Introduction
Cystic fibrosis (CF) is an autosomal recessive (AR) disorder that commonly affects the White population with an annual incidence of approximately 1 in 3,500 live births.[1][2][3] This multisystem disorder is characterized by genetic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene on chromosome 7, which encrypts a protein essential for the regulation of transmembrane chloride reabsorption.[1][3] CFTR, a chloride channel, is expressed in secretory epithelial cells lining the airways, digestive system, reproductive system, and the skin.[2] Homozygous mutations in the CFTR gene impairs the transport of chloride ions and the movement of water into and out of cells resulting in the inspissation of secretions leading to organ dysfunction. Although CF commonly affects the airways, approximately 10% to 15% of patients with CF demonstrate Cystic fibrosis-associated liver disease (CFLD).[4] The improvement in the diagnostic modalities and management of CF that has had a positive impact on the life expectancy of patients with CF. Consequently, there has been a significant emergence of liver dysfunction in CF complicating the clinical course of the disease.[5][1] CFTR dysfunction has a significant effect on cholangiocyte function causing an alteration in the final bile composition that causes chronic damage to the biliary epithelium resulting in the development of a broad spectrum of hepatobiliary complications ranging from cholestasis, progressive periportal fibrosis, biliary obstruction causing focal biliary cirrhosis which may progress to multinodular cirrhosis, portal hypertension, and hepatic decompensation.[6][7][8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of cystic fibrosis-associated liver disease has been attributed to an alteration in hydration and alkalinity of the bile composition due to mutation of the CFTR gene expressed in the cholangiocytes that regulate transmembrane chloride exchange. Approximately 2000 CFTR mutations classified into five different groups based on the involved functional defect (Class I: Defective protein production; class II: Defective protein processing; class III: defective gating of the channel; class IV: decreased chloride conductance; class V: Decreased protein synthesis) have been identified in patients with CF with the most common mutation being the deletion of a phenylalanine residue at position 508 (F508del) on chromosome 7 which is a class II mutation seen in 80% to 90% patients with CF.[8] Currently, there are no specific CFTR mutations that have been associated with CFLD, but studies have demonstrated risk factors like male sex, class I-III mutations, associated pancreatic insufficiency, and meconium ileus at birth. In a small prospective study, F508 was noted to be in 51% to 55% of the patients with CFLD.[9] Patients with CF with a specific CFTR genotype exhibit a variable liver phenotype, implicating influence of environmental factors or other possible causes such as medications used in the treatment of CF, chronic infection, and malnutrition that might be important in the evolution of CFLD.[6][10]
Epidemiology
The prevalence of CF differs by geographical background and ethnicity. It is commonly seen in Whites with an incidence of approximately 1 in 2000 to 3000 live births compared to an incidence of 1 in 4000 to10 000 and 1 in 15000 to 20000 live births in Latin American and African American populations, respectively. The prevalence of the disease in other racial groups is not well studied.[3][11] The incidence of cystic fibrosis-associated liver disease in patients with CF is approximately 10% to 15%. In a prospective study, the incidence of CF was noted to be 2.5 per 100 patient-years through the first decade of life with a significant decline in the second decade.[9] Approximately 5% to 10% of patients with CF develop multilobular cirrhosis during the first decade of life.[12] CFLD is the third most common cause of death in patients with CF, with an overall mortality rate of 2.5%.[4]
Pathophysiology
A precise pathogenetic mechanism linking the development and progression of liver disease in patients with CF has not been well identified. Classically, the development of liver disease in patients with CF has been attributed to ductal cholestasis caused by CFTR dysfunction. CFTR is a cyclic adenosine monophosphate (cAMP) dependent chloride channel expressed in secretory epithelial cells of various organs. In the liver, CFTR is expressed by the cholangiocytes (bile duct cells) lining the luminal membrane of the biliary epithelium.[13]
