Introduction
Endophthalmitis is a rare but severe disease of ocular inflammation involving vitreous or aqueous fluids and other intraocular tissues that can result in blindness. Fungal endophthalmitis is caused by intraocular fungal infections originating either exogenously from penetrating trauma and surgeries or endogenously from hematogenous spread. The highly variable clinical picture of fungal endophthalmitis presents as a diagnostic challenge as the features of the disease overlap with bacterial, non-infectious, and neoplastic etiologies.
A lack of clinical suspicion and the empiric use of corticosteroids without proper antifungal coverage increases the risk of significant visual loss. Therefore, it is imperative for healthcare providers to recognize fungal endophthalmitis and diagnose it early for prompt initiation of effective antifungal therapy.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Exogenous Endophthalmitis
Exogenous endophthalmitis is due to infectious pathogens of the ocular surface or external environment complicating intraocular surgery or from penetrating ocular injury or the contiguous spread from adjacent tissues. The three forms of exogenous fungal endophthalmitis are postoperative, posttraumatic, and fungal keratitis-derived.
In general, postoperative endophthalmitis is the most common type of endophthalmitis, accounting for 40%-80% of cases of endophthalmitis. It is rarely fungal, but Aspergillus and Candida species are the most commonly reported fungal organisms implicated in postoperative endophthalmitis.[2][3][4][5] Posttraumatic endophthalmitis is the second most common type, accounting for 25% of cases of endophthalmitis.[6] Bacteria are responsible for most cases of posttraumatic endophthalmitis, and Aspergillus are the most prevalent fungal cause.[7] The contiguous spread of fungal keratitis is the rarest etiology of fungal endophthalmitis.[8]
Endogenous Endophthalmitis
Endogenous endophthalmitis is rare, compromising only 5%–15% of endophthalmitis. It most often occurs via hematogenous spread secondary to compromised blood–ocular barrier. Patients with endogenous endophthalmitis are primarily immunocompromised or intravenous drug users that develop fungemia. In contrast to other types of endophthalmitis where bacteria are the most prevalent pathogens, the most common cause of endogenous endophthalmitis is fungi. Candida albicans is the leading pathogen for endogenous fungal endophthalmitis. Other Candida species have also been reported, including C. tropicalis, C. glabrata, C. krusei, C. parapsilosis, and C. pelliculosa.[9][10][11] Aspergillus and Fusarium are the most common causative molds.[1][12]
Epidemiology
Endogenous Endophthalmitis
Disseminated Candida infection is the most significant cause of endogenous endophthalmitis. It has been reported that the incidence of endophthalmitis in patients with candidemia ranges from 9% to 40%.[13][14][15][16][17][18] However, recent studies have shown the incidence to be as low as 0 to 2.2%, which could demonstrate that the current practice of prophylaxis and prompt treatment after positive blood cultures has decreased ocular complications of candidemia dramatically.[10][13][19][20][21]
The fungal infection usually seeds the choroid first since it is highly vascular then progresses anteriorly from the outer to inner retinal layers. As a consequence, endogenous fungal endophthalmitis often presents with choroiditis or chorioretinitis then involves the vitreous as the infection worsens. The nature of progression is typically slow.
