Introduction
The heart is the center of the body's circulation, the engine that drives oxygen received in the lungs to the tissues and organs as well as to exchange carbon dioxide. The oxygenated blood travels in the organism to organs and tissues through the aorta. This large artery brings nourishment to the whole body by dividing into various branches and capillaries, each smaller and smaller. Blood with carbon dioxide waste returns to the heart through the venous system.
The aortic valve is one of four heart valves and is the final one encountered by oxygenated blood as it leaves the heart. It is also called aortic semilunar due to its semilunar shape. It is between the left ventricle and the aorta to ensure that oxygen-rich blood does not flow back into the left ventricle. The aortic valve is typically made up of three membranes (cusps) made up mainly of collagen; the valve is placed on a muscle ring and connected to the heart wall.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
The aortic valve is a semilunar valve (diameter of about 20 mm) that generally has three leaflets. Common congenital differences include a bicuspid valve. More rarely, unicuspid valves can be present. The valve can be visualized via ultrasound. The valve divides one of the highest pressure differentials of the cardiopulmonary system and, as such, is subject to wear and tear injury. The leaflets of the aortic valve connect to the aortic root via the aortic annulus. They are also suspended by fibrous structures called commissures. The leaflets are also referred to as cusps. The leaflets and their corresponding sinuses traditionally derive their names from the coronary arteries that arise from the sinuses (left coronary, right coronary, and non-coronary). However, normal anatomical variations can obfuscate this naming convention. Anatomic descriptions usually name the leaflets/cusps/sinuses as the left posterior instead of the left coronary, anterior instead of right coronary, and right posterior instead of non-coronary.[1][2][3][4]
The aortic valve is often described relative to the aortic root. The aortic root consists of the left ventricular outflow tract until it meets with the ascending aorta. The following descriptions detail the anatomy of the aortic root as they relate to the aortic valve.[2][4]
Annulus
The aortic annulus serves as an anchor point for the valve.[2][3][1][5]
Interleaflet Fibrous Triangles
The interleaflet fibrous triangles (also known as just interleaflet triangles, intervalvular trigone, fibrous trigones, inter-annular trigones, or Aranzio's nodules) are attached to the left ventricular wall between the bases of the sinuses.[1] These triangles form a boundary between the extracardiac space and the left ventricular cavity as they extend to the level of the sinotubular junction.[1] The interleaflet fibrous triangle located between the right and left coronary sinuses is attached to the septal part of the right ventricular outflow tract and faces the pulmonary valve. The interleaflet fibrous triangle lies between the non-coronary sinus and the right coronary sinus. It faces the right atrium and is continuous with the septum, which is significant because the bundle of His comes in through the ventricular septum under this triangle. The last triangle is between the left and the non-coronary sinus. It continues directly with the aortic leaflet of the mitral valve. These three triangles separate the three sinuses.[4]
Sinus of Valsalva
The aortic sinuses are also called the sinus of Valsalva, named after Italian anatomist Antonio Valsalva. They ensure that the aortic leaflets do not occlude the ostia of the coronary arteries during systole.[5]
These sinuses are mostly made up of the wall of the aorta. At the base of the two coronary sinuses is ventricular musculature. In the non-coronary sinus, the base consists of a fibrous piece that continues from the aortic and mitral valves.[6]
Sinotubular Junction
The sinotubular junction lies at the top of the sinuses. This point marks the transition from the aortic root to the ascending aorta. The sinotubular junction also marks the top of the attachments of the aortic leaflets.[4]
Function
When the pressure in the ventricle is greater than that of the aorta, the aortic valve leaflets open, allowing blood to exit the ventricle and enter the ascending aorta. In a healthy heart, this happens during systole. Conversely, the aortic valve leaflets close when the left ventricle pressure decreases to less than the ascending aorta; this typically occurs during diastole. The sinuses ensure that the ostia (openings) of the coronary arteries are not occluded when the aortic valve leaflets are open.[2]
Cusps
The cusps are made up of three layers:
- Ventricularis
- Spongiosa
- Fibrosa
Embryology
The semilunar valves develop from the endocardial cushions around the 5th week of embryonic development. The aortic valve begins as a swelling of the endocardial cushions. The valve then undergoes additional remodeling in the presence of ventricular ejection streams.[7][8]
Endothelial cells and vascular smooth muscle cells will form the aortic root and aortic valve; this results from the embryological collaboration between the splanchnic mesoderm and the neural crest, deriving from the second heart field.