Normally, cholangiocytes serve to perform an array of intracellular transport functions that regulate the transmembrane chloride and bicarbonate reabsorption, thereby determining the secretion and composition of bile.[7] CFTR dysfunction has a major effect on the cholangiocyte dysfunction, which causes decreased bile alkalinity and flow, as demonstrated in cholangiocytes isolated from explanted livers of patients with cystic fibrosis-associated liver disease and animal studies involving CFTR knockout mice.[7] Prolonged cholestasis due to CFTR dysfunction predisposes the biliary epithelium to cytotoxic damage from inflammatory mediators like cytokines and chemokines, causing a state of chronic inflammation resulting in periportal fibrosis which ultimately progresses into focal and multilobar cirrhosis. However, this classic view is not fully supported in experimental studies and clinically as commonly viscous bile and jaundice are late findings in patients with CFLD.[13]
Besides its function on the regulation of transmembrane chloride conductance, newer studies have demonstrated the effect of CFTR in relation to toll-like receptor 4 (TLR4) activity. CFTR normally regulates TLR4 dependent inflammatory responses by inhibiting the activity of Rous sarcoma oncogene cellular homolog (Src), which is a non-receptor protein tyrosine kinase. Mutations in the CFTR gene lead to self-activation of Src and phosphorylation of TLR4, resulting in the generation of pro-inflammatory cytokines resulting in disruption of the epithelial barrier by inflammatory cells and translocation of bacteria into portal circulation resulting in chronic inflammation and fibrosis.[8][13]
CFTR dysfunction affects the gut microbiota causing intestinal inflammation and symbiosis with an overall increase in pathogenic bacteria favoring translocation of bacteria into the portal circulation.[13] However, the pathophysiology of the gut microbiome, causing hepatic inflammation in CF, is not well understood and requires further studies. Recent studies have identified noncirrhotic portal hypertension with focal nodular hyperplasia in adults implicating a different alternate mechanism in the pathophysiology of CFLD.[14]
Histopathology
Microscopic exam of liver biopsies performed in patients with cystic fibrosis-associated liver disease demonstrate a wide spectrum of histopathological changes ranging from pan acinar steatosis, cholestasis, bile duct proliferation, portal inflammation to fibrosis of varying degrees of severity and nodular regenerative hyperplasia. Neutrophils were the most predominant inflammatory cells. While hepatic steatosis and focal biliary cirrhosis are common findings in pediatric patients with CFLD, focal biliary cirrhosis is not as common in adult patients with CFLD. Portal hypertension in patients with CFLD is not entirely due to fibrosis or cirrhosis, but non-cirrhotic portal hypertension (NCPH) also plays a role with demonstrated evidence of presinusoidal type portal hypertension because of obliterative venopathy with fibrosis.[15][16]
History and Physical
Pathological involvement of the hepatobiliary system in patients with CF typically develops before or during puberty with an incidence of 15% to 17%, clinically manifesting in a diverse manner ranging from asymptomatic elevation in liver function tests to advanced liver disease with decompensation. In infants with CFTR dysfunction, liver dysfunction may manifest in the form of cholestasis, which is relatively rare but is seen in approximately 50% of newborns with CF when it cooccurs with meconium ileus.[17] However, the most common clinical finding in cystic fibrosis-associated liver disease is hepatomegaly noted on routine physical examination, often but not always concomitant with abnormal liver function tests. Hepatomegaly may be attributable to either fatty infiltration of the liver or focal biliary fibrosis.[6][18] Common bile duct obstruction and cholecystitis may occur in 1% to 10% of patients presenting with abdominal pain and jaundice.[19] Severe CFLD occurs in 7% of patients with CF characterized by biliary multilobar cirrhosis with or without portal hypertension and has a damaging effect on the respiratory function, thereby worsening morbidity and mortality.[13]
Evaluation
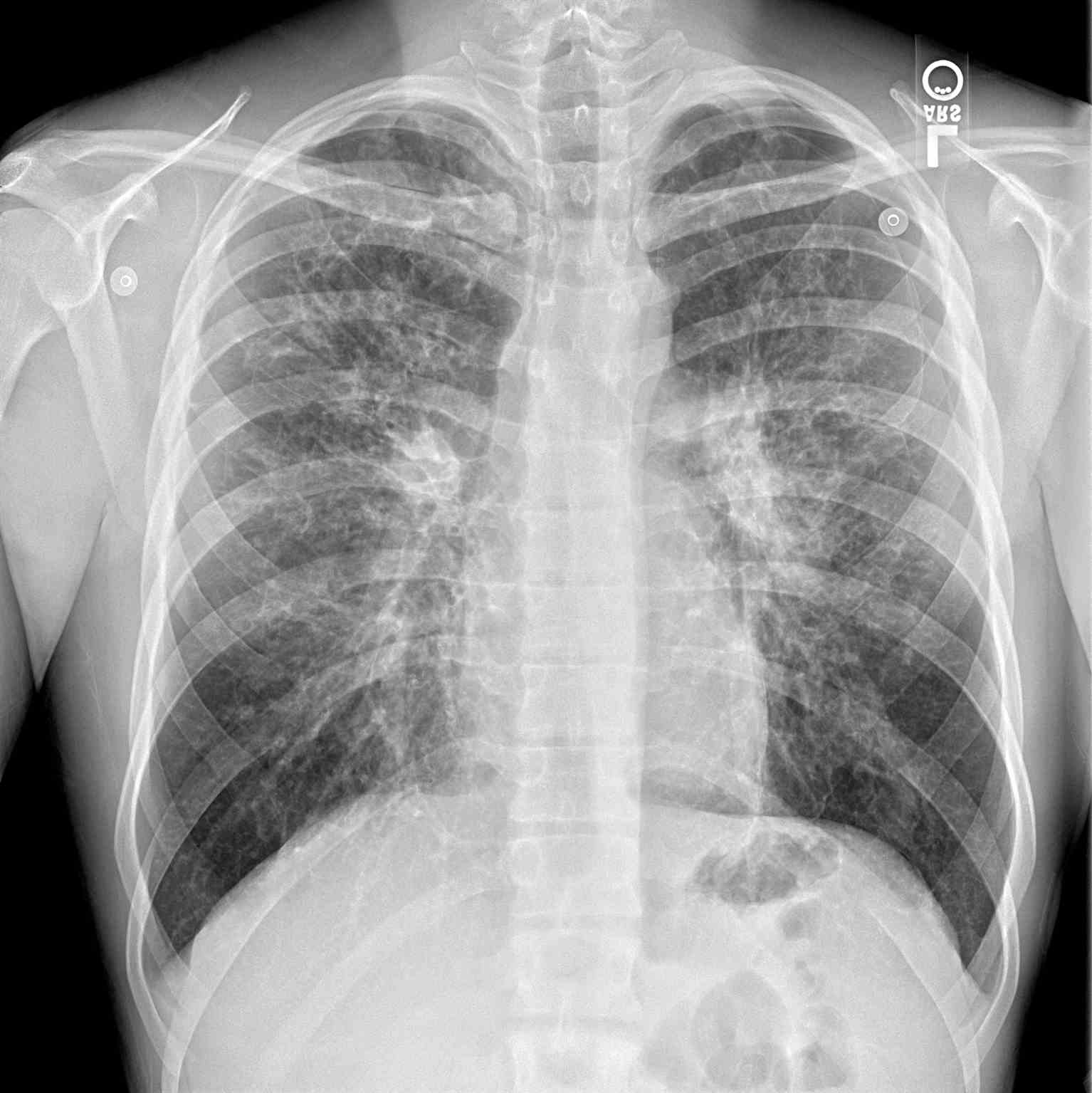
