Introduction
Maintaining blood in a liquid state is critical for homeostasis. It allows blood to supply adequate oxygen and nutrients to tissues while also eliminating carbon dioxide and other waste products. On the other hand, the ability of blood to convert from a liquid to a solid state, in other words, to coagulate, underlies the mechanism that protects the body from life-threatening exsanguination. This process of thrombosis is normally a localized event at the site of vascular injury while the rest of the circulating blood remains in a liquid state. Thrombosis is a dynamic process that includes associated thrombolysis to maintain or restore blood flow through vessels once an injury has been sealed. These unique properties of blood are largely determined by a complex and active balance between pro-coagulation factors, anticoagulants, and fibrinolysis. Two major pathologic conditions are commonly associated with disequilibrium of this intricate system: bleeding and vessel thrombosis.
Major bleeding is a serious medical complication that may be caused by external trauma, surgery, invasive procedures, or an underlying medical condition such as aneurysm rupture or peptic ulcer disease. According to the World Health Organization (WHO), injuries are responsible for 5.8 million deaths per year worldwide, with the associated bleeding responsible for about 30% to 40% of these deaths.[1] Several congenital disorders associated with a coagulation factor deficiency, such as Von Willebrand disease, hemophilia A or B, may cause significant bleeding even with minor injuries. Also, prescribed anticoagulants and antiplatelet agents may create a coagulopathic state that may lead to excessive bleeding either associated with trauma or medical procedures. Finally, major acute blood loss can lead to coagulopathy due to a loss of coagulation factors. Predictably, trauma-related coagulopathy has been associated with significantly higher mortality.[2] Patients with ongoing or expected major bleeding would benefit from an accurate assessment of the functional state of the hemostatic system to provide optimal care, providing cost-effective replacement of only the needed blood components.
Venous thromboembolism (VTE) is another common and serious condition that is associated with abnormal blood coagulation. In these cases, systemic hypercoagulability shifts the body’s homeostatic mechanisms toward a pro-thrombotic state. In particular patients, however, a definitive cause for the VTE may be unclear. Routine coagulation testing has not been shown to predict such events, and in many cases, even a detailed hypercoagulability investigation fails to identify an underlying disorder. Many people take anticoagulants and antiplatelet agents regularly, which impacts the accuracy of the results of many laboratory coagulation studies.[3][4] An accurate and cost-efficient method of monitoring antithrombotic activity would be helpful to maintain an acceptable risk/benefit ratio in such patients. Inadequate anticoagulation or antiplatelet therapy can lead to devastating thromboembolic conditions.
Several commonly used blood tests assess blood coagulation. These tests include prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), platelet count, fibrinogen concentration, D-dimer level, activated clotting time, and whole blood bleeding time (BT). These tests are usually used for the clinical diagnosis of coagulopathy and a possible prothrombotic state, to monitor anticoagulation therapy, and to assist in treating bleeding episodes. More specific factor analyses, such as Factor V, proteins C and S, anti-thrombin III, anticardiolipin antibodies, and prothrombin gene mutation, are useful but not as readily available in emergency clinical situations. Despite being very effective for specific clinical needs, such as anticoagulation monitoring, the first group of usual diagnostic tests has limitations. Their main disadvantage in circumstances of acute major bleeding is the long turnaround time. Furthermore, they do not provide a complete picture of hemostasis due to their inability to assess some coagulation factors (such as Factor XIII), platelet function, and the activity of the fibrinolytic system. Platelet concentration, easily measured as part of a complete blood count, does not necessarily reflect their function, especially in the presence of elements known to affect platelet reactivity, such as non-steroidal anti-inflammatory drugs, antiplatelet agents, uremia, malignancy, or alcohol intake. Bleeding time has a low sensitivity and high inconsistency in detecting platelet disorders.[5] Delayed or inadequate diagnosis of coagulopathy in a bleeding patient may lead to an excessive and improperly balanced transfusion of scarce blood components with increased morbidity, treatment costs, and mortality.[6]
Thromboelastography (TEG) is a promising diagnostic modality that offers several advantages compared to the other tests that have been mentioned above. TEG was developed and first described by Dr. Hellmut Hartert at the University of Heidelberg (Germany) in 1948.[7] The first reported clinical application of the test occurred during the Vietnam War in an attempt to guide transfusions of blood components in injured soldiers.[8] In the 1980s, TEG was found to be beneficial in liver transplant patients, and in the 1990s, it was demonstrated to be useful in cardiac surgery.[9][10] Since then, TEG has evolved into a more commonly used test as more evidence for its clinical efficacy has been attained. A brief search in PubMed using keywords “thromboelastography” and “thromboelastometry” results in about 6000 publications. This article will describe the general principles of TEG, methodology, normal values, along with the current evidence and clinical applications, as well as limitations and future research directions.
Pathophysiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Pathophysiology
TEG is a non-invasive test that quantitatively measures the ability of whole blood to form a clot. The principle of this in vitro test is to detect and quantify dynamic changes of the viscoelastic properties of a blood sample during clotting under low shear stress. The test is performed in a specially designed system called a thromboelastograph. The system consists of 2 chambers simultaneously examining a blood sample in duplicate to reduce the risk of sampling and measurement errors. Each chamber consists of a platform with a disposable cup where a blood sample is placed and a detection pin suspended in its center. The cup oscillates around the detection pin in a limited arc of plus or minus (+/-) 4 degrees 45' every 5 seconds. Induced pin movement is recorded, and changes are measured as a function of time. Initially, there is little movement of the pin since liquid blood possesses minimal viscosity, and the oscillations of the cup are not transmitted to the pin.