Risk factors for endogenous fungal endophthalmitis are mainly related to immunosuppression and other factors that increase the colonization or bloodborne infection of Candida. Systemic immunosuppression per se is not sufficient for the development of intraocular candidiasis because it does not increase the access of Candida to the bloodstream. However, in the presence of candidemia, immunosuppression is likely to increase the risk and severity of the ocular infection. Established risk factors for endogenous fungal endophthalmitis include:[22][23]
- Human immunodeficiency virus (HIV) and other immunosuppressive diseases
- Underlying malignancy
- Broad-spectrum antibiotic therapy
- Corticosteroid therapy and other immunosuppressive therapies
- GI trauma or surgery
- Intravenous hyperalimentation
- Indwelling intravenous catheters
- Intravenous drug abuse
- Neutropenia
- Diabetes mellitus
- Renal insufficiency
- Recent hospitalization
- Organ transplant
- Hemodialysis
- Pregnancy, abortion, postpartum state
- Lung disease (specific to Aspergillus)
Two studies found that 90% of patients with endogenous fungal endophthalmitis had one or more underlying medical conditions that predisposed them to the disease.[24][25] However, endogenous fungal endophthalmitis has been seldom reported in immunocompetent patients without underlying predisposing conditions. The disease may be the first manifestation of an occult infection that is focal, so systemic cultures are still negative.[23][25][26][27][28][29][30] It is of note that, despite the very high incidence of mucosal candidiasis, endophthalmitis due to candidemia in patients with HIV infection or acquired immune deficiency syndrome (AIDS) is very uncommon in the absence of other risk factors.[31]
Candida species are the fourth most common cause of nosocomial bloodstream infections, and C. albicans is the most common cause of endogenous fungal endophthalmitis. It is still unknown as to why the eye is a common end-organ target of fungemia. In an experimental study using a rabbit model of C. albicans endophthalmitis, there was evidence to believe that C. albicans had a marked tropism for the eye similar to its tropism for kidneys and endothelium.[22][32]
Neonatal Endophthalmitis
Neonatal endophthalmitis is very rare. Moshfeghi et al. reported a decreasing trend of neonatal endophthalmitis in the United States and determined that the incidence rate was 0.0045% in 2006. Unlike endophthalmitis in adults, neonatal cases are overwhelmingly endogenously sourced with Candida as the most common cause. Retinopathy of prematurity (ROP) is a significant risk factor for and closely associated with neonatal endophthalmitis. It is theorized that fungal infections may cause the release of proinflammatory cytokines that promote retinal neovascularization and progression of ROP. Low birth weight and prolonged hospital stay are also important risk factors.[33][34][35][36]
Exogenous Endophthalmitis
In contrast to endogenous endophthalmitis, exogenous endophthalmitis occurs in otherwise healthy people. Risk factors for exogenous endophthalmitis include intraocular surgery, penetrating trauma, and fungal keratitis. Because of the pathogenesis of exogenous fungal endophthalmitis, the aqueous humor is usually involved first in contrast to endogenous endophthalmitis.[1][22]