The diagnosis of cystic fibrosis-associated liver disease remains a challenge due to the non-availability of a sensitive and specific test. Most hepatic manifestations are not seen until the development of cirrhosis and portal hypertension. Nevertheless, the early diagnosis of CFLD relies on comprehensive clinical assessments, biochemical tests, and imaging modalities (see Image. Periapical Chest Radiograph, Cystic Fibrosis).[13] Approximately 40% to 50% of patients with CF demonstrate intermittent elevations in serum transaminases without any symptoms and are not predictive of the presence of underlying fibrosis. Persistent elevation in serum transaminases is uncommon and should be extensively evaluated to rule out acute/chronic viral hepatitis, alpha-1 antitrypsin deficiency, autoimmune hepatitis, celiac disease, Wilson disease, and hemochromatosis. Considering, CFLD is predominantly a cholestatic liver disorder, other diseases with similar etiology like primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), and choledocholithiasis should be excluded.[13] Newer noninvasive tests that employ the use of serum biomarkers like aspartate aminotransferase to platelet ratio index (APRI) and Fibrosis-4 (FIB-4) index should be considered early to determine liver fibrosis.[20]
Ultrasonography plays a significant role in the diagnosis of CFLD and should be considered early as it is more sensitive in detecting hepatic steatosis, focal biliary fibrosis, multilobular cirrhosis, and biliary duct defects. However, a normal ultrasound examination does not translate as a low risk for the development of fibrosis, as patients with CF with normal ultrasound examinations have been shown to progress to cirrhosis.[21][16] Other radiologic tests like CT and MRI are valuable in differentiating fibrosis from steatosis and are valuable in detecting biliary ductal abnormalities. Although not well studied in CFLD, vibration controlled transient elastography (VCTE) is emerging as a valuable, noninvasive, inexpensive test in determining the stage of liver fibrosis.[20] Utilization of noninvasive serum biomarkers like aspartate aminotransferase to platelet ratio index (APRI) and FIB-4 index in conjunction with transient elastography can be considered to determine liver fibrosis.[20][22][23] A recent prospective study employing supersonic shear-wave elastography (SSWE) in combination with APRI showed good diagnostic accuracy in detecting CFLD in children with CF.[24]
Although liver biopsy remains the gold standard for diagnosis and staging of CFLD, it is not routinely recommended owing to the invasive nature of the test and patchy nature of the disease.[6] A prospective study demonstrated an increase of 22% in the detection of fibrosis with a dual pass needle core liver biopsy compared to a single pass in patients with suspected CFLD.[25] Debray et al. and Koh et al. proposed two separate diagnostic criteria for CFLD employing multiple variables like physical examination, laboratory tests, imaging studies, transient elastography, noninvasive biomarkers, and liver biopsy, as summarized in Table 1.[6][26]
Treatment / Management
Currently, there is no available treatment that has proven to be efficacious or delay the progression of cystic fibrosis-associated liver disease. Best practice guidelines for the prevention and management of advanced liver disease in CF are lacking.[27] Once the diagnosis of CFLD is made, the primary goal of management is to mitigate the complications of portal hypertension and cirrhosis.(A1)
Portal hypertension is a relatively common complication in patients with end-stage liver disease, and its diagnosis and management are very crucial. Patients with cirrhosis should be assessed for portal hypertension with an abdominal exam to check for splenomegaly and ascites, ultrasound examination with Doppler velocity, and biochemical tests showing platelet counts of <150,000 units x 10/mL.[28] Esophageal and gastric varices occur as a result of portal hypertension and commonly manifest as acute bleeding. Specific guidelines related to the prevention and management of acute variceal bleeding due to portal hypertension in people with CFLD are not available.[27] Nonetheless, all patients with CFLD with cirrhosis should be screened for esophageal varices (EV) with an esophagogastroduodenoscopy (EGD) examination.[6] The presence of large esophageal varices on EGD examination requires variceal prophylaxis, which can be pharmacologic, endoscopic, surgical, or by interventional radiology.[27] The safety and benefits of time tested pharmaceutical intervention of initiating non-selective beta-blockers (NSBB) as primary variceal prophylaxis have not been completely evaluated in patients with CFLD and are avoided due to bronchoconstriction.[27] Endoscopic variceal band ligation (EVBL) is considered the management of choice of primary and secondary variceal prophylaxis.[28][29] However, EVBL carries a risk of rebleeding, in which case patients should be considered for placement of a transjugular intrahepatic portosystemic stent (TIPS) as a bridge to liver transplantation.[27] An alternative measure is the surgical placement of a portosystemic (PS) shunt and should be reserved for refractory cases only as it causes the risk of development of worsening hepatic encephalopathy and acute liver failure.[6][27](A1)