As the blood coagulates, it begins to adhere to both the cup and the pin, and movement of the cup induces motion on the pin. These gradually increasing viscoelastic mechanical properties of the blood reflect the developing 3-dimensional fibrin mesh and platelet components of the clot. The greater the viscoelasticity of the clot, the higher the amplitude of the pin motion. As fibrinolysis starts, the fibrin-platelet structure begins to dissolve gradually, and the clot loses its contact with the detection pin, which has less induced motion. The thromboelastogram (Figure 1) is a graphical image of the recorded amplitude of movement of the pin as a function of time. Analytical software measures and quantifies these changes. Therefore, TEG measures the functional ability of the blood to make a hemostatic plug. A newer version replaces the cup rotation method with a resonance technique wherein the blood sample is subjected to vibration, and the vertical movement of the blood meniscus is measured under LED illumination. The system uses pre-measured cartridges that do not require pipetting and allows the simultaneous performance of four blood tests.
Specimen Requirements and Procedure
The blood sample is collected via venipuncture in a plastic vial with 3.2% buffered sodium citrate with a citrate-to-blood ratio of 1:9. The vial is inverted several times to mix the blood and citrate. Maintaining this citrate-blood ratio is crucial for test accuracy. Citrate binds calcium, an important cofactor of coagulation, preventing the blood from clotting before the beginning of the test. A clotted specimen, reflecting a vial overfilled with blood, cannot be used. For TEG testing, the collected non-clotted samples are considered stable and usable for up to 2 hours at room temperature. Non-citrated whole blood (native blood TEG or NATEM) can also be tested, but it must be used immediately. The test and reagents used are at room temperature. A volume of 340 uL of citrated blood is pipetted to the study cup, recalcified by the addition of 20 uL of 0.2M calcium chloride, and then activated with a kaolin-cephalin reagent. Cephalins, or phosphatidylethanolamines, are a class of phospholipids commonly present in membranes of human cells. They are an important cofactor of the coagulation cascade, enabling the assembly of tenase and prothrombinase complexes on the surface of platelets that are critical for thrombin generation. Kaolin is a mineral primarily composed of hydrated aluminum silicate, a negatively charged molecule that can initiate the intrinsic coagulation pathway by activating Factor XII. Precise proportioning of the blood and kaolin-cephalin reagent is important for accurate and reproducible TEG results.[11] Non-activated TEG is also possible, but the lack of activators significantly prolongs clotting time and the testing process, which is not desirable in a clinical emergency.
Several modifications of the classic TEG assay have been developed to improve its diagnostic value. Rapid TEG (r-TEG) utilizes tissue factor instead of the kaolin-cephalin reagent to activate blood coagulation. Because tissue factor triggers the extrinsic coagulation pathway, which involves a smaller number of coagulation factors, the test can be performed faster than conventional TEG. Rapid TEG can be completed within 15 minutes and thus helps manage massive transfusions in trauma patients.[12][13] The TEG platelet mapping assay was developed to predict the inhibitory effect of antiplatelet agents such as aspirin and clopidogrel. This is accomplished by evaluating platelet aggregation in the presence of adenosine diphosphate or arachidonic acid. TEG with added heparinase (hTEG) measures the effect of heparin reversal on blood coagulation.
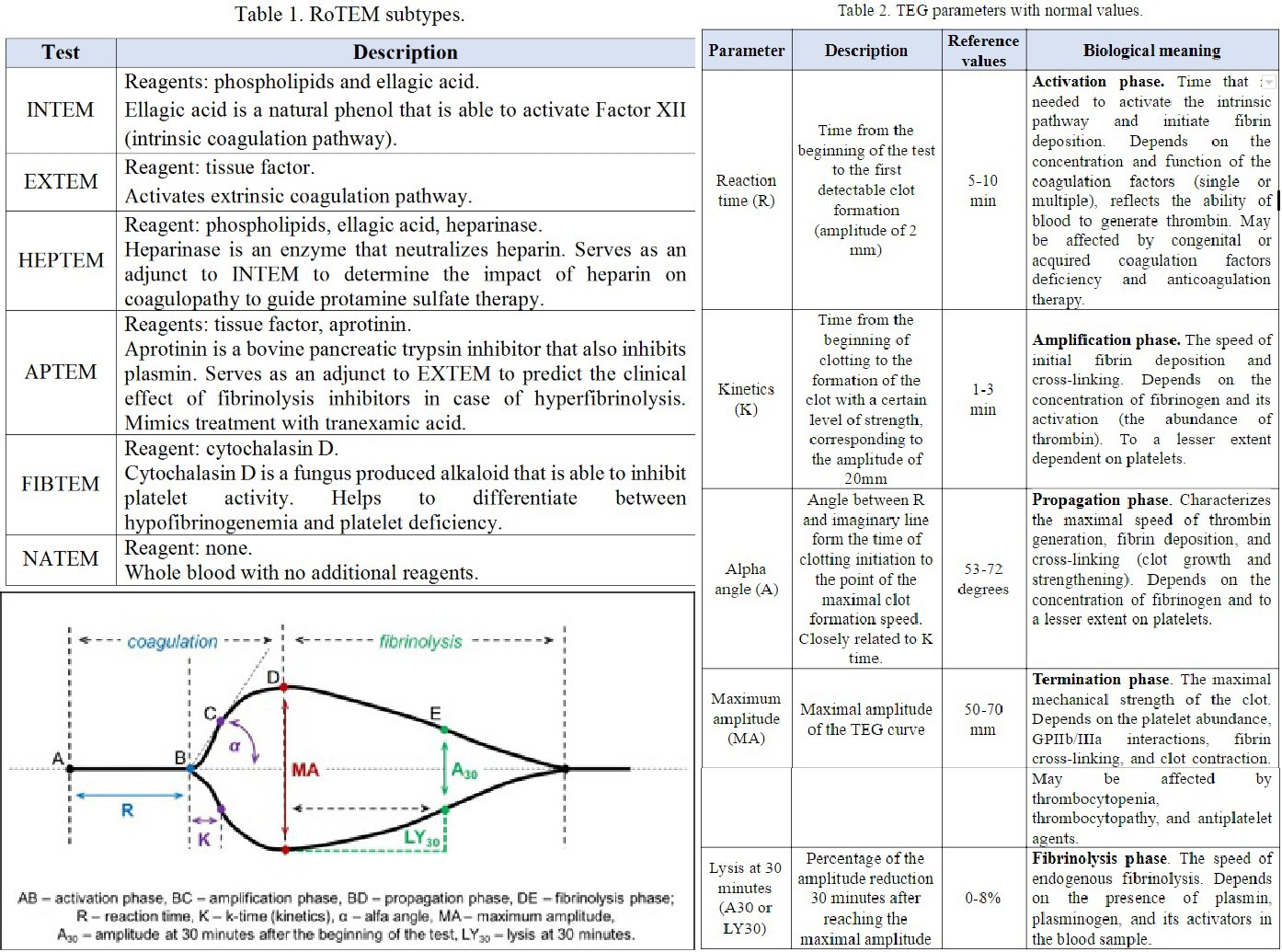