Blood Supply and Lymphatics
Heart valves are metabolically active, as is the aortic valve; the cusps contain blood vessels for oxygen supply and removal of metabolic waste. The vessels exist in a higher percentage in the basal third of the cusps; these vessels continue from the commissures to the portion of the free edge. This microcirculation of the valve is essential for any intrinsic repairs of the cusps tissue. The distribution of oxygen in the cusps depends on the relationship between the thickness of the valves and the tension of the ventricle/aorta.[9] Within the tissues of the cusps, one can find, in a lesser percentage, the presence of lymphatic vessels.
Nerves
Currently, there is no shared agreement on cardiac plexus formation. Sympathetic fibers derive from C8-T5; vagal fibers involve, in particular, the ventricles and, with less extension, the atria. Some studies show that some phrenic nerve branches may be involved in the cardiac plexus. In the animal model, it is clear that the cusps receive innervation by the autonomic system, where sympathetic stimulation increases the tone of the cusps. The nerve fibers that go to the aortic valve derive from the ventricular endocardial plexuses and others, to a lesser extent, from the aortic adventitial wall. The most innervated layer of the cusps is the ventricularis.[10][11][12]
Muscles
Unlike the mitral and tricuspid valves, the aortic valve has no associated papillary muscle. The valve is attached to the heart muscle via the annulus.[6][13]
Physiologic Variants
One percent of the population has an aortic valve with only two leaflets. This condition is referred to as a bicuspid aortic valve. The bicuspid valve cusps are not symmetrical the majority of the time. Possible complications of a bicuspid aortic valve include aortic stenosis or aortic regurgitation, usually due to early calcification. This abnormality is also associated with an increased risk of infection endocarditis. While senile calcification occurs late in life, early calcification due to a bicuspid valve usually presents by age 60 and can present much earlier. Typical treatment begins once the condition is symptomatic and usually involves valve replacement.[14][15]
The presence of a quadricuspid aortic valve has a very rare percentage of findings, but it can be found during an ultrasound or a more invasive examination such as a transesophageal examination. Generally, this type of cusps leads to valve failure.
Surgical Considerations
Balloon valvuloplasty or aortic valve replacement are indicated procedures for symptomatic aortic stenosis or aortic regurgitation. A bioprosthetic valve (i.e., cadaveric, xenograft, or Ross procedure) or mechanical valve represent options. Following the placement of a mechanical valve, lifelong anticoagulation therapy is necessary. An additional valvular option is in development called Tissue-engineered heart valves.[15]
Cardiac surgery on the aortic valve falls into two basic groups: those of "plastic" or "repair," which maintains the native valve, and "replacement" interventions, in which the valve is replaced with a prosthesis (biological or mechanical). Aortic plastic interventions consist of repairing the valve to correct its pathology (insufficiency or stenosis) without replacing it. Whenever possible, it is preferable to repair a valve rather than replace it because proper valve function is associated with better maintenance of cardiac function, better survival, and less risk of endocarditis; moreover, there is often no need for anticoagulant treatment.