Ascites, if demonstrated on physical examination and or imaging, is considered to be a poor prognostic sign. Management includes sodium and fluid restrictive diet and initiation of diuretics. Referral for liver transplant evaluation should be considered.[6][28] Hepatic encephalopathy is considered to be a rare finding in cirrhosis associated with CFLD and usually occurs after portosystemic shunting for portal hypertension.[28] Patients with CFLD with cirrhosis need imaging studies (ultrasound, CT scan, or MRI of the liver) every six months to screen for hepatocellular carcinoma. Most patients with CFLD are at higher risk for developing severe malnutrition secondary to fat malabsorption and protein loss. Optimal nutritional support should be provided by increasing the protein and fat intake, pancreatic enzyme replacement, and fat-soluble vitamin supplementation.[6] Patients with CFLD should receive full immunization against Hepatitis A and B, if not immune already. Patients should be educated to abstain from alcohol and avoid using hepatotoxic drugs, NSAIDs, and salicylates to decrease the risk of bleeding from portal hypertensive gastropathy.[6]
The role of ursodeoxycholic acid (UDCA) in CFLD is controversial, with multiple clinical trials showing conflicting results. UDCA prescribed at 20 to 30 mg/kg body weight/day has shown to improve biochemical parameters, but there is no strong evidence that the administration of UDCA alters the natural course of CFLD.[17][30][31][32] Newer FDA approved medications like lumacaftor and ivacaftor that directly target CFTR protein have shown promising results, but their efficacy in CFLD has not been well studied.[33][34] More long-term follow-up and validation studies are needed.(A1)
Liver transplantation (LT) confers a substantial survival advantage in patients with CFLD, but the clinical criteria and timing of liver transplantation in CFLD are not well established.[35] Debray et al. recommended liver transplantation should be considered if patients with CFLD demonstrate progressive hepatic failure characterized by progressive hypoalbuminemia and coagulopathy, worsening jaundice and ascites, variceal bleeding not controlled by conventional means, hepatopulmonary and portopulmonary syndromes, severe malnutrition despite intensive nutritional provision, worsening pulmonary function (FEV1/FVC <50%) and declining quality of life linked to advanced liver disease.[6] Studies have shown that worsening hepatic decompensation in patients with CF negatively influences nutritional status affecting the overall prognosis, and liver transplantation has long term beneficial effects on nutritional status.[36] Combined lung and liver transplantation (CLLT) should be considered for patients with advanced pulmonary and liver disease.[28] The outcomes of LT in CFLD have been favorable with 1-year patient and graft survival at 89% and 83%, respectively, and 5-year patient survival rates at 85.8%.[37][38] Analytical studies of the CF registry have shown no significant change in the rate of decline in FEV1 in 3 years following LT in patients with CF with end-stage liver disease compared to CF control patients without liver disease, which indicates that LT does not necessarily improve long term pulmonary outcome.[39](B2)
Differential Diagnosis
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder characterized by inflammation and fibrosis of the intrahepatic and extrahepatic biliary ducts that ultimately progress to end-stage liver disease. It is commonly associated with IBD. Like CFLD, PSC results from prolonged cholestasis and the histological features and radiological features are similar to those seen in the liver of patients with cystic fibrosis-associated liver disease which has been explained by possible interaction between bile acids receptors such as Farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (TGR5).[32] CFLD only affects the intrahepatic bile ducts in contrast to PSC, which can involve the intrahepatic, extrahepatic bile ducts, or both and lead to end-stage liver disease.