Rotational thromboelastography, also known as rotational thromboelastometry (RoTEM), utilizes an oscillating pin that rotates +/- 4 degrees 45' every 6 seconds while maintaining the cup in a stable position. In this assay, some different activator reagents are utilized to investigate specific components of the coagulation pathway (Table 1).
Results, Reporting, and Critical Findings
A normal thromboelastogram is schematically represented in Figure 1. Prompt qualitative analysis of the TEG tracing can be performed during the test. The quantitative analysis of TEG includes the measurement of the 5 parameters listed and described in Table 1. The manufacturer has also suggested a coagulation index to assess the overall coagulation status. The coagulation index (CI) for whole blood may be calculated as follows:
- CI = -0.2454R+ 0.0184K + 0.1655MA - 0.0241a - 5.0220
Normal values of the coagulation index lie within -3.0 and +3.0, which is three standard deviations from the mean of zero. A hypercoagulable state is defined as CI greater than +3.0 and coagulopathy as CI less than -3.0. Previous studies demonstrated a significantly elevated CI in the postoperative period after general surgery and in cancer patients, suggesting a prothrombotic state.[14][15] However, this index is not widely used, and its clinical usefulness is not yet validated. Several other parameters may be calculated based on the thromboelastogram, such as projected maximal amplitude, time to maximal amplitude, the G parameter (shear elastic modulus strength, or clot strength), and a thrombodynamic potential index. While providing interesting information, these variables are rarely used in clinical practice.
Normal TEG values are presented in Table 2, although some patient-related factors may affect these values. Elderly patients tend towards more pro-coagulable TEG results, suggesting a need to correct these reference values in the elderly.[16] In some circumstances, such as in patients undergoing cardiac surgery and liver transplantation, specific reference values are less important because the principal application of TEG is to compare the patient’s own baseline to changes during the intraoperative and postoperative periods. In other clinical scenarios, such as trauma or postoperative bleeding, reference values are important for interpreting the results as no baseline data is available. There is also some variability in testing results. A study on 118 healthy volunteers revealed at least one abnormal parameter in 19% of specimens, and a coagulopathy (defined as at least 2 abnormal parameters) in 9%, leading to a calculated specificity of 81%.[17] A larger prospective trial of a more diverse group of subjects would be helpful to establish analyzer-specific and reagent-specific reference values in selected subgroups of patients.
Deviation of each of these TEG parameters from the reference values suggests specific disturbances of hemostasis and coagulation. Prolongation of the R time reflects a quantitative or qualitative deficiency of coagulation factors that may be corrected by fresh frozen plasma (FFP) transfusion, prothrombin complex, or anticoagulant reversal. Prolongation of the K time, or a decrease of the alfa angle, suggests a deficiency of fibrinogen and may be corrected by cryoprecipitate or lyophilized fibrinogen concentrate. Low MA indicates a quantitative or functional deficiency of platelets and could be corrected by platelet concentrate transfusion or desmopressin. Finally, an increased LY value implies activated fibrinolysis that may be treated by fibrinolysis inhibitors (aminocaproic or tranexamic acid). The opposite changes in TEG parameters suggest a prothrombotic state. This interpretive approach represents a convenient but rather simplified view on disturbances of blood coagulation. It is important to remember that these TEG parameters are interrelated due to the complex nature of hemostasis.
