptying process, further interfering with normal bladder development and urinary voiding in the long run.[88]
Fogarty catheter ablation: Another technique that disrupts the obstructing posterior urethral valves. This method can be performed under cystoscopic visualization and fluoroscopic control and involves inflating the Fogarty catheter balloon and then withdrawing it through the obstructing membranes, effectively disrupting them.[89][90][91][92] Unlike vesicostomy or long-term catheterization, this approach avoids the need for invasive procedures. While the results are generally favorable, the reported experience with this technique is somewhat limited, leading to its lack of preference in many cases.[90][91][92][93] Nevertheless, it may be considered a reasonable option in regions where specialized neonatal cystoscopes are not readily available.[90][92]
Regardless of the chosen treatment modality, patients with posterior urethral valves face a notable risk of chronic kidney disease, underscoring the need for ongoing monitoring of renal function and obstructive urinary symptoms.
Management of voiding dysfunction: Management after surgery is usually based on urodynamic findings and clinical bladder functional parameters. Often, this entails long-term intermittent self-catheterization, alpha-blockers, anticholinergics, or a blend of these therapies.[14] In specific instances, overnight Foley catheterization can be considered, particularly for young children who have reached maximum tolerated dosages of anticholinergics, aiming to minimize bladder pressure.
Consistent adherence to intermittent self-catheterization is crucial for safeguarding and sustaining renal function, although this regimen may pose challenges for some children.[94] Sensitivity in the area, coupled with a hypertrophic bladder neck and an enlarged posterior urethral lumen, can complicate the process of intermittent self-catheterization, especially for young children. In such cases, a continent, catheterizable cystostomy can be created surgically as an alternative channel for self-catheterization.[95]
The role of botulinum toxin bladder injections in treating intractable hyperactive bladders following posterior urethral valve surgery appears promising in some instances where anticholinergic therapy proves insufficient. However, administering botulinum toxin into hypertrophic bladder necks did not demonstrate efficacy in a singular investigation.[96]
Surgical bladder augmentation is rarely necessary for individuals with posterior urethral valves. In cases where bladder augmentation is considered, careful evaluation and counseling regarding the potential risks and benefits are essential.
Follow-up Examinations
Post-surgery for posterior urethral valves is crucial for assessing treatment efficacy and monitoring patient progress. A VCUG or cystoscopy 3 months after surgery helps ascertain the adequacy of valve ablative therapy, while yearly follow-up visits are advised to assess renal function and bladder activity.[14]
Differential Diagnosis
The differential diagnosis for posterior urethral valves includes the following:
- Anterior urethral valves
- Bilateral ureterovesical junction (UVJ) stenosis
- Detrusor sphincter dyssynergia [97]
- Ectopic obstructive ureteral implantation
- Fibroepithelial polyp of the posterior urethra [98]
- Incomplete surgical ablation of posterior urethral valves
- Megalourethra
- Megacystis (defined by a bladder size >7 mm at gestational week 12 in the first trimester. In approximately 60% of cases, posterior urethral valves are identified as the cause) [99]
- Neurogenic or atonic bladder
- Primary megaureter
- Prune Belly syndrome [100]
- Ureterocele
- Urethral atresia, stenosis, or stricture
- Vesicoureteral reflux [101]
Prognosis
The prognosis of posterior urethral valves varies depending on the degree of obstruction and prenatal complications. Studies have shown a strong correlation between creatinine levels, particularly the creatinine nadir, and long-term bladder function in affected boys.[102][103] Elevated creatinine levels above 1 mg/dL during the first year of life raise significant concerns, suggesting a heightened risk for ESRD and renal failure.[102][103][104]
The prognosis for patients diagnosed with posterior urethral valves before 24 weeks gestation, evidence of renal dysplasia, or severe bilateral hydronephrosis with oligohydramnios is very guarded. There is a high risk of chronic kidney failure, ESRD, and early mortality in such cases.[105][106] Perinatal mortality rates can be as high as 90% or more if oligohydramnios from posterior urethral valves are identified during the second trimester antenatally.[107][108]
Most patients diagnosed with posterior urethral valves antenatally and presenting with average or unremarkable amniotic fluid volumes at 24 weeks of gestational age are expected to have normal renal function at birth. However, up to a third of these individuals may still require some form of renal replacement treatment later in life due to kidney failure.[109]
Most infants who survive the neonatal period often develop chronic kidney disease, with many experiencing ongoing bladder dysfunction. Generally, about 15% to 20% of patients with posterior urethral valves will progress to ESRD, but this could be as high as 29%.[18][32][110] Risk factors for ESRD include: [110][111]
- Corticomedullary differentiation
- Decreased glomerular filtration rate (GFR) at 1 year of age (P<0.001)
- ≥4 documented UTIs with fever
- Higher baseline creatinine, nadir creatinine, and proteinuria
- Prenatal diagnosis with bilateral vesicoureteral reflux and oligohydramnios
- Renal volume below the third percentile
In contrast, a renal volume greater than 88.2 mL/m² body surface area was protective against stage 5 renal failure.[110] This suggests that adequate renal growth during development may play a role in mitigating the risk of severe renal dysfunction in patients with posterior urethral valves.
Interestingly, the development of prenatal urinary extravasation seems to contribute to the preservation of renal function in patients with significant posterior urethral valve disease.[24][112][113] This phenomenon underscores the complex interplay between prenatal urinary dynamics and renal outcomes in affected individuals.