Clinical Significance
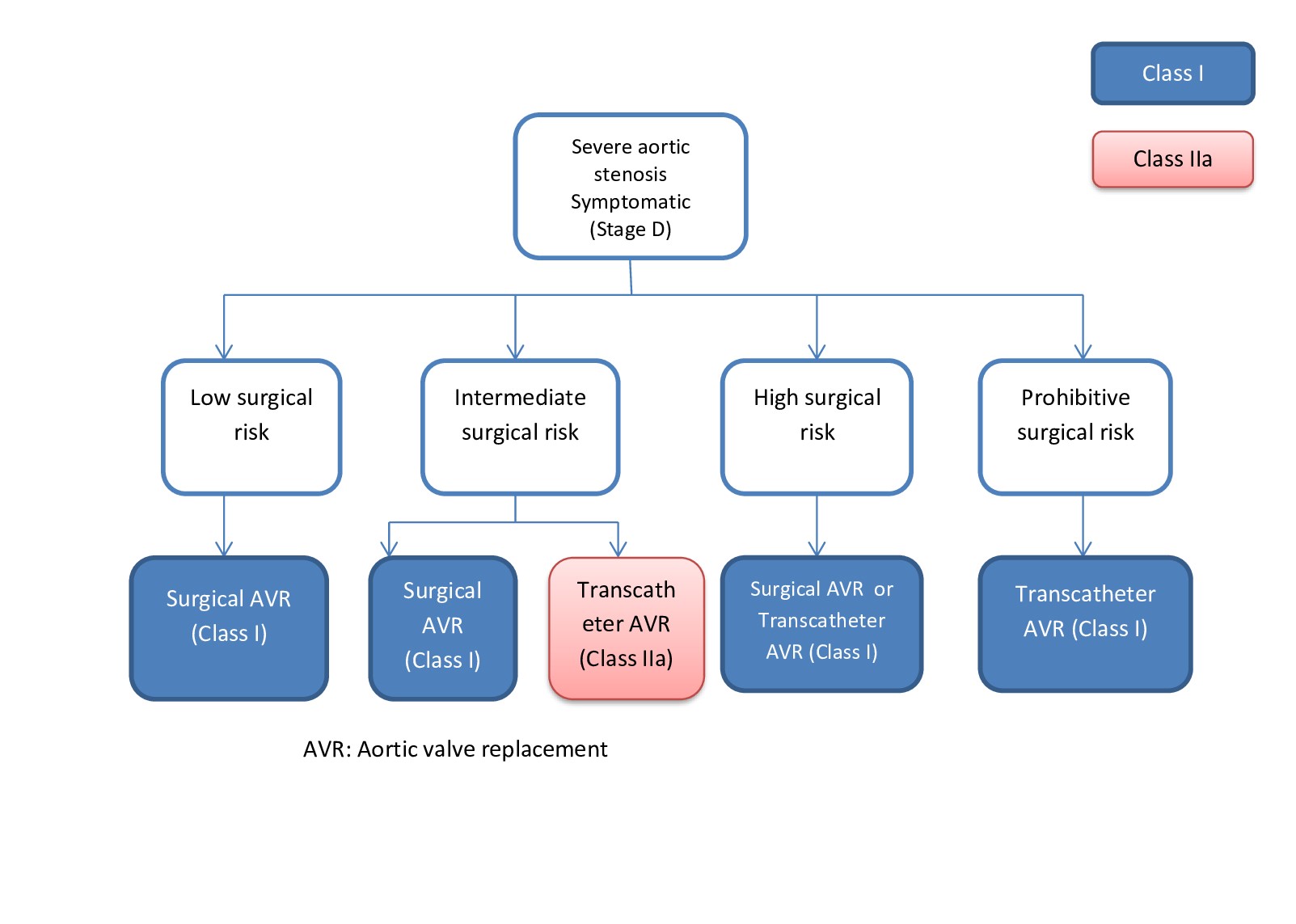
Aortic stenosis is defined as the narrowing of the aortic valve to impair blood flow out of the left ventricle and can be confirmed by an echocardiogram. This condition can occur in up to 10% of the 80+ population. Clinical exam findings typically include a crescendo decrescendo murmur that peaks during systole. This murmur is also known as a systolic ejection murmur and can be auscultated in the right sternal border at the second intercostal space. Typically, the murmur will peak later during systole as the stenosis progresses. Additional clinical findings can include symptoms of heart failure, angina, and left ventricular concentric hypertrophy due to excessive ejection pressures.