A different nomenclature is used for RoTEM assays to define the same TEG parameters: clotting time (CT) instead of R, clot formation time (CFT) instead of K, maximum clot firmness (MCF) instead of MA, and CL instead of LY. Reference values for RoTEM have been established in a multicenter study on 500 healthy volunteers.[18] Depending on the specific TEG analyzer (TEG versus RoTEM) and reagents being used, differing results may be obtained from the same blood sample, potentially affecting clinical decision-making.[19],[20] Some of these differences are not well understood. For example, NATEM may be more sensitive to hyperfibrinolysis than INTEM and EXTEM.[21] A systematic review of four clinical trials comparing TEG and RoTEM found clinically significant differences between the 2 tests with a lack of comparability of the results.[22] A recent systematic review did not discover a sufficient number of well-designed studies to compare the results of TEG and RoTEM in healthy subjects.[23] Hence, the results of different modifications of TEG and RoTEM cannot be considered interchangeable until head-to-head prospective comparative studies are performed.
Clinical Significance
The main advantage of TEG testing is its potential to deliver immediate goal-oriented and individualized care to a bleeding patient:
- Global assessment of blood coagulability, including coagulation cascade, platelet function, and fibrinolysis
- Rapid real-time bedside test with a simple methodology (point-of-care testing)
- Diagnosis of coagulopathic bleeding
- Guide transfusion therapy and decrease the use of blood products
- Detect dynamic changes in blood coagulation during resuscitation
- Predict the clinical efficacy of therapeutic agents affecting blood coagulability
TEG has convincingly demonstrated its usefulness to help improve outcomes in cardiac surgery. A meta-analysis of 17 randomized controlled trials (RCTs) demonstrated that TEG decreases blood product transfusions and surgical re-exploration due to postoperative bleeding in cardiac surgery patients.[24] These effects were associated with a lower incidence of acute kidney injury and thromboembolic events. Another systematic review of 17 RCTs involving 1493 patients, mainly elective on-pump cardiac surgery, revealed that TEG/RoTEM decreases transfusion of blood components and reduces overall mortality.[25] The quality of the included studies, however, was considered to be low.[25] A recent RCT found that intraoperative correction of coagulopathy guided by EXTEM and FIBTEM can reduce postoperative bleeding, blood transfusions, and duration of critical care in pediatric cardiac surgery patients.[26] TEG is also a more cost-effective method compared to standard coagulation tests in the diagnosis of coagulopathy in cardiac surgery.[27]
There is conflicting evidence for TEG's usefulness in trauma patients. A recent Cochrane database systematic review found insufficient data to compare the accuracy of TEG and RoTEM versus PT/INR in the diagnosis of trauma-induced coagulopathy.[28] The review concluded that these tests are still in the phase of clinical research. However, it is questionable whether PT/INR can be considered a good reference standard to diagnose coagulopathy. In major trauma, r-TEG has been found to be better in predicting the need for transfusion of FFP, RBCs, and platelets compared to conventional coagulation tests of PT, aPTT, INR, platelet count, and fibrinogen.[29] Based on another large systematic review, although with evidence limited to cohort studies with a moderate to high risk of bias, TEG/RoTEM can diagnose coagulopathy and may predict blood components transfusion and mortality in trauma patients.[30] Another review of 13 cohort studies involving only RoTEM in 2835 adult trauma patients came to the same conclusions.[31] However, there was no improvement in patient morbidity or mortality.[30] Another Cochrane database systematic review of 9 RCTs with a total of 776 participants, mainly cardiac surgery patients, found a decreased amount of bleeding when TEG or RoTEM were utilized but also without a decrease in morbidity or mortality.[22] This inability of TEG/RoTEM testing to significantly reduce mortality may become a barrier to widespread clinical use. However, it is important to realize that overall mortality in hospitalized patients with bleeding is relatively low and thus would require large clinical trials to detect a statistically significant impact of TEG on mortality. Furthermore, these are complex patients, and the overall treatment strategy, rather than diagnostic testing, will have a greater role in affecting overall morbidity and mortality.
There is expanding evidence of using different TEG and RoTEM assays in other additional clinical scenarios. A small RCT has demonstrated the ability of TEG to better guide anticoagulation during ECMO compared to aPTT, reducing the dose of heparin.[32] TEG platelet mapping can detect platelet inhibition by clopidogrel and aspirin in surgical patients.[33] A novel TEG-based scoring system has been suggested to diagnose disseminated intravascular coagulation.[34] TEG may detect possible coagulopathy in patients with intracranial bleeding and hematoma enlargement.[35] TEG may also have an application in liver disease patients. It is known that conventional coagulation tests are commonly abnormal in liver disease. At the same time, TEG/RoTEM results are normal in many patients despite an abnormal INR or platelet count due to adjustments in the system of hemostasis or rebalanced hemostasis.[36] Thus, TEG/RoTEM may provide a better insight into the risk of bleeding in patients with liver disease than conventional coagulation tests.[37] EXTEM has been found helpful to detect intraoperative coagulopathy in liver transplant patients.[38] There are other clinical situations when TEG has demonstrated potential benefit, but listing all of them is beyond the scope of this review.