Secondary Sclerosing Cholangitis
Secondary sclerosing cholangitis (SSC) is a chronic cholestatic liver disorder that is morphologically similar to PSC and is caused by an identified pathological process.[39] Considering CFLD and PSC have similar features, CFLD itself can be considered as a type of SSC. Recurrent pancreatitis, which is not uncommon in patients with CF, is also considered to be a well-described cause of SSC. Other types of cholangiopathies like choledocholithiasis, ischemic cholangitis, and recurrent pyogenic cholangitis need to be ruled out before a diagnosis of CFLD is considered.[40]
Drug-induced Liver Injury
Drug-induced liver injury (DILI) is a concern in these patients as they tend to be on antibiotics frequently for recurrent bacterial sinus infections. Newer treatment agents (ivacaftor and lumacaftor) that directly modulate the CFTR function can cause a mild elevation in serum transaminases.[41][34]
Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) is seen in approximately 10% to 20% of the general pediatric population incorporating a wide spectrum of chronic liver disorders ranging from hepatic steatosis to non-alcoholic steatohepatitis (NASH) leading to end-stage liver disease.[42] NAFLD, by far, is the most common cause of hepatic fibrosis and cirrhosis in children of the Western world. Hepatic steatosis is the most common hepatic manifestation seen in patients with CF with a prevalence rate of 20% to 60% and is not well characterized compared to other CFLD manifestations like focal biliary and multinodular cirrhosis.[43][44] The pathophysiology of the development of hepatic steatosis in patients with CF is largely unknown, and long term prospective studies are needed.[44]
Prognosis
With an increase in the life expectancy of patients with CF, the increased prevalence of liver involvement has emerged as a significant healthcare burden.[45] Cystic fibrosis-associated liver disease should be considered as an early manifestation of cystic fibrosis, considering it involves more than one-fourth of patients with CF. Risk factors for the development of liver disease in cystic fibrosis are unclear, but active surveillance of hepatic manifestations should be focused in the first decade of life, especially in patients with class I-III mutations, male sex, meconium ileus at birth and associated pancreatic insufficiency.[43][46] A prospective study evaluating the prognosis of patients with CFLD showed worsening of weight percentile with no significant difference in the incidence of respiratory failure, need for oxygen therapy, and frequency of hospitalizations.[9] Patients with CF with liver disease did not demonstrate higher mortality when compared to cystic fibrosis patients without liver disease, but patients with CFLD had a significant impact on overall morbidity. The progression of CFLD after lung transplant is not well known, but few studies have shown CFLD does not appear to be influenced by lung transplantation. For patients with progressive disease, liver transplantation either alone or in combination with lung transplant is a feasible alternative with improved outcomes. Consideration must be given for early identification of CFLD, which can have a significant impact on overall morbidity and mortality.
Complications
Cystic fibrosis-associated liver disease, as a disease entity itself, is a complication of CF. Approximately 5% to 10% of patients with CF develop multilobar cirrhosis during the first decade of life, and it remains to be the single most important non-pulmonary cause of death, accounting for 2.5% of overall CF mortality.[9][47] Most patients develop signs of portal hypertension during their second decade of life and primarily present with upper GI bleeding secondary from esophageal varices.[12][48] Other common complications of decompensated cirrhosis are the development ascites which is considered to be a poor prognostic sign, hepatic encephalopathy, hepatopulmonary syndrome, portopulmonary syndrome, thrombocytopenia, leukopenia, and hepatocellular carcinoma (HCC).[28]
Deterrence and Patient Education
Cystic fibrosis is a progressive autosomal recessive disorder that involves multiple organ systems. To develop cystic fibrosis, a child must inherit one copy of the cystic fibrosis transmembrane conductance regulator (CFTR) gene mutation from each parent. Caucasians have a higher risk of carrying a mutation of the CFTR gene. The life expectancy of individuals with cystic fibrosis has dramatically improved in the last decade and continues to increase with improved treatment protocols. CFLD (cystic fibrosis-associated liver disease) is now the third leading cause of death, only behind pulmonary and transplant-related complications. Cystic fibrosis-associated liver disease accounts for 5% of deaths in patients with CF. Risk factors for the development of CFD include male sex, meconium ileus, and severe phenotype CF. Until recently, most patients with CFLD presented in childhood; however, recent studies show the second wave of this chronic liver disease become evident in adulthood, the etiology of which remains unclear. Currently, there is no consensus methodology for the diagnosis of CFLD, particularly given its variable presentation and patchy nature of the disease. Annual screening for CFLD is recommended for early identification to help prevent or delay its complications to reduce morbidity and mortality. Early identification of progressive CFLD allows monitoring for portal hypertension and prompt management of its complications. LT should be considered in patients with progressive hepatic failure characterized by portal hypertension-related complications, progressive hypoalbuminemia and coagulopathy, severe malnutrition, worsening pulmonary function, and declining quality of life.