Acute-onset postoperative endophthalmitis is rarely fungal,[2] except in tropical regions, such as India, where up to 22% of cases of postoperative endophthalmitis are due to fungi.[3] Chronic postoperative endophthalmitis, a fairly uncommon inflammatory process, is a result of fungal infection in 16% to 27% of cases.[2] Likewise, post-keratoplasty endophthalmitis is rare and occurs in only 0.67% of keratoplasty cases, but up to 33% of cases of post-keratoplasty endophthalmitis are due to fungi.[5] Fungal endophthalmitis following trabeculectomies or intravitreal injections is extremely rare, but there are reported cases due to contamination of the injected compounds.[37][38][39]
Fungal keratitis progresses to endophthalmitis by direct extension of the infection as the fungus invades through the corneal stroma and Descemet’s membrane into the anterior chamber. Contact lens use, trauma with organic matter, and laser-assisted in situ keratomileusis (LASIK) are risk factors for fungal keratitis-derived endophthalmitis. In a retrospective study performed by Henry et al., only 0.5% of eyes with keratitis progressed to culture-proven endophthalmitis. However, fungi accounted for 78% of cases, suggesting that fungal infection may increase the risk of keratitis developing into endophthalmitis. A majority of cases in the study were due to molds, most commonly Fusarium.[8]
History and Physical
Endogenous Endophthalmitis
Patients with endogenous fungal endophthalmitis present with variable clinical signs and symptoms, which can make diagnosis difficult. Patients most commonly present with a subacute onset of decreased vision and floaters that may be associated with a red, painful eye, ocular discomfort, and photophobia. They often have a history of immunosuppression or related aforementioned risk factors. Early or peripheral fungal lesions may be asymptomatic with a patient’s referral for ocular consultation on the basis of positive blood culture or diagnosis of systemic Candida infection.
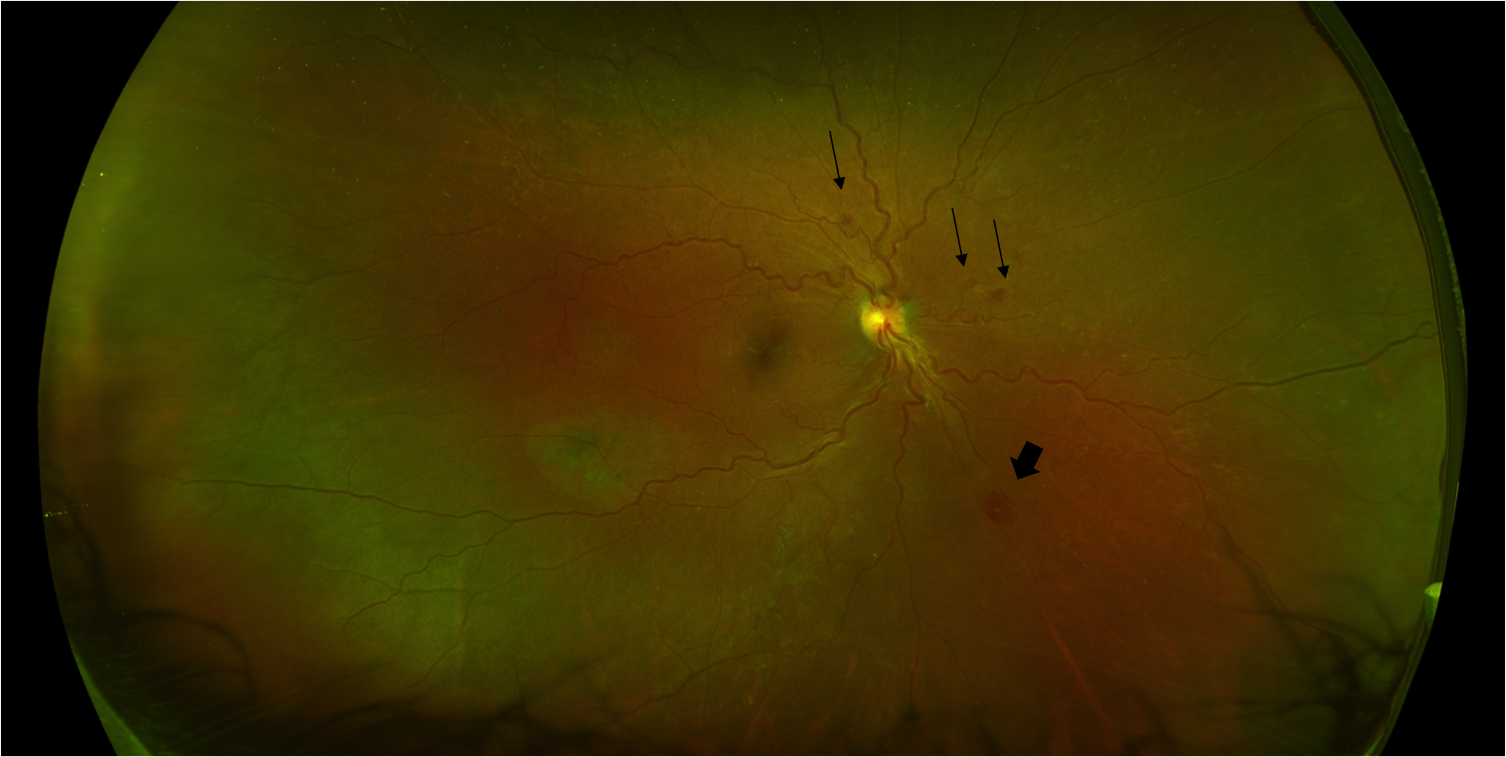
Physical examination may reveal eyelid edema, conjunctival and circumcorneal congestion, anterior chamber inflammation with or without a hypopyon, absent red reflex, and vitreous exudates and haze. Intraretinal hemorrhages, nerve fiber layer infarcts, Roth spots (Figure 1), and cotton wool spots are nonspecific findings on fundus exam, which can be limited by intraocular inflammation obscuring the view of the fundus.[6][22]
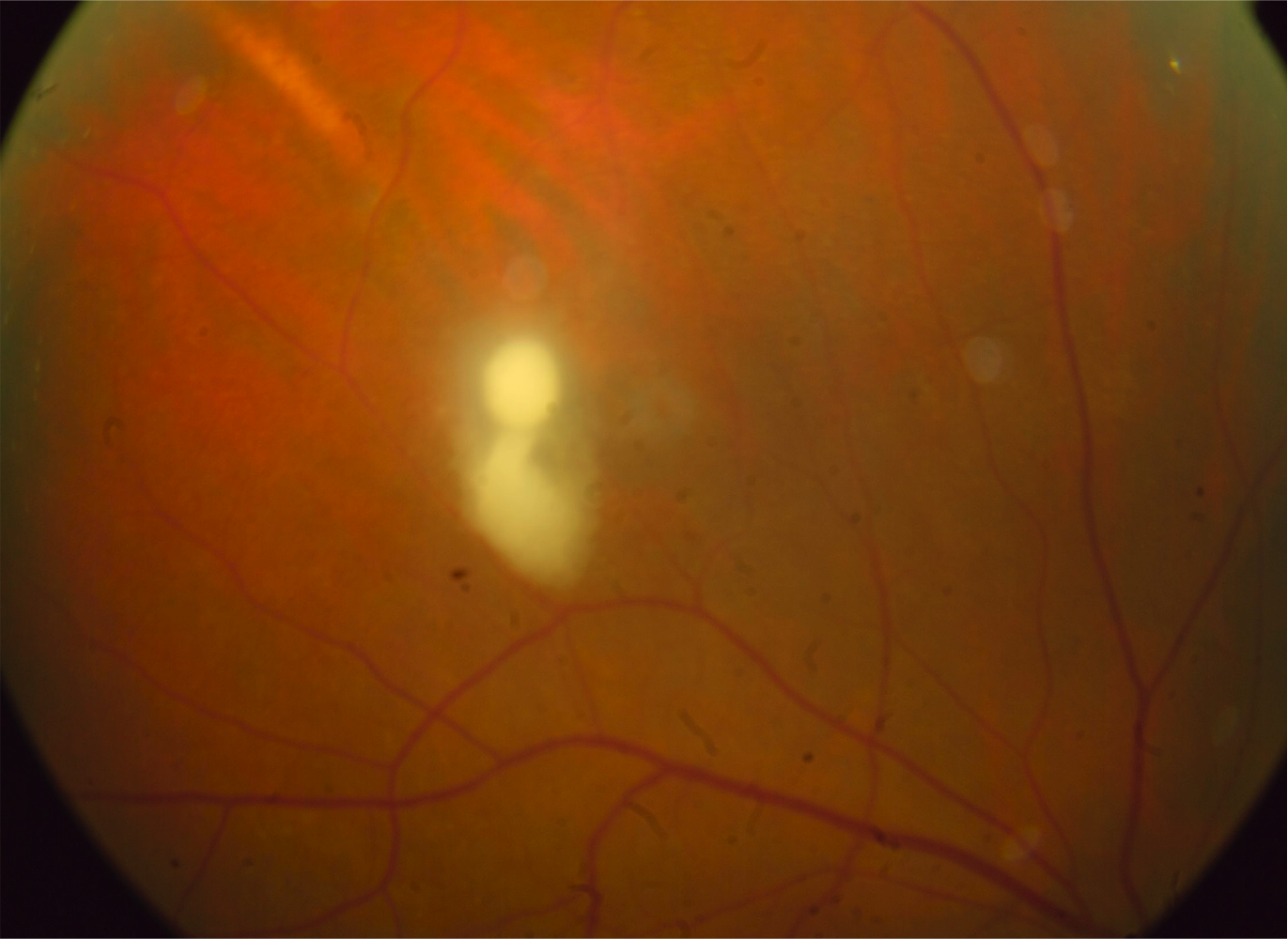
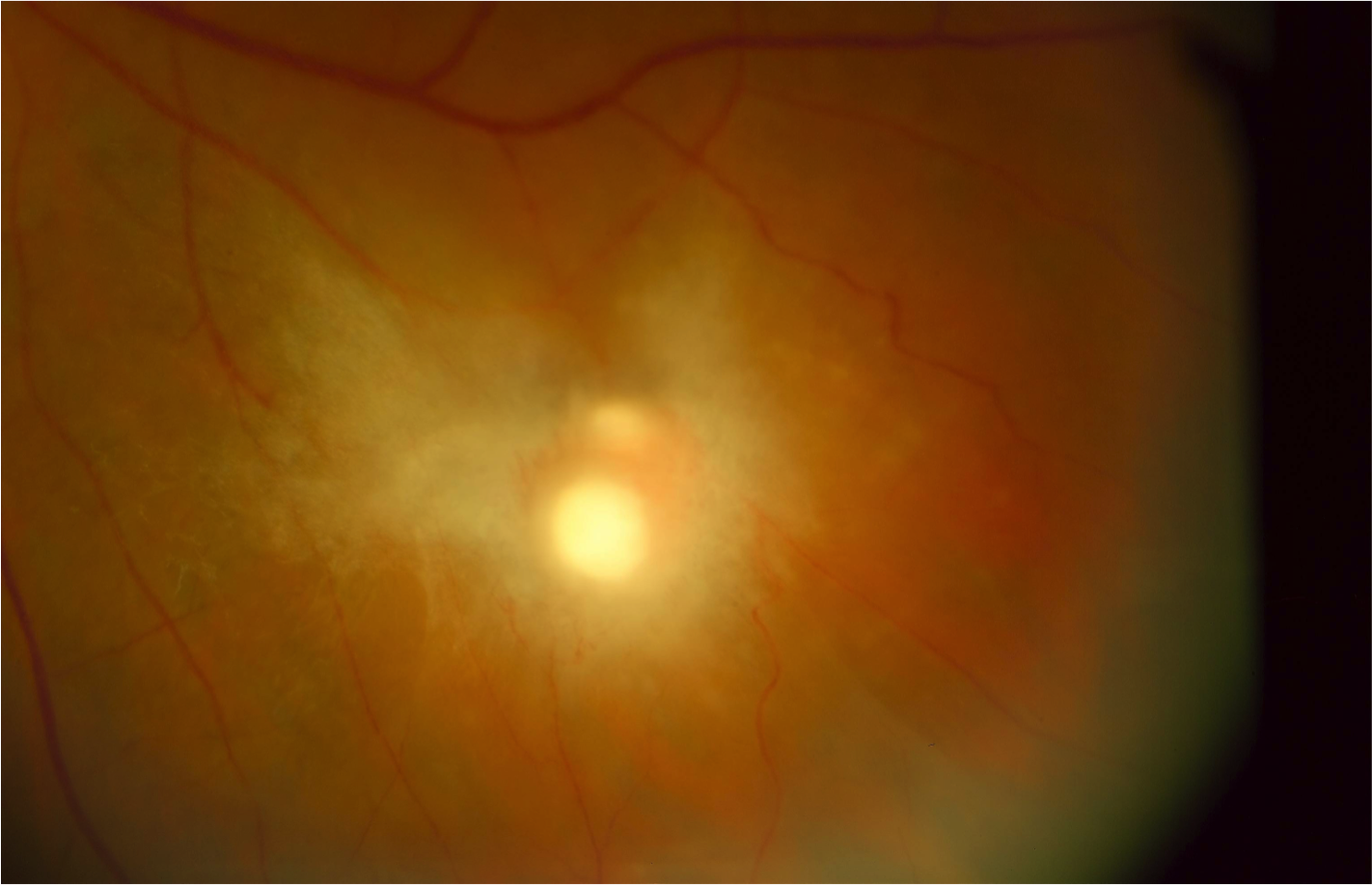
The hallmark for the diagnosis of Candida chorioretinitis is the presence of creamy white lesions at the level of the choroid and retina that is usually associated with vitreous haze. Early lesions are flat in the choroid with no or minimal vitritis. Untreated, they penetrate the blood-retina barrier and extend into the retina and vitreous. At this stage, the lesions become significantly elevated (Figure 2) and are associated with significant vitritis and later heal with scarring (Figure 3) that may result in retinal traction and tractional detachment. The lesions are commonly multiple and can be bilateral in two-thirds of cases.[17] Candida endophthalmitis can also present with vitreal abscesses, which appear as puff ball-like lesions in the vitreous cavity.[6][22]
Aspergillus endophthalmitis presents with yellow-white chorioretinal lesions involving the posterior pole that are often larger, more severe, and more likely to be hemorrhagic. Aspergillus commonly invades the retinal and choroidal vessels, which can lead to ischemic infarcts or full-thickness necrosis of the retina.[39][40]