Clinical Guidelines
NICE guidelines recommend thromboelastography to help detect, manage and monitor hemostasis in cardiac surgery patients. Other clinical guidelines do not currently strongly recommend TEG for use in other settings due to the lack of high-quality evidence. Recently updated guidelines of the European Society of Anesthesiology recommended viscoelastic hemostatic assays (TEG/RoTEM) to guide the management of perioperative bleeding and manage severe peripartum hemorrhages, albeit with a low level of evidence.[39]
Limitations
An ideal test on blood coagulation does not yet exist. TEG measures blood coagulation in vitro, with or without an additional activator. An important component of the coagulation cascade, tissue factor, cannot be quantified in vitro. Moreover, blood coagulation potential is only one component in such complex processes as clinical thrombosis and bleeding. Blood coagulation also depends on the size of the injured vessel, blood flow characteristics, and local vessel wall biology that determines the quantity and functional activity of the membrane-bound pro- and anticoagulation factors. In other words, there are significant aspects of coagulation that are not components of the blood. An abnormal TEG in a patient without clinically relevant bleeding does not require transfusion of blood components. A single test or patient-related factor rarely guides the decision to transfuse blood components or initiate/correct antithrombotic therapy.
TEG has a sensitivity and specificity that may vary significantly in different populations. Patients taking anticoagulants and antiplatelet agents are a major concern in the trauma setting. Warfarin is a commonly prescribed medication [40] that has been associated with increased mortality in trauma patients [41]. In about half of patients on warfarin therapy, R-time may be normal in both TEG and rapid TEG tests, with a poor correlation between TEG and INR.[11] This is a good example of how TEG may miss a clinically significant coagulopathic state. Hence, INR is still the gold standard for monitoring warfarin therapy. Several important blood tests also cannot be currently replaced by TEG, such as P2Y12 platelet function assay to guide clopidogrel therapy, D-Dimer to exclude VTE in low-risk outpatients, and advanced thrombophilia diagnostic tests.
Future Directions: Guiding Anticoagulation and Antiplatelet Therapy
Anticoagulation therapy is a field where TEG may become more applicable pending future clinical studies. Up to one-third of patients on warfarin therapy may have subtherapeutic anticoagulation at some point of treatment.[42] One of the advantages of direct oral anticoagulants (DOAKs) is no requirement to monitor anticoagulation therapy. However, they may be problematic in certain groups of patients, such as those with renal failure, liver failure, pregnancy, extremes of body weight, high bleeding risk, thrombosis progression, or recurrence on anticoagulation.[43] High plasma concentration of DOAKs has been associated with higher bleeding risk.[44][45] In these situations, TEG may provide the ability to adjust the level of anticoagulation during the same office visit. Since TEG implies activation of an intrinsic coagulation pathway with kaolin, it is sensitive to heparin and low molecular weight heparin therapy (R time). An interesting case of an emergent heparin reversal under TEG control in a patient with intracranial bleeding has been reported.[46]
Prediction of platelet inhibition by antiplatelet agents (such as aspirin, clopidogrel, abciximab, eptifibatide, or tirofiban) is another promising avenue for TEG application. Most antiplatelet agents are used with a standard dose despite several known issues associated with this approach. For example, up to 25% of patients with STEMI may be resistant to clopidogrel, increasing the risk of recurrent cardiovascular events.[47] Aspirin resistance has been associated with increased myocardial infarction, stroke, or death in patients with cardiovascular disease.[48] Hence, there is significant variability in individual response to antiplatelet therapy. Available evidence does not support the use of usual laboratory testing to guide the dose of aspirin or clopidogrel.[49] Future studies may determine if TEG can measure the effect of antiplatelet therapy, detect hyporesponsiveness, and predict the risk of bleeding or thromboembolic complications.
A novel concept of individualized health care applies to both anticoagulation and antiplatelet therapy monitoring. Using a standard dose of the same medications to treat patients with different medical conditions and comorbidities may not be an ideal approach. The potential of TEG to improve the quality of antithrombotic therapy is a promising avenue for experimental and clinical research.
Future Directions: Prevention of Venous Thromboembolism
Another potential application of TEG is to improve the diagnosis, prevention, and treatment of patients with venous thromboembolism. The most appropriate evidence-based practice to prevent VTE is to stratify patients based on the VTE risk using one of the risk prediction models elaborated for surgical and medical patients. None of these models includes conventional blood coagulation tests since they do not predict VTE. A number of initial reports suggest that TEG may be a useful tool to help with risk stratification. TEG may have a VTE predictive value in critically ill patients, gynecological oncology patients, and prostate cancer.[50][51][52][53] A large prospective cohort of adult trauma patients revealed a 2-fold higher risk of VTE in patients with hypercoagulable TEG parameters on arrival to the trauma bay.[54] However, other reports did not find TEG of value in predicting VTE in selected patients, such as orthopedic surgery.[55]
Despite an appropriate prophylaxis, VTE is still a frequent concern in hospitalized patients. In one study, the R time of TEG was significantly shorter in critically ill patients on LMWH prophylaxis who develop DVT compared to those patients who do not.[56] Thus, TEG may be helpful to predict VTE that occurs despite standard pharmacological prophylaxis.
Quality Control and Lab Safety
TEG/RoTEM requires experienced personnel for the best results as the precision of the tests may vary broadly depending on the user.[57] Thus, regular external quality assessment and proficiency testing have been suggested.[57] For the appropriate use of the test, please refer to user manuals.