Enhancing Healthcare Team Outcomes
Enhancing positive outcomes in patients with cystic fibrosis-associated liver disease requires an interprofessional team approach and closed-loop communication by primary care providers, hepatologists, pulmonologists, nutritionists, radiologists, and surgeons at times, all collaborating across disciplines to achieve optimal patient results. Besides, the overall clinical management of this chronic liver disease by involved specialists, the prescribed medications should be vetted through a board-certified pharmacist who can collaborate on agent selection, verify dosing and oversee the medication regimen for drug interactions. Nursing staff who have a good understanding of this disease should administer medications, monitor treatment effectiveness, and alert the clinicians to any possible adverse effects from treatment. Such an integrated approach to the care of these patients can help to achieve the best possible outcomes.
Media
References
Athanazio RA, Silva Filho LVRF, Vergara AA, Ribeiro AF, Riedi CA, Procianoy EDFA, Adde FV, Reis FJC, Ribeiro JD, Torres LA, Fuccio MB, Epifanio M, Firmida MC, Damaceno N, Ludwig-Neto N, Maróstica PJC, Rached SZ, Melo SFO, Grupo de Trabalho das Diretrizes Brasileiras de Diagnóstico e Tratamento da Fibrose Cística.. Brazilian guidelines for the diagnosis and treatment of cystic fibrosis. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2017 May-Jun:43(3):219-245. doi: 10.1590/S1806-37562017000000065. Epub [PubMed PMID: 28746534]
Boëlle PY, Debray D, Guillot L, Clement A, Corvol H, French CF Modifier Gene Study Investigators. Cystic Fibrosis Liver Disease: Outcomes and Risk Factors in a Large Cohort of French Patients. Hepatology (Baltimore, Md.). 2019 Apr:69(4):1648-1656. doi: 10.1002/hep.30148. Epub 2018 Dec 28 [PubMed PMID: 30058245]
O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet (London, England). 2009 May 30:373(9678):1891-904. doi: 10.1016/S0140-6736(09)60327-5. Epub 2009 May 4 [PubMed PMID: 19403164]
Level 3 (low-level) evidenceParanjape SM,Mogayzel PJ Jr, Cystic fibrosis in the era of precision medicine. Paediatric respiratory reviews. 2018 Jan; [PubMed PMID: 28372929]
Keogh RH, Szczesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: A longitudinal study using UK patient registry data. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2018 Mar:17(2):218-227. doi: 10.1016/j.jcf.2017.11.019. Epub 2018 Jan 6 [PubMed PMID: 29311001]
Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2011 Jun:10 Suppl 2():S29-36. doi: 10.1016/S1569-1993(11)60006-4. Epub [PubMed PMID: 21658639]
Zsembery A, Jessner W, Sitter G, Spirlí C, Strazzabosco M, Graf J. Correction of CFTR malfunction and stimulation of Ca-activated Cl channels restore HCO3- secretion in cystic fibrosis bile ductular cells. Hepatology (Baltimore, Md.). 2002 Jan:35(1):95-104 [PubMed PMID: 11786964]
Fiorotto R,Strazzabosco M, Cystic Fibrosis-Related Liver Diseases: New Paradigm for Treatment Based on Pathophysiology. Clinical liver disease. 2016 Nov; [PubMed PMID: 31041076]
Colombo C, Battezzati PM, Crosignani A, Morabito A, Costantini D, Padoan R, Giunta A. Liver disease in cystic fibrosis: A prospective study on incidence, risk factors, and outcome. Hepatology (Baltimore, Md.). 2002 Dec:36(6):1374-82 [PubMed PMID: 12447862]
Narkewicz MR. Markers of cystic fibrosis-associated liver disease. Journal of pediatric gastroenterology and nutrition. 2001 Apr:32(4):421-2 [PubMed PMID: 11396806]
Hamosh A, FitzSimmons SC, Macek M Jr, Knowles MR, Rosenstein BJ, Cutting GR. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. The Journal of pediatrics. 1998 Feb:132(2):255-9 [PubMed PMID: 9506637]
Level 2 (mid-level) evidenceDebray D,Lykavieris P,Gauthier F,Dousset B,Sardet A,Munck A,Laselve H,Bernard O, Outcome of cystic fibrosis-associated liver cirrhosis: management of portal hypertension. Journal of hepatology. 1999 Jul; [PubMed PMID: 10424286]
Fiorotto R, Strazzabosco M. Pathophysiology of Cystic Fibrosis Liver Disease: A Channelopathy Leading to Alterations in Innate Immunity and in Microbiota. Cellular and molecular gastroenterology and hepatology. 2019:8(2):197-207. doi: 10.1016/j.jcmgh.2019.04.013. Epub 2019 May 7 [PubMed PMID: 31075352]