The most common cause of aortic stenosis is due to age-related reasons such as a calcified aortic valve. A bicuspid valve can present with stenosis secondary to calcification much earlier.
A history of rheumatic fever can elevate a patient's lifetime risk of stenosis. The aortic stenosis, if left untreated, can lead to left ventricular hypertrophy. Severe aortic stenosis is a serious medical condition, and valve replacement is usually indicated in eligible patients.
Aortic regurgitation is the retrograde blood flow from the aorta into the left ventricle. The net effect is left ventricular volume overload and subsequent chamber dilation. Because of the heart's ability to compensate, it can maintain this hypertrophy for a prolonged period. The prevalence of aortic regurgitation is estimated at 4.9% and increases with age until the sixth decade. Aortic regurgitation is a common finding in patients with aortic stenosis. On physical exam, aortic regurgitation can be suspected due to wide pulse pressure and an early diastolic murmur (over the right sternal border at the second intercostal space.)
The murmur associated with aortic regurgitation is usually referred to as a diastolic decrescendo murmur. The duration of the murmur positively correlates with the severity of the disease. Other physical exam findings and complaints can be a bounding pulse, headbobbing (Corrigan pulse), pulsing fingernails (Quincke's sign) as well as uvular pulsing (Muller's sign). Aortic regurgitation can be auscultated as an early diastolic murmur. An Austin Flint murmur may be auscultated in some cases of aortic regurgitation. Like aortic stenosis, aortic regurgitation can present with symptoms of heart failure as well.
Similar to aortic stenosis, the etiology of aortic regurgitation is most impacted by age and congenital abnormalities, such as a bicuspid valve. Rheumatic fever is a common risk factor for aortic regurgitation.
The acuity and severity of the regurgitation are the primary drivers for management. Management includes medical therapy or valve replacement.[16][17][18]
Other Issues
The aortic valve signifies the point between the left ventricle and the aorta. Aortic stenosis and regurgitation are two valve dysfunctions that can eventually lead to heart failure and are among the primary indications for heart valve replacement.[12]
Media
(Click Image to Enlarge)
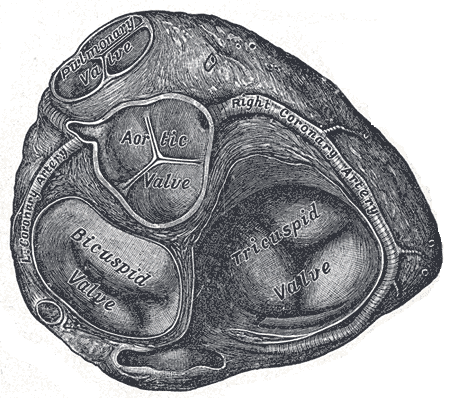
Valves of the Heart. The bicuspid valve, aortic valve, pulmonary valve, tricuspid valve, and right coronary artery are illustrated.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
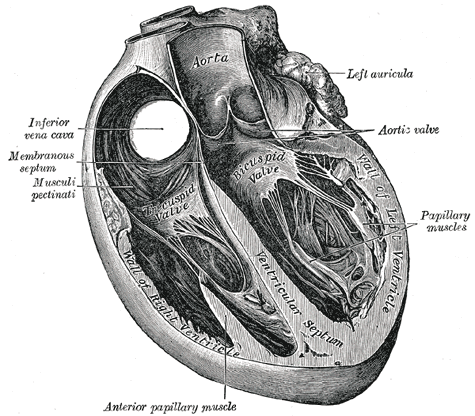
Trans Sagittal Cross section of the Heart, Aorta, Left Auricula, Aortic Valve, Papillary muscles, Left Ventricle, Bicuspid Valve, Ventricular Septum, Inferior Vena Cava, Membranous septum, Musculi pectinati, Anterior Papillary Muscles, Tricuspid Valve
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