Exogenous Endophthalmitis
Postoperative fungal endophthalmitis manifests several weeks or months after surgery with symptoms similar to endogenous endophthalmitis.
Posttraumatic fungal endophthalmitis may occur within hours or several weeks after the traumatic injury, particularly with soil, dirty objects, or vegetable matter. Patients present with decreased vision, eye pain out of proportion to exam, edema of the eyelid, corneal ring ulcer, anterior chamber inflammation, hypopyon, vitritis, or frank purulence. The injury may be apparent, and the intraocular inflammation can appear clumped rather than diffuse on the exam. All clinical findings must be evaluated with regard to the degree of injury.
On examination of the cornea in suspected cases of fungal keratitis, the appearance of infiltrates with fuzzy or feathery borders and satellite lesions are highly suggestive of a mold infection. Frond-like projections extending from the back of the cornea into the aqueous or thick clumped material in the aqueous are concerning for the progression of fungal keratitis into endophthalmitis.[1][6]
Evaluation
Diagnosing fungal endophthalmitis requires a thorough history and ophthalmic exam with a high index of suspicion. It is difficult to clinically diagnose fungal endophthalmitis, as there are no pathognomonic signs. Exogenous fungal endophthalmitis warrants inquiries about ocular surgeries or penetrating ocular injuries. For endogenous fungal endophthalmitis, a recent history of infections, surgery, hospitalizations, immunosuppressant use, and intravenous drug use should be noted. A diagnosis of fungal endophthalmitis can be reached by performing the following:
- Ultrasound B-scan is routinely used to evaluate endophthalmitis, especially if the view of the posterior segment is obscured. It is nonspecific but provides information, such as the severity of posterior involvement, the foci of the infection, and the presence of retinal detachment or abscesses, which aid ophthalmologists in formulating appropriate surgical plans.[41]
- Cultures of intraocular fluids aid in the diagnosis of patients with negative blood cultures that present with clinical findings consistent with fungal endophthalmitis. Vitreous cultures are limited by low sensitivity. Tanaka et al. found that only 38% of vitreous specimens in their study generated positive cultures.[42] Vitreous samples can be obtained by needle aspiration or diagnostic vitrectomy, which is more likely to have a higher diagnostic yield, especially if it is performed early.[43] Aqueous samples are also obtained by needle aspiration but have an even lower yield for cultures.[1]
- Blood cultures and complete workup for systemic infection, including echocardiography to look for heart valve vegetations, should be performed for all suspected cases of endogenous endophthalmitis. It is important to note that negative blood cultures cannot exclude endogenous fungal endophthalmitis. In endogenous Candida endophthalmitis, only 50%-75% of patients have positive blood cultures.[44] Cases with negative blood cultures are presumed to be in the setting of transient or intermittent fungemia.[20]
- Every patient diagnosed with systemic fungemia should promptly be referred to an ophthalmologist for a formal ophthalmic exam. If the exam is negative, a repeat exam should be conducted two weeks later.