Witters P, Libbrecht L, Roskams T, De Boeck K, Dupont L, Proesmans M, Vermeulen F, Maleux G, Monbaliu D, Pirenne J, Cassiman D. Liver disease in cystic fibrosis presents as non-cirrhotic portal hypertension. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2017 Sep:16(5):e11-e13. doi: 10.1016/j.jcf.2017.03.006. Epub 2017 Mar 25 [PubMed PMID: 28347603]
Lewindon PJ, Pereira TN, Hoskins AC, Bridle KR, Williamson RM, Shepherd RW, Ramm GA. The role of hepatic stellate cells and transforming growth factor-beta(1) in cystic fibrosis liver disease. The American journal of pathology. 2002 May:160(5):1705-15 [PubMed PMID: 12000722]
Mueller-Abt PR,Frawley KJ,Greer RM,Lewindon PJ, Comparison of ultrasound and biopsy findings in children with cystic fibrosis related liver disease. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2008 May; [PubMed PMID: 17904429]
Level 2 (mid-level) evidenceKobelska-Dubiel N, Klincewicz B, Cichy W. Liver disease in cystic fibrosis. Przeglad gastroenterologiczny. 2014:9(3):136-41. doi: 10.5114/pg.2014.43574. Epub 2014 Jun 26 [PubMed PMID: 25097709]
Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ, American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology (Baltimore, Md.). 2010 Feb:51(2):660-78. doi: 10.1002/hep.23294. Epub [PubMed PMID: 20101749]
Diwakar V, Pearson L, Beath S. Liver disease in children with cystic fibrosis. Paediatric respiratory reviews. 2001 Dec:2(4):340-9 [PubMed PMID: 12052306]
Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterology & hepatology. 2012 Sep:8(9):605-7 [PubMed PMID: 23483859]
Lenaerts C, Lapierre C, Patriquin H, Bureau N, Lepage G, Harel F, Marcotte J, Roy CC. Surveillance for cystic fibrosis-associated hepatobiliary disease: early ultrasound changes and predisposing factors. The Journal of pediatrics. 2003 Sep:143(3):343-50 [PubMed PMID: 14517517]
Level 2 (mid-level) evidenceWitters P, De Boeck K, Dupont L, Proesmans M, Vermeulen F, Servaes R, Verslype C, Laleman W, Nevens F, Hoffman I, Cassiman D. Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2009 Dec:8(6):392-9. doi: 10.1016/j.jcf.2009.08.001. Epub 2009 Sep 4 [PubMed PMID: 19733131]
Level 1 (high-level) evidenceSadler MD,Crotty P,Fatovich L,Wilson S,Rabin HR,Myers RP, Noninvasive methods, including transient elastography, for the detection of liver disease in adults with cystic fibrosis. Canadian journal of gastroenterology [PubMed PMID: 25855877]
Calvopina DA, Noble C, Weis A, Hartel GF, Ramm LE, Balouch F, Fernandez-Rojo MA, Coleman MA, Lewindon PJ, Ramm GA. Supersonic shear-wave elastography and APRI for the detection and staging of liver disease in pediatric cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2020 May:19(3):449-454. doi: 10.1016/j.jcf.2019.06.017. Epub 2019 Jul 11 [PubMed PMID: 31303380]
Lewindon PJ, Shepherd RW, Walsh MJ, Greer RM, Williamson R, Pereira TN, Frawley K, Bell SC, Smith JL, Ramm GA. Importance of hepatic fibrosis in cystic fibrosis and the predictive value of liver biopsy. Hepatology (Baltimore, Md.). 2011 Jan:53(1):193-201. doi: 10.1002/hep.24014. Epub 2010 Nov 17 [PubMed PMID: 21254170]
Koh C, Sakiani S, Surana P, Zhao X, Eccleston J, Kleiner DE, Herion D, Liang TJ, Hoofnagle JH, Chernick M, Heller T. Adult-onset cystic fibrosis liver disease: Diagnosis and characterization of an underappreciated entity. Hepatology (Baltimore, Md.). 2017 Aug:66(2):591-601. doi: 10.1002/hep.29217. Epub 2017 Jun 26 [PubMed PMID: 28422310]
Palaniappan SK,Than NN,Thein AW,Moe S,van Mourik I, Interventions for preventing and managing advanced liver disease in cystic fibrosis. The Cochrane database of systematic reviews. 2017 Aug 29; [PubMed PMID: 28850173]
Level 1 (high-level) evidenceFlass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2013 Mar:12(2):116-24. doi: 10.1016/j.jcf.2012.11.010. Epub 2012 Dec 20 [PubMed PMID: 23266093]
Funakoshi N, Duny Y, Valats JC, Ségalas-Largey F, Flori N, Bismuth M, Daurès JP, Blanc P. Meta-analysis: beta-blockers versus banding ligation for primary prophylaxis of esophageal variceal bleeding. Annals of hepatology. 2012 May-Jun:11(3):369-83 [PubMed PMID: 22481457]
Level 1 (high-level) evidenceColombo C,Crosignani A,Assaisso M,Battezzati PM,Podda M,Giunta A,Zimmer-Nechemias L,Setchell KD, Ursodeoxycholic acid therapy in cystic fibrosis-associated liver disease: a dose-response study. Hepatology (Baltimore, Md.). 1992 Oct; [PubMed PMID: 1398498]