Advances in prenatal ultrasonography have enabled early detection of posterior urethral valves in utero, paving the way for selective fetal surgical interventions. However, despite these aggressive treatments, beneficial long-term outcomes and improved survival rates have yet to be demonstrated.[14]
Implementing early surgical ablative treatment shortly after birth, coupled with comprehensive management strategies including assisted bladder-emptying techniques, timed and double voiding strategies, pharmacotherapy, appropriate antibiotics, and optimized intermittent self-catheterization protocols, provides the most effective protection against deteriorating bladder and renal function in these patients.[75] Regular monitoring of renal function and bladder activity through follow-up examinations is essential to ensure timely interventions and adjustments to the management plan.
Complications
Complications of posterior urethral valves can lead to significant morbidity and long-term health challenges. While the severity of complications varies, they often arise due to urinary obstruction caused by abnormal valves. These complications can affect renal function, bladder health, and overall quality of life. Understanding these potential complications is crucial for timely diagnosis, intervention, and management to mitigate their impact and improve patient outcomes.
Bladder Dysfunction
A multicenter case series involving 119 patients, initially managed with drainage followed by cystoscopic ablation, revealed that a third developed severely abnormal bladder function. As a result, these patients required clean intermittent self-catheterization with or without anticholinergics to manage their bladder dysfunction.[32]
Given the significant prevalence of this complication, integrating urodynamic studies into the ongoing management of patients with posterior urethral valves is crucial. Additional studies have revealed that up to 41% of patients exhibit symptoms of bladder dysfunction, although, among the 55 children who were toilet trained in this study, 42 managed to maintain continence. In both groups of patients, treatment with alpha-blockers was initiated to reduce postvoid residual volume if indicated following urodynamic studies.[18]
Renal Failure
In a study involving 274 patients receiving initial treatment within the first 90 days of life at 5 different hospitals, findings revealed that 16% required renal replacement therapy within 10 years.[114] These patients were risk-stratified utilizing the lowest creatinine levels recorded within the first year as a critical parameter for classification.[114]
Vesicoureteric Reflux and Urinary Tract Infections
Due to elevated intravesical pressure, at least a third of patients diagnosed with posterior urethral valves will develop vesicoureteric reflux. Typically, this condition is managed with antibiotics to reduce the risk of renal damage, scarring, and infections. However, alpha-blockers and anticholinergics may additionally be utilized to assist with voiding.
When recurrent urinary tract infections lead to renal damage and severe reflux, which in turn impairs bladder emptying, urinary reconstruction may become necessary. Even without vesicoureteric reflux, patients with persistently increased postvoid residual volumes are prone to recurrent infections due to stagnant urine. The risk is further heightened in cases with dilated upper urinary tracts.[6]
Other complications of posterior urethral valves include:
- Chronic renal failure
- ESRD
- Incomplete ablation of the posterior urethral valves
- Incomplete voiding
- Incontinence
- Permanent bladder dysfunction
- Recurrent UTIs
- Urethral strictures
- Vesicoureteral reflux
Deterrence and Patient Education
Many complications associated with posterior urethral valves persist despite initial valve ablation. Consequently, patients often require clean intermittent self-catheterization, a skill that parents and later children need to be taught for home management. Patient education is critical in optimizing ongoing outcomes and preventing chronic kidney disease.
Empowering patients with knowledge about their condition enables them to advocate for themselves effectively, essential in managing any long-term health issue, such as posterior urethral valves.[115] Regular follow-up visits with healthcare providers are crucial for monitoring the condition's progression and adjusting management strategies as needed.
Pearls and Other Issues
Key facts to keep in mind about posterior urethral valves include the following:
- Posterior urethral valves are the most common cause of lower urinary tract obstruction in male neonates, occurring in approximately 1 in 5,000 to 1 in 8,000 live births.
- Neonates with posterior urethral valves typically present with a triad of symptoms: 1) urinary retention, 2) palpable bladder, and 3) respiratory distress due to oligohydramnios and Potter sequence.
- Failure of a newborn to void within the first 24 hours after birth is a "red flag" for possible posterior urethral valves and other significant abnormalities.
- Diagnosis is usually suspected prenatally based on ultrasound findings showing bilateral hydronephrosis, dilated posterior urethra, and a distended bladder.
- Ultrasonography should be used to evaluate suspected posterior urethral valves. Confirmatory imaging is done with a VCUG or direct visualization via cystoscopy.
- Posterior urethral valves should be considered in children beyond the neonatal stage who demonstrate a UTI or any persistent abnormal urinary symptoms.
- Nonurological symptoms would include unexplained lethargy, fatigue, poor eating, or failure to thrive.
- Complications of posterior urethral valves include renal impairment, hydronephrosis, vesicoureteral reflux, urinary tract infections, and bladder dysfunction.
- Definitive treatment involves endoscopic ablation of the valves via cystoscopy. This procedure aims to relieve the obstruction and prevent long-term renal damage. In severe cases, vesicostomy or urinary diversion may be necessary.
- Early postoperative urodynamic studies are crucial for optimizing bladder health, minimizing additional detrusor damage, and maximizing renal function following surgical ablation of posterior urethral valves.[54]
Enhancing Healthcare Team Outcomes
Posterior urethral valves present a complex congenital urological condition with diverse manifestations across life stages. From critically ill neonates with pulmonary hypoplasia and urinary retention to children managing chronic kidney disease in community settings, the spectrum of presentation underscores the challenges in the diagnosis and management of chronic kidney disease in children in the community. Transitioning from pediatric to adult healthcare services is pivotal for ensuring continuity of care and optimizing long-term outcomes.[116]
Diagnosing and treating posterior urethral valves necessitate a multidisciplinary approach, engaging obstetricians, neonatologists, nurse midwives, pediatric nephrologists, radiologists, pediatric urologists, nurses, and other healthcare professionals. Collaborative communication and coordinated efforts among team members are essential for delivering comprehensive and effective patient care.