- Polymerase chain reaction (PCR) has limited clinical use, but several studies have described its diagnostic potential in fungal endophthalmitis and its advantages over cultures. PCR produces rapid results with better specificity and greater sensitivity, as it can identify culture-negative cases without fear of contamination yielding false-positive results.[45][46][47] PCR may also detect fungal DNA in aqueous samples through anterior chamber paracentesis, which is less invasive than vitreous aspiration or biopsy.[48]
- Measuring levels of intraocular fluid beta-D-glucan was described by Chen et al. in 2020 as having a close association with fungal endophthalmitis and being more sensitive than culture methods. It is limited by an inability to confirm specific fungal species.[49]
Treatment / Management
Patients with fungal endophthalmitis should be promptly referred to a uveitis specialist after the disease is suspected or diagnosed. Mild cases that have flat choroidal lesions and minimal retinal or vitreous involvement can be managed with systemic antifungal therapy as the infection is predominantly outside the blood-retina barrier. Moderate or severe cases with raised retinal lesions and more significant involvement of the vitreous are best managed with combined intraocular and systemic antifungal therapies. Early vitrectomy decreases the incidence of late retinal detachment and is recommended in patients with severe vitreous involvement.
Because intraocular Candida infections are commonly part of a systemic infection, systemic antifungal therapy is mandatory. The agents must be able to cross the blood-retina barrier and achieve high ocular concentration, so fluconazole is the preferred agent.[50][51][52] Voriconazole, a second-generation triazole, is another option for primary treatment of endogenous Candida endophthalmitis, particularly in patients with suspected or proven resistance to fluconazole including C. krusei, C. glabrata, and molds infection.[40][43][53] It is of note that while voriconazole has a very extended fungal spectrum, the drug is expensive and requires that drug blood levels are checked periodically to adjust the dose. It is also associated with a higher level of liver toxicity compared to fluconazole. The utility of systemic amphotericin B (AMB) is limited by an inability to penetrate ocular tissues, systemic toxicity, and insufficient activity against non-Candida albicans species and molds.(A1)
Intravitreal therapy in fungal endophthalmitis relies on the use of intravitreal AMB or intravitreal voriconazole. While both are effective against fungal endophthalmitis, intravitreal voriconazole is associated with a lower risk of retinal toxicity, has a wider spectrum, and is currently the drug of choice.[40][54] Intravitreal AMB remains a reasonable alternative in eyes with fungal endophthalmitis due to yeast when voriconazole use is not feasible.(B3)
The role of vitrectomy surgery to obtain a vitreous biopsy for diagnosis and for the treatment of complications of fungal endophthalmitis, such as persistent vitreous opacities and tractional retinal detachment, is well established. However, the therapeutic benefit of early vitrectomy to control inflammation remains unclear. According to a retrospective study of 44 eyes with endogenous Candida endophthalmitis by Sallam et al., early vitrectomy, within one week of presentation, did not significantly reduce the risk of profound visual loss (postoperative Snellen acuity of ≤ 20/200), yet it decreased the risk of retinal detachment by fivefold.[51] The same results were replicated in a larger retrospective study of 66 eyes that compared eyes undergoing immediate vitrectomy at the time of initial injection of antimicrobial agents to delayed vitrectomy and found no difference in the proportions of eyes reaching a postoperative acuity of ≥20/200.[55](B2)
Differential Diagnosis
Candida chorioretinitis and endophthalmitis should be considered in the differential diagnosis of posterior uveitis or endophthalmitis in a patient who is immunosuppressed, with a recent history of hospitalization or intravenous drug abuse. Important differential diagnoses include:
- Noninfectious uveitis
- Sarcoidosis
- Behçet syndrome
- Sympathetic ophthalmia
- Juvenile idiopathic arthritis
- Vogt-Koyanagi-Harada disease
- Infectious chorioretinitis
- Bacterial endophthalmitis
- Coagulase-negative staphylococci
- Staphylococcus aureus
- Streptococci
- Tuberculosis
- Syphilis
- Viral endophthalmitis
- Herpes simplex virus
- Varicella-zoster virus
- Epstein-Barr virus
- Cytomegalovirus
- Toxoplasma Chorioretinitis
- Bacterial endophthalmitis
- Malignancy
- Intraocular lymphoma
- Retinoblastoma in children
Prognosis
In general, endogenous fungal endophthalmitis does not have a favorable prognosis and results in complete vision loss, especially if diagnosis and treatment are delayed.[24] Sallam et al. determined that poor visual outcomes, worse initial visual acuity, and centrally located lesion were associated with poor visual outcomes.[51] Virulence of the pathogen is also a significant prognostic factor. Ocular infections by Aspergillus and other molds have a worse visual prognosis than yeasts because they result in more aggressive cases of endophthalmitis.[55][56][57][58]
Complications
While patients with Candida endophthalmitis often achieve a final visual acuity of 6/60 or more,[42][59] patients with candidemia have a high overall mortality rate,[60] which reflects the extent and severity of both the fungal infection and underlying medical problems. Among the etiologies of exogenous fungal endophthalmitis, posttraumatic infections have the worst visual outcomes.[61] Wykoff et al. reported that 70% of eyes with open-globe-associated endophthalmitis required enucleation.[4]
Deterrence and Patient Education
Patients diagnosed with fungal endophthalmitis should be counseled on the importance of medication adherence and the consequences of inadequate treatment. The discomfort of intravitreal injections and the cost of antifungal agents, especially voriconazole, are among the patient factors that inhibit treatment compliance. Patients should be made aware that early and aggressive treatment of fungal endophthalmitis can lead to better outcomes. Otherwise, ocular fungal infections will persist and lead to worsening outcomes.