Level 1 (high-level) evidenceLamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. Journal of hepatology. 2004 Dec:41(6):920-5 [PubMed PMID: 15582124]
Level 2 (mid-level) evidenceStaufer K, Halilbasic E, Trauner M, Kazemi-Shirazi L. Cystic fibrosis related liver disease--another black box in hepatology. International journal of molecular sciences. 2014 Aug 4:15(8):13529-49. doi: 10.3390/ijms150813529. Epub 2014 Aug 4 [PubMed PMID: 25093717]
Barry PJ, Plant BJ, Nair A, Bicknell S, Simmonds NJ, Bell NJ, Shafi NT, Daniels T, Shelmerdine S, Felton I, Gunaratnam C, Jones AM, Horsley AR. Effects of ivacaftor in patients with cystic fibrosis who carry the G551D mutation and have severe lung disease. Chest. 2014 Jul:146(1):152-158. doi: 10.1378/chest.13-2397. Epub [PubMed PMID: 24522694]
Level 2 (mid-level) evidenceWark PAB,Cookson K,Thiruchelvam T,Brannan J,Dorahy DJ, Lumacaftor/ Ivacaftor improves exercise tolerance in patients with Cystic Fibrosis and severe airflow obstruction. BMC pulmonary medicine. 2019 Jun 17; [PubMed PMID: 31208380]
Freeman AJ, Sellers ZM, Mazariegos G, Kelly A, Saiman L, Mallory G, Ling SC, Narkewicz MR, Leung DH. A Multidisciplinary Approach to Pretransplant and Posttransplant Management of Cystic Fibrosis-Associated Liver Disease. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2019 Apr:25(4):640-657. doi: 10.1002/lt.25421. Epub 2019 Mar 20 [PubMed PMID: 30697907]
Colombo C, Costantini D, Rocchi A, Romano G, Rossi G, Bianchi ML, Bertoli S, Battezzati A. Effects of liver transplantation on the nutritional status of patients with cystic fibrosis. Transplant international : official journal of the European Society for Organ Transplantation. 2005 Feb:18(2):246-55 [PubMed PMID: 15691279]
Level 2 (mid-level) evidenceLu BR, Esquivel CO. A review of abdominal organ transplantation in cystic fibrosis. Pediatric transplantation. 2010 Dec:14(8):954-60. doi: 10.1111/j.1399-3046.2010.01412.x. Epub 2010 Oct 14 [PubMed PMID: 20946451]
Gridelli B. Liver: Benefit of liver transplantation in patients with cystic fibrosis. Nature reviews. Gastroenterology & hepatology. 2011 Apr:8(4):187-8. doi: 10.1038/nrgastro.2011.39. Epub 2011 Mar 8 [PubMed PMID: 21386811]
Miller MR, Sokol RJ, Narkewicz MR, Sontag MK. Pulmonary function in individuals who underwent liver transplantation: from the US cystic fibrosis foundation registry. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012 May:18(5):585-93. doi: 10.1002/lt.23389. Epub [PubMed PMID: 22271602]
Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology (Baltimore, Md.). 2006 Nov:44(5):1063-74 [PubMed PMID: 17058222]
Jong T,Geake J,Yerkovich S,Bell SC, Idiosyncratic reactions are the most common cause of abnormal liver function tests in patients with cystic fibrosis. Internal medicine journal. 2015 Apr; [PubMed PMID: 25644776]
Level 2 (mid-level) evidenceTemple JL, Cordero P, Li J, Nguyen V, Oben JA. A Guide to Non-Alcoholic Fatty Liver Disease in Childhood and Adolescence. International journal of molecular sciences. 2016 Jun 15:17(6):. doi: 10.3390/ijms17060947. Epub 2016 Jun 15 [PubMed PMID: 27314342]
Feranchak AP,Sokol RJ, Cholangiocyte biology and cystic fibrosis liver disease. Seminars in liver disease. 2001 Nov; [PubMed PMID: 11745036]
Ayoub F, Trillo-Alvarez C, Morelli G, Lascano J. Risk factors for hepatic steatosis in adults with cystic fibrosis: Similarities to non-alcoholic fatty liver disease. World journal of hepatology. 2018 Jan 27:10(1):34-40. doi: 10.4254/wjh.v10.i1.34. Epub [PubMed PMID: 29399276]
FitzSimmons SC. The changing epidemiology of cystic fibrosis. Current problems in pediatrics. 1994 May-Jun:24(5):171-9 [PubMed PMID: 8070278]
Colombo C, Apostolo MG, Ferrari M, Seia M, Genoni S, Giunta A, Sereni LP. Analysis of risk factors for the development of liver disease associated with cystic fibrosis. The Journal of pediatrics. 1994 Mar:124(3):393-9 [PubMed PMID: 8120708]
Lindblad A,Glaumann H,Strandvik B, Natural history of liver disease in cystic fibrosis. Hepatology (Baltimore, Md.). 1999 Nov; [PubMed PMID: 10534335]
Gooding I, Dondos V, Gyi KM, Hodson M, Westaby D. Variceal hemorrhage and cystic fibrosis: outcomes and implications for liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005 Dec:11(12):1522-6 [PubMed PMID: 16258952]
Level 2 (mid-level) evidence