Enhancing Healthcare Team Outcomes
All interprofessional healthcare team members who deal with patients who require precise hemostatic control should be familiar with thromboelastography and how it can be used in the diagnosis and guide subsequent interventions for these patients. This includes clinicians, specialists, mid-level practitioners, nurses, and pharmacists, as they will all need to understand the ramifications of this diagnostic information so they can inform the team and assist in making therapeutic decisions, leading to better patient outcomes. [Level 5]
Media
References
Sobrino J, Shafi S. Timing and causes of death after injuries. Proceedings (Baylor University. Medical Center). 2013 Apr:26(2):120-3 [PubMed PMID: 23543966]
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003 Jun:54(6):1127-30 [PubMed PMID: 12813333]
Level 2 (mid-level) evidenceBarnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. The American journal of medicine. 2015 Dec:128(12):1300-5.e2. doi: 10.1016/j.amjmed.2015.05.044. Epub 2015 Jul 2 [PubMed PMID: 26144101]
Luepker RV, Steffen LM, Duval S, Zantek ND, Zhou X, Hirsch AT. Population Trends in Aspirin Use for Cardiovascular Disease Prevention 1980-2009: The Minnesota Heart Survey. Journal of the American Heart Association. 2015 Dec 23:4(12):. doi: 10.1161/JAHA.115.002320. Epub 2015 Dec 23 [PubMed PMID: 26702085]
Level 3 (low-level) evidenceRodgers RP, Levin J. A critical reappraisal of the bleeding time. Seminars in thrombosis and hemostasis. 1990 Jan:16(1):1-20 [PubMed PMID: 2406907]
Johnson DJ, Scott AV, Barodka VM, Park S, Wasey JO, Ness PM, Gniadek T, Frank SM. Morbidity and Mortality after High-dose Transfusion. Anesthesiology. 2016 Feb:124(2):387-95. doi: 10.1097/ALN.0000000000000945. Epub [PubMed PMID: 26569167]
HARTERT H. [Blood clotting studies with Thrombus stressography; a new Investigation procedure]. Klinische Wochenschrift. 1948 Oct 1:26(37-38):577-83 [PubMed PMID: 18101974]
Walsh M, Fritz S, Hake D, Son M, Greve S, Jbara M, Chitta S, Fritz B, Miller A, Bader MK, McCollester J, Binz S, Liew-Spilger A, Thomas S, Crepinsek A, Shariff F, Ploplis V, Castellino FJ. Targeted Thromboelastographic (TEG) Blood Component and Pharmacologic Hemostatic Therapy in Traumatic and Acquired Coagulopathy. Current drug targets. 2016:17(8):954-70 [PubMed PMID: 26960340]
Kang YG, Martin DJ, Marquez J, Lewis JH, Bontempo FA, Shaw BW Jr, Starzl TE, Winter PM. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesthesia and analgesia. 1985 Sep:64(9):888-96 [PubMed PMID: 3896028]
Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesthesia and analgesia. 1999 Feb:88(2):312-9 [PubMed PMID: 9972747]
Level 1 (high-level) evidenceQuarterman C, Shaw M, Johnson I, Agarwal S. Intra- and inter-centre standardisation of thromboelastography (TEG®). Anaesthesia. 2014 Aug:69(8):883-90. doi: 10.1111/anae.12748. Epub 2014 May 20 [PubMed PMID: 24841452]
Cotton BA, Faz G, Hatch QM, Radwan ZA, Podbielski J, Wade C, Kozar RA, Holcomb JB. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. The Journal of trauma. 2011 Aug:71(2):407-14; discussion 414-7. doi: 10.1097/TA.0b013e31821e1bf0. Epub [PubMed PMID: 21825945]
Level 3 (low-level) evidencePezold M, Moore EE, Wohlauer M, Sauaia A, Gonzalez E, Banerjee A, Silliman CC. Viscoelastic clot strength predicts coagulation-related mortality within 15 minutes. Surgery. 2012 Jan:151(1):48-54. doi: 10.1016/j.surg.2011.06.023. Epub 2011 Sep 6 [PubMed PMID: 21899867]
Caprini JA, Zuckerman L, Cohen E, Vagher JP, Lipp V. The identification of accelerated coagulability. Thrombosis research. 1976 Aug:9(2):167-80 [PubMed PMID: 788222]
Caprini JA, Arcelus JI, Laubach M, Size G, Hoffman KN, Coats RW 2nd, Blattner S. Postoperative hypercoagulability and deep-vein thrombosis after laparoscopic cholecystectomy. Surgical endoscopy. 1995 Mar:9(3):304-9 [PubMed PMID: 7597604]
Ng KF. Changes in thrombelastograph variables associated with aging. Anesthesia and analgesia. 2004 Aug:99(2):449-54, table of contents [PubMed PMID: 15271723]
Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, Huang H, Pinto R, Speers V, Reis M, Rizoli SB. Normal range values for thromboelastography in healthy adult volunteers. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2009 Dec:42(12):1210-7 [PubMed PMID: 19882085]
Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2005 Jun:16(4):301-10 [PubMed PMID: 15870552]
Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. Journal of cardiothoracic and vascular anesthesia. 2006 Aug:20(4):548-53 [PubMed PMID: 16884987]
Nielsen VG. A comparison of the Thrombelastograph and the ROTEM. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2007 Apr:18(3):247-52 [PubMed PMID: 17413761]