Enhancing Healthcare Team Outcomes
Interprofessional communication involving healthcare providers from multiple disciplines is key for the coordinated care of patients with fungal endophthalmitis. Consultations with internists or infectious disease specialists should be considered in all cases of fungal endophthalmitis in order to help facilitate the diagnostic workup for systemic fungal infections. Likewise, all cases of fungemia should be referred for ophthalmologic and optometry consultation to rule out ocular involvement. [Level 4][50]
Other specialists that are often involved in the care of patients with fungal endophthalmitis include cardiologists, pulmonologists, and nephrologists. Consultation with cardiologists is required to perform echocardiography to assess for fungal vegetations on heart valves. In cases of Aspergillus endophthalmitis, a pulmonologist will help manage infections of the lungs. Nephrology consultations are necessary when the source of infection is chronic indwelling hemodialysis catheters. Practicing a collaborative and interprofessional team approach will promote improved outcomes for patients with fungal endophthalmitis. Coordinated follow-up with an optometrist working with an ophthalmologist will lead to the best outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Durand ML. Bacterial and Fungal Endophthalmitis. Clinical microbiology reviews. 2017 Jul:30(3):597-613. doi: 10.1128/CMR.00113-16. Epub [PubMed PMID: 28356323]
Vaziri K, Schwartz SG, Kishor K, Flynn HW Jr. Endophthalmitis: state of the art. Clinical ophthalmology (Auckland, N.Z.). 2015:9():95-108. doi: 10.2147/OPTH.S76406. Epub 2015 Jan 8 [PubMed PMID: 25609911]
Gupta A, Gupta V, Gupta A, Dogra MR, Pandav SS, Ray P, Chakraborty A. Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian journal of ophthalmology. 2003 Jun:51(2):139-45 [PubMed PMID: 12831144]
Wykoff CC, Flynn HW Jr, Miller D, Scott IU, Alfonso EC. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology. 2008 Sep:115(9):1501-7, 1507.e1-2. doi: 10.1016/j.ophtha.2008.02.027. Epub 2008 May 16 [PubMed PMID: 18486220]
Level 2 (mid-level) evidenceChen JY, Jones MN, Srinivasan S, Neal TJ, Armitage WJ, Kaye SB, NHSBT Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Audit Study 18). Endophthalmitis after penetrating keratoplasty. Ophthalmology. 2015 Jan:122(1):25-30. doi: 10.1016/j.ophtha.2014.07.038. Epub 2014 Sep 26 [PubMed PMID: 25264028]
Level 2 (mid-level) evidenceKernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clinical ophthalmology (Auckland, N.Z.). 2010 Mar 24:4():121-35 [PubMed PMID: 20390032]
Level 3 (low-level) evidenceLong C, Liu B, Xu C, Jing Y, Yuan Z, Lin X. Causative organisms of post-traumatic endophthalmitis: a 20-year retrospective study. BMC ophthalmology. 2014 Mar 25:14():34. doi: 10.1186/1471-2415-14-34. Epub 2014 Mar 25 [PubMed PMID: 24661397]
Level 2 (mid-level) evidenceHenry CR, Flynn HW Jr, Miller D, Forster RK, Alfonso EC. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012 Dec:119(12):2443-9. doi: 10.1016/j.ophtha.2012.06.030. Epub 2012 Aug 1 [PubMed PMID: 22858123]
Level 2 (mid-level) evidenceFeman SS, Nichols JC, Chung SM, Theobald TA. Endophthalmitis in patients with disseminated fungal disease. Transactions of the American Ophthalmological Society. 2002:100():67-70; discussion 70-1 [PubMed PMID: 12545679]
Level 3 (low-level) evidenceShin SU, Yu YH, Kim SS, Oh TH, Kim SE, Kim UJ, Kang SJ, Jang HC, Park KH, Jung SI. Clinical characteristics and risk factors for complications of candidaemia in adults: Focus on endophthalmitis, endocarditis, and osteoarticular infections. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2020 Apr:93():126-132. doi: 10.1016/j.ijid.2020.01.049. Epub 2020 Jan 30 [PubMed PMID: 32007642]
Dozier CC, Tarantola RM, Jiramongkolchai K, Donahue SP. Fungal eye disease at a tertiary care center: the utility of routine inpatient consultation. Ophthalmology. 2011 Aug:118(8):1671-6. doi: 10.1016/j.ophtha.2011.01.038. Epub 2011 May 6 [PubMed PMID: 21550121]
Level 2 (mid-level) evidenceVilela RC, Vilela L, Vilela P, Vilela R, Motta R, Pôssa AP, de Almeida C, Mendoza L. Etiological agents of fungal endophthalmitis: diagnosis and management. International ophthalmology. 2014 Jun:34(3):707-21. doi: 10.1007/s10792-013-9854-z. Epub 2013 Oct 1 [PubMed PMID: 24081913]
Griffin JR, Pettit TH, Fishman LS, Foos RY. Blood-borne Candida endophthalmitis. A clinical and pathologic study of 21 cases. Archives of ophthalmology (Chicago, Ill. : 1960). 1973 Jun:89(6):450-6 [PubMed PMID: 4706441]
Level 3 (low-level) evidenceDonahue SP, Greven CM, Zuravleff JJ, Eller AW, Nguyen MH, Peacock JE Jr, Wagener MW, Yu VL. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology. 1994 Jul:101(7):1302-9 [PubMed PMID: 8035995]
Level 2 (mid-level) evidenceMcDonnell PJ, McDonnell JM, Brown RH, Green WR. Ocular involvement in patients with fungal infections. Ophthalmology. 1985 May:92(5):706-9 [PubMed PMID: 3874382]
Brooks RG. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Archives of internal medicine. 1989 Oct:149(10):2226-8 [PubMed PMID: 2802888]
Bross J, Talbot GH, Maislin G, Hurwitz S, Strom BL. Risk factors for nosocomial candidemia: a case-control study in adults without leukemia. The American journal of medicine. 1989 Dec:87(6):614-20 [PubMed PMID: 2589396]
Level 2 (mid-level) evidenceKrishna R, Amuh D, Lowder CY, Gordon SM, Adal KA, Hall G. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye (London, England). 2000 Feb:14 ( Pt 1)():30-4 [PubMed PMID: 10755096]
Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. The British journal of ophthalmology. 2008 Apr:92(4):466-8. doi: 10.1136/bjo.2007.133405. Epub [PubMed PMID: 18369061]
Level 2 (mid-level) evidenceKannangara S, Shindler D, Kunimoto DY, Sell B, DeSimone JA. Candidemia complicated by endophthalmitis: a prospective analysis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2007 Nov:26(11):839-41 [PubMed PMID: 17668252]
Sallam A, Lynn W, McCluskey P, Lightman S. Endogenous Candida endophthalmitis. Expert review of anti-infective therapy. 2006 Aug:4(4):675-85 [PubMed PMID: 17009945]
Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and parasitic infections of the eye. Clinical microbiology reviews. 2000 Oct:13(4):662-85 [PubMed PMID: 11023963]
Level 3 (low-level) evidenceSadiq MA, Hassan M, Agarwal A, Sarwar S, Toufeeq S, Soliman MK, Hanout M, Sepah YJ, Do DV, Nguyen QD. Endogenous endophthalmitis: diagnosis, management, and prognosis. Journal of ophthalmic inflammation and infection. 2015 Dec:5(1):32. doi: 10.1186/s12348-015-0063-y. Epub 2015 Nov 3 [PubMed PMID: 26525563]
Okada AA, Johnson RP, Liles WC, D'Amico DJ, Baker AS. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994 May:101(5):832-8 [PubMed PMID: 8190467]
Level 2 (mid-level) evidenceWu ZH, Chan RP, Luk FO, Liu DT, Chan CK, Lam DS, Lai TY. Review of Clinical Features, Microbiological Spectrum, and Treatment Outcomes of Endogenous Endophthalmitis over an 8-Year Period. Journal of ophthalmology. 2012:2012():265078. doi: 10.1155/2012/265078. Epub 2012 Feb 23 [PubMed PMID: 23533699]
Mamandhar A, Bajracharya L. Endogenous Aspergillus endophthalmitis in a healthy individual. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2012 Jan-Jun:4(1):179-83. doi: 10.3126/nepjoph.v4i1.5873. Epub [PubMed PMID: 22344019]
Level 3 (low-level) evidenceLogan S, Rajan M, Graham E, Johnson E, Klein J. A case of aspergillus endophthalmitis in an immuncompetent woman: intra-ocular penetration of oral voriconazole: a case report. Cases journal. 2010 Jan 18:3():31. doi: 10.1186/1757-1626-3-31. Epub 2010 Jan 18 [PubMed PMID: 20205770]
Level 3 (low-level) evidenceAgarwal M, Biswas J, Mathur U, Sijwali MS, Singh AK. Aspergillus iris granuloma in a young male: a case report with review of literature. Indian journal of ophthalmology. 2007 Jan-Feb:55(1):73-4 [PubMed PMID: 17189896]
Level 3 (low-level) evidenceLee JH, Kim JS, Park YH. Diagnosis and treatment of postpartum Candida endophthalmitis. The journal of obstetrics and gynaecology research. 2012 Sep:38(9):1220-2. doi: 10.1111/j.1447-0756.2012.01854.x. Epub 2012 May 8 [PubMed PMID: 22563724]
Level 3 (low-level) evidenceShankar K, Gyanendra L, Hari S, Narayan SD. Culture proven endogenous bacterial endophthalmitis in apparently healthy individuals. Ocular immunology and inflammation. 2009 Nov-Dec:17(6):396-9. doi: 10.3109/09273940903216891. Epub [PubMed PMID: 20001259]
Level 2 (mid-level) evidenceTsirouki T, Steffen J, Dastiridou A, Praidou A, Androudi S. Endophthalmitis in HIV Infection. Ocular immunology and inflammation. 2020 Oct 2:28(7):1060-1065. doi: 10.1080/09273948.2019.1699580. Epub 2020 Jan 16 [PubMed PMID: 31944150]
Edwards JE Jr, Montgomerie JZ, Foos RY, Shaw VK, Guze LB. Experimental hematogenous endophthalmitis caused by Candida albicans. The Journal of infectious diseases. 1975 Jun:131(6):649-57 [PubMed PMID: 48529]
Level 3 (low-level) evidenceMoshfeghi AA, Charalel RA, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Declining incidence of neonatal endophthalmitis in the United States. American journal of ophthalmology. 2011 Jan:151(1):59-65.e1. doi: 10.1016/j.ajo.2010.07.008. Epub [PubMed PMID: 20970776]
Level 2 (mid-level) evidenceAziz HA, Berrocal AM, Sisk RA, Hartley K, Diaz-Barbosa M, Johnson RA, Hess D, Dubovy SR, Murray TG, Flynn HW Jr. Intraocular infections in the neonatal intensive care unit. Clinical ophthalmology (Auckland, N.Z.). 2012:6():733-7. doi: 10.2147/OPTH.S26362. Epub 2012 May 14 [PubMed PMID: 22654500]
Level 3 (low-level) evidenceBasu S, Kumar A, Kapoor K, Bagri NK, Chandra A. Neonatal endogenous endophthalmitis: a report of six cases. Pediatrics. 2013 Apr:131(4):e1292-7. doi: 10.1542/peds.2011-3391. Epub 2013 Mar 11 [PubMed PMID: 23478867]
Level 3 (low-level) evidenceStone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investigative ophthalmology & visual science. 1996 Feb:37(2):290-9 [PubMed PMID: 8603833]
Level 3 (low-level) evidenceKaburaki T, Sato S, Kawashima H, Sakurai M, Numaga J, Fujino Y, Araie M. A hypopyon is a sign of post-trabeculectomy endophthalmitis or not? Eye (London, England). 2005 Jun:19(6):692-3 [PubMed PMID: 15184940]
Level 3 (low-level) evidenceSheyman AT, Cohen BZ, Friedman AH, Ackert JM. An outbreak of fungal endophthalmitis after intravitreal injection of compounded combined bevacizumab and triamcinolone. JAMA ophthalmology. 2013 Jul:131(7):864-9. doi: 10.1001/jamaophthalmol.2013.88. Epub [PubMed PMID: 23640384]
Rao NA, Hidayat A. A comparative clinicopathologic study of endogenous mycotic endophthalmitis: variations in clinical and histopathologic changes in candidiasis compared to aspergillosis. Transactions of the American Ophthalmological Society. 2000:98():183-93; discussion 193-4 [PubMed PMID: 11190022]