<p>Valveguru, <a href="https://creativecommons.org/licenses/by-sa/3.0">Public Domain</a>, via Wikimedia Commons</p>
(Click Image to Enlarge)
References
Loukas M, Bilinsky E, Bilinsky S, Blaak C, Tubbs RS, Anderson RH. The anatomy of the aortic root. Clinical anatomy (New York, N.Y.). 2014 Jul:27(5):748-56. doi: 10.1002/ca.22295. Epub 2013 Sep 2 [PubMed PMID: 24000000]
Ho SY. Structure and anatomy of the aortic root. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009 Jan:10(1):i3-10. doi: 10.1093/ejechocard/jen243. Epub [PubMed PMID: 19131496]
Underwood MJ, El Khoury G, Deronck D, Glineur D, Dion R. The aortic root: structure, function, and surgical reconstruction. Heart (British Cardiac Society). 2000 Apr:83(4):376-80 [PubMed PMID: 10722531]
Misfeld M, Sievers HH. Heart valve macro- and microstructure. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2007 Aug 29:362(1484):1421-36 [PubMed PMID: 17581807]
Bass D, Tivakaran VS. Sinus of Valsalva Aneurysm. StatPearls. 2023 Jan:(): [PubMed PMID: 28846266]
Anderson RH. Clinical anatomy of the aortic root. Heart (British Cardiac Society). 2000 Dec:84(6):670-3 [PubMed PMID: 11083753]
Mathew P, Bordoni B. Embryology, Heart. StatPearls. 2023 Jan:(): [PubMed PMID: 30725998]
Maron BJ, Hutchins GM. The development of the semilunar valves in the human heart. The American journal of pathology. 1974 Feb:74(2):331-44 [PubMed PMID: 4811758]
Weind KL, Boughner DR, Rigutto L, Ellis CG. Oxygen diffusion and consumption of aortic valve cusps. American journal of physiology. Heart and circulatory physiology. 2001 Dec:281(6):H2604-11 [PubMed PMID: 11709429]
Level 3 (low-level) evidenceRehman I, Rehman A. Anatomy, Thorax, Heart. StatPearls. 2023 Jan:(): [PubMed PMID: 29262022]
El-Hamamsy I, Yacoub MH, Chester AH. Neuronal regulation of aortic valve cusps. Current vascular pharmacology. 2009 Jan:7(1):40-6 [PubMed PMID: 19149639]
Level 3 (low-level) evidenceChester AH, Kershaw JDB, Sarathchandra P, Yacoub MH. Localisation and function of nerves in the aortic root. Journal of molecular and cellular cardiology. 2008 Jun:44(6):1045-1052. doi: 10.1016/j.yjmcc.2008.03.014. Epub 2008 Mar 29 [PubMed PMID: 18485360]
Level 3 (low-level) evidenceSanchez Vaca F, Bordoni B. Anatomy, Thorax, Mitral Valve. StatPearls. 2023 Jan:(): [PubMed PMID: 31751074]
Mubarik A, Sharma S, Law MA. Bicuspid Aortic Valve. StatPearls. 2024 Jan:(): [PubMed PMID: 30480953]
Rajput FA, Zeltser R. Aortic Valve Replacement. StatPearls. 2023 Jan:(): [PubMed PMID: 30725821]
Wenn P, Zeltser R. Aortic Valve Disease. StatPearls. 2023 Jan:(): [PubMed PMID: 31194362]
Joseph J, Naqvi SY, Giri J, Goldberg S. Aortic Stenosis: Pathophysiology, Diagnosis, and Therapy. The American journal of medicine. 2017 Mar:130(3):253-263. doi: 10.1016/j.amjmed.2016.10.005. Epub 2016 Nov 1 [PubMed PMID: 27810479]
Akinseye OA, Pathak A, Ibebuogu UN. Aortic Valve Regurgitation: A Comprehensive Review. Current problems in cardiology. 2018 Aug:43(8):315-334. doi: 10.1016/j.cpcardiol.2017.10.004. Epub 2017 Nov 2 [PubMed PMID: 29174586]