Durila M. Nonactivated thromboelastometry able to detect fibrinolysis in contrast to activated methods (EXTEM, INTEM) in a bleeding patient. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2016 Oct:27(7):828-830 [PubMed PMID: 26656899]
Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. The Cochrane database of systematic reviews. 2016 Aug 22:2016(8):CD007871. doi: 10.1002/14651858.CD007871.pub3. Epub 2016 Aug 22 [PubMed PMID: 27552162]
Level 1 (high-level) evidenceAdler M, Ivic S, Bodmer NS, Ten Cate H, Bachmann LM, Wuillemin WA, Nagler M. Thromboelastometry and Thrombelastography Analysis under Normal Physiological Conditions - Systematic Review. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2017 Apr:44(2):78-83. doi: 10.1159/000464297. Epub 2017 Mar 8 [PubMed PMID: 28503123]
Level 1 (high-level) evidenceDeppe AC, Weber C, Zimmermann J, Kuhn EW, Slottosch I, Liakopoulos OJ, Choi YH, Wahlers T. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. The Journal of surgical research. 2016 Jun 15:203(2):424-33. doi: 10.1016/j.jss.2016.03.008. Epub 2016 Mar 26 [PubMed PMID: 27363652]
Level 1 (high-level) evidenceWikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2017 Apr:72(4):519-531. doi: 10.1111/anae.13765. Epub 2017 Jan 4 [PubMed PMID: 28052313]
Level 1 (high-level) evidenceNakayama Y, Nakajima Y, Tanaka KA, Sessler DI, Maeda S, Iida J, Ogawa S, Mizobe T. Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. British journal of anaesthesia. 2015 Jan:114(1):91-102. doi: 10.1093/bja/aeu339. Epub 2014 Oct 10 [PubMed PMID: 25303988]
Level 1 (high-level) evidenceWhiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, Misso K, Ross J, Severens J, Kleijnen J. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health technology assessment (Winchester, England). 2015 Jul:19(58):1-228, v-vi. doi: 10.3310/hta19580. Epub [PubMed PMID: 26215747]
Level 1 (high-level) evidenceHunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. The Cochrane database of systematic reviews. 2015 Feb 16:2015(2):CD010438. doi: 10.1002/14651858.CD010438.pub2. Epub 2015 Feb 16 [PubMed PMID: 25686465]
Level 2 (mid-level) evidenceHolcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, Adams PR, McCarthy JJ, Cotton BA. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Annals of surgery. 2012 Sep:256(3):476-86. doi: 10.1097/SLA.0b013e3182658180. Epub [PubMed PMID: 22868371]
Level 2 (mid-level) evidenceDa Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Critical care (London, England). 2014 Sep 27:18(5):518. doi: 10.1186/s13054-014-0518-9. Epub 2014 Sep 27 [PubMed PMID: 25261079]
Level 1 (high-level) evidenceVeigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scandinavian journal of trauma, resuscitation and emergency medicine. 2016 Oct 3:24(1):114 [PubMed PMID: 27716278]
Level 1 (high-level) evidencePanigada M, E Iapichino G, Brioni M, Panarello G, Protti A, Grasselli G, Occhipinti G, Novembrino C, Consonni D, Arcadipane A, Gattinoni L, Pesenti A. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: a safety and feasibility pilot study. Annals of intensive care. 2018 Jan 16:8(1):7. doi: 10.1186/s13613-017-0352-8. Epub 2018 Jan 16 [PubMed PMID: 29340875]
Level 2 (mid-level) evidenceCollyer TC, Gray DJ, Sandhu R, Berridge J, Lyons G. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by Thrombelastography Platelet Mapping. British journal of anaesthesia. 2009 Apr:102(4):492-8. doi: 10.1093/bja/aep039. Epub [PubMed PMID: 19286767]
Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. American journal of clinical pathology. 2010 Jul:134(1):97-102. doi: 10.1309/AJCPPZ4J6CAFYDVM. Epub [PubMed PMID: 20551273]
Kawano-Castillo J, Ward E, Elliott A, Wetzel J, Hassler A, McDonald M, Parker SA, Archeval-Lao J, Tremont C, Cai C, Pivalizza E, Rahbar MH, Grotta JC. Thrombelastography detects possible coagulation disturbance in patients with intracerebral hemorrhage with hematoma enlargement. Stroke. 2014 Mar:45(3):683-8. doi: 10.1161/STROKEAHA.113.003826. Epub 2014 Jan 14 [PubMed PMID: 24425123]
Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010 Aug 12:116(6):878-85. doi: 10.1182/blood-2010-02-261891. Epub 2010 Apr 16 [PubMed PMID: 20400681]
Mallett SV. Clinical Utility of Viscoelastic Tests of Coagulation (TEG/ROTEM) in Patients with Liver Disease and during Liver Transplantation. Seminars in thrombosis and hemostasis. 2015 Jul:41(5):527-37. doi: 10.1055/s-0035-1550434. Epub 2015 Jun 6 [PubMed PMID: 26049072]
Roullet S, Pillot J, Freyburger G, Biais M, Quinart A, Rault A, Revel P, Sztark F. Rotation thromboelastometry detects thrombocytopenia and hypofibrinogenaemia during orthotopic liver transplantation. British journal of anaesthesia. 2010 Apr:104(4):422-8. doi: 10.1093/bja/aeq022. Epub 2010 Feb 25 [PubMed PMID: 20185519]
Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, Haas T, Jacob M, Lancé MD, Pitarch JVL, Mallett S, Meier J, Molnar ZL, Rahe-Meyer N, Samama CM, Stensballe J, Van der Linden PJF, Wikkelsø AJ, Wouters P, Wyffels P, Zacharowski K. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. European journal of anaesthesiology. 2017 Jun:34(6):332-395. doi: 10.1097/EJA.0000000000000630. Epub [PubMed PMID: 28459785]
Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Archives of internal medicine. 2007 Jul 9:167(13):1414-9 [PubMed PMID: 17620536]
Dossett LA, Riesel JN, Griffin MR, Cotton BA. Prevalence and implications of preinjury warfarin use: an analysis of the National Trauma Databank. Archives of surgery (Chicago, Ill. : 1960). 2011 May:146(5):565-70. doi: 10.1001/archsurg.2010.313. Epub 2011 Jan 17 [PubMed PMID: 21242422]
Level 2 (mid-level) evidenceRose AJ, Ozonoff A, Grant RW, Henault LE, Hylek EM. Epidemiology of subtherapeutic anticoagulation in the United States. Circulation. Cardiovascular quality and outcomes. 2009 Nov:2(6):591-7. doi: 10.1161/CIRCOUTCOMES.109.862763. Epub 2009 Sep 22 [PubMed PMID: 20031897]
Level 2 (mid-level) evidenceFavaloro EJ, Lippi G. Laboratory testing in the era of direct or non-vitamin K antagonist oral anticoagulants: a practical guide to measuring their activity and avoiding diagnostic errors. Seminars in thrombosis and hemostasis. 2015 Mar:41(2):208-27. doi: 10.1055/s-0035-1546827. Epub 2015 Feb 19 [PubMed PMID: 25703514]
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2009 Sep 17:361(12):1139-51. doi: 10.1056/NEJMoa0905561. Epub 2009 Aug 30 [PubMed PMID: 19717844]
Level 1 (high-level) evidenceGiugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM, ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine. 2013 Nov 28:369(22):2093-104. doi: 10.1056/NEJMoa1310907. Epub 2013 Nov 19 [PubMed PMID: 24251359]
Level 1 (high-level) evidenceFigueiredo S, Vigué B, Benhamou D, Duranteau J. Emergency reversal of heparin overdose in a neurosurgical patient guided by thromboelastography. British journal of anaesthesia. 2013 Aug:111(2):303-4. doi: 10.1093/bja/aet245. Epub [PubMed PMID: 23858079]
Level 3 (low-level) evidenceMatetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004 Jun 29:109(25):3171-5 [PubMed PMID: 15184279]
Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. Journal of the American College of Cardiology. 2003 Mar 19:41(6):961-5 [PubMed PMID: 12651041]
Michelson AD, Bhatt DL. How I use laboratory monitoring of antiplatelet therapy. Blood. 2017 Aug 10:130(6):713-721. doi: 10.1182/blood-2017-03-742338. Epub 2017 Jun 9 [PubMed PMID: 28600334]
Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, Pezold M, Lawrence J, Biffl WL, Cothren CC, Johnson JL. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009 Oct:146(4):764-72; discussion 772-4. doi: 10.1016/j.surg.2009.06.054. Epub [PubMed PMID: 19789037]
Level 2 (mid-level) evidenceTartamella F, Vassallo MC, Berlot G, Grassi P, Testa F. Thromboelastographic predictors of venous thromboembolic events in critically ill patients: are we missing something? Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2016 Oct:27(7):804-811 [PubMed PMID: 26895213]
Liu J, Wang N, Chen Y, Lu R, Ye X. Thrombelastography coagulation index may be a predictor of venous thromboembolism in gynecological oncology patients. The journal of obstetrics and gynaecology research. 2017 Jan:43(1):202-210. doi: 10.1111/jog.13154. Epub 2016 Oct 20 [PubMed PMID: 27762468]
Toukh M, Siemens DR, Black A, Robb S, Leveridge M, Graham CH, Othman M. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thrombosis research. 2014 Jan:133(1):88-95. doi: 10.1016/j.thromres.2013.10.007. Epub 2013 Oct 12 [PubMed PMID: 24246296]
Level 2 (mid-level) evidenceBrill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. The journal of trauma and acute care surgery. 2017 Sep:83(3):413-419. doi: 10.1097/TA.0000000000001618. Epub [PubMed PMID: 28598908]
Parameswaran A, Krishnamoorthy VP, Oommen AT, Jasper A, Korula RJ, Nair SC, Poonnoose PM. Is pre-operative assessment of coagulation profile with Thrombelastography (TEG) useful in predicting venous thromboembolism (VTE) following orthopaedic surgery? Journal of clinical orthopaedics and trauma. 2016 Oct-Dec:7(Suppl 2):225-229. doi: 10.1016/j.jcot.2016.08.003. Epub 2016 Aug 24 [PubMed PMID: 28053389]
Van PY, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. The Journal of trauma. 2009 Jun:66(6):1509-15; discussion 1515-7. doi: 10.1097/TA.0b013e3181a51e33. Epub [PubMed PMID: 19509608]
Level 3 (low-level) evidenceKitchen DP, Kitchen S, Jennings I, Woods T, Walker I. Quality assurance and quality control of thrombelastography and rotational Thromboelastometry: the UK NEQAS for blood coagulation experience. Seminars in thrombosis and hemostasis. 2010 Oct:36(7):757-63. doi: 10.1055/s-0030-1265292. Epub 2010 Oct 26 [PubMed PMID: 20978996]
Level 2 (mid-level) evidence