Level 2 (mid-level) evidenceRiddell Iv J, McNeil SA, Johnson TM, Bradley SF, Kazanjian PH, Kauffman CA. Endogenous Aspergillus endophthalmitis: report of 3 cases and review of the literature. Medicine. 2002 Jul:81(4):311-20 [PubMed PMID: 12169886]
Level 3 (low-level) evidenceDavis JL. Diagnostic dilemmas in retinitis and endophthalmitis. Eye (London, England). 2012 Feb:26(2):194-201. doi: 10.1038/eye.2011.299. Epub 2011 Nov 25 [PubMed PMID: 22116459]
Tanaka M, Kobayashi Y, Takebayashi H, Kiyokawa M, Qiu H. Analysis of predisposing clinical and laboratory findings for the development of endogenous fungal endophthalmitis. A retrospective 12-year study of 79 eyes of 46 patients. Retina (Philadelphia, Pa.). 2001:21(3):203-9 [PubMed PMID: 11421007]
Level 2 (mid-level) evidenceLingappan A, Wykoff CC, Albini TA, Miller D, Pathengay A, Davis JL, Flynn HW Jr. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. American journal of ophthalmology. 2012 Jan:153(1):162-6.e1. doi: 10.1016/j.ajo.2011.06.020. Epub 2011 Sep 13 [PubMed PMID: 21917234]
Level 2 (mid-level) evidenceDenning DW, Evans EG, Kibbler CC, Richardson MD, Roberts MM, Rogers TR, Warnock DW, Warren RE. Guidelines for the investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. British Society for Medical Mycology. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 1997 Jun:16(6):424-36 [PubMed PMID: 9248745]
Level 1 (high-level) evidenceSugita S, Kamoi K, Ogawa M, Watanabe K, Shimizu N, Mochizuki M. Detection of Candida and Aspergillus species DNA using broad-range real-time PCR for fungal endophthalmitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2012 Mar:250(3):391-8. doi: 10.1007/s00417-011-1819-1. Epub 2011 Sep 27 [PubMed PMID: 21947326]
Level 3 (low-level) evidenceSandhu HS, Hajrasouliha A, Kaplan HJ, Wang W. Diagnostic Utility of Quantitative Polymerase Chain Reaction versus Culture in Endophthalmitis and Uveitis. Ocular immunology and inflammation. 2019:27(4):578-582. doi: 10.1080/09273948.2018.1431291. Epub 2018 Feb 22 [PubMed PMID: 29470930]
Sowmya P, Madhavan HN. Diagnostic utility of polymerase chain reaction on intraocular specimens to establish the etiology of infectious endophthalmitis. European journal of ophthalmology. 2009 Sep-Oct:19(5):812-7 [PubMed PMID: 19787602]
Kharel Sitaula R, Janani MK, Madhavan HN, Biswas J. Outcome of polymerase chain reaction (PCR) analysis in 100 suspected cases of infectious uveitis. Journal of ophthalmic inflammation and infection. 2018 Jan 10:8(1):2. doi: 10.1186/s12348-017-0144-1. Epub 2018 Jan 10 [PubMed PMID: 29322275]
Level 3 (low-level) evidenceChen L, Tao Y, Hu X. Utility of Intraocular Fluid β-D-glucan Testing in Fungal Endophthalmitis: A Series of 5 Cases. The American journal of case reports. 2020 Mar 23:21():e921188. doi: 10.12659/AJCR.921188. Epub 2020 Mar 23 [PubMed PMID: 32201431]
Level 3 (low-level) evidencePappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Feb 15:62(4):e1-50. doi: 10.1093/cid/civ933. Epub 2015 Dec 16 [PubMed PMID: 26679628]
Level 1 (high-level) evidenceSallam A, Taylor SR, Khan A, McCluskey P, Lynn WA, Manku K, Pacheco PA, Lightman S. Factors determining visual outcome in endogenous Candida endophthalmitis. Retina (Philadelphia, Pa.). 2012 Jun:32(6):1129-34. doi: 10.1097/IAE.0b013e31822d3a34. Epub [PubMed PMID: 22298012]
Level 2 (mid-level) evidenceSilva RA, Sridhar J, Miller D, Wykoff CC, Flynn HW Jr. Exogenous fungal endophthalmitis: an analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990-2010). American journal of ophthalmology. 2015 Feb:159(2):257-64.e1. doi: 10.1016/j.ajo.2014.10.027. Epub 2014 Nov 1 [PubMed PMID: 25449001]
Sen P, Gopal L, Sen PR. Intravitreal voriconazole for drug-resistant fungal endophthalmitis: case series. Retina (Philadelphia, Pa.). 2006 Oct:26(8):935-9 [PubMed PMID: 17031296]
Level 2 (mid-level) evidenceHariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. The British journal of ophthalmology. 2008 Jul:92(7):871-8. doi: 10.1136/bjo.2007.136515. Epub [PubMed PMID: 18577634]
Level 3 (low-level) evidenceZhang H, Liu Z. Endogenous endophthalmitis: a 10-year review of culture-positive cases in northern China. Ocular immunology and inflammation. 2010 Apr:18(2):133-8. doi: 10.3109/09273940903494717. Epub [PubMed PMID: 20370344]
Level 2 (mid-level) evidenceSridhar J, Flynn HW Jr, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: risk factors, clinical features, and treatment outcomes in mold and yeast infections. Journal of ophthalmic inflammation and infection. 2013 Sep 20:3(1):60. doi: 10.1186/1869-5760-3-60. Epub 2013 Sep 20 [PubMed PMID: 24053550]
Duan F, Yang Y, Yuan Z, Zheng Y, Cheng Z, Lin X. Clinical Features and Visual Acuity Outcomes in Culture-Positive Endogenous Fungal Endophthalmitis in Southern China. Journal of ophthalmology. 2017:2017():3483497. doi: 10.1155/2017/3483497. Epub 2017 Aug 13 [PubMed PMID: 28884023]
Behera UC, Budhwani M, Das T, Basu S, Padhi TR, Barik MR, Sharma S. ROLE OF EARLY VITRECTOMY IN THE TREATMENT OF FUNGAL ENDOPHTHALMITIS. Retina (Philadelphia, Pa.). 2018 Jul:38(7):1385-1392. doi: 10.1097/IAE.0000000000001727. Epub [PubMed PMID: 28541964]
Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine. 2003 Mar:82(2):97-105 [PubMed PMID: 12640186]
Level 2 (mid-level) evidenceHorn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Jun 15:48(12):1695-703. doi: 10.1086/599039. Epub [PubMed PMID: 19441981]
Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. American journal of ophthalmology. 2004 Apr:137(4):725-31 [PubMed PMID: 15059712]
Level 2 (mid-level) evidence