Introduction
Classic tetralogy of Fallot (TOF) is a congenital heart defect (CHD) that is comprised of 4 anatomical alterations: a large, anteriorly malaligned ventricular septal defect (VSD), an overriding aorta which results in infundibular (ie, sub-pulmonary) right ventricular outflow tract obstruction (RVOTO), and consequent right ventricular hypertrophy secondary to chronic systemic pressures. The pulmonary valve annulus is often hypoplastic, with a pulmonary valve that is dysplastic and stenotic. The VSD is most frequently located in the perimembranous septum; however, the defect can extend to the muscular septum, and infrequently, there might be additional muscular VSDs.[1] A right aortic arch is observed in 20% to 25% of TOF.[2]
The clinical presentation will also depend on the associated cardiovascular anomalies in roughly 40% of patients with TOF. These may include atrial septal defects, patent ductus arteriosus, supravalvar pulmonary stenosis, branch pulmonary artery stenoses, hypoplastic branch pulmonary arteries, pulmonary valve atresia which may develop during fetal life as the subpulmonary infundibular narrowing progresses, a disconnected left pulmonary artery that originates from the ascending aorta formerly known as hemitruncus, a left pulmonary artery arising from the ductus arteriosus, absent left pulmonary artery, absent pulmonary valve, anomalous coronary arteries, anomalous pulmonary venous return, aortic incompetence, aortopulmonary window, and atrioventricular septal defect (AVSD).[3]
Patients with TOF and pulmonary atresia may have a remnant of a pulmonary artery trunk with different calibers of the central pulmonary arteries and variable pulmonary tree anatomy. In approximately 50% of these patients, the right and left pulmonary arteries are confluent, and blood flow is ductal-dependent. In the other 50%, pulmonary flow is from multiple collateral vessels, usually from the descending thoracic aorta, and is not ductal-dependent. Occasionally, collateral arteries might arise from the head and neck, abdominal aorta, or coronary arteries. Surgical repair requires unifocalization of the many aortopulmonary collaterals, which can be quite challenging.[2]
TOF associated with rudimentary pulmonary valve leaflets, which occurs in 3% to 6% of cases, is known as “TOF with absent pulmonary valve.” In these patients, the main and branch pulmonary arteries are aneurysmal. Some degree of RVOTO is due to the presence of a small pulmonary annulus. The ductus arteriosus is usually absent, and approximately 50% have a right-sided aortic arch. Occasionally, one of the branch pulmonary arteries may arise from the aorta or may be absent.[2] Approximately 2% of patients with TOF have an associated atrioventricular septal defect (AVSD). Because the RVOTO limits pulmonary overcirculation, these patients usually do not display symptoms of congestive heart failure. Therefore, primary surgical repair can be performed within the first few months of life.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
TOF is the most frequently occurring conotruncal or outflow tract malformation, sharing this classification with other CHD (eg, truncus arteriosus, interrupted aortic arch, transposition of the great arteries, and double outlet right ventricle). In conotruncal malformations, suppression of developmental networks such as the NOTCH and WNT pathways during early embryogenesis produces malfunction of downstream regulatory pathways that lead to faulty structural cardiac development.[4]
Approximately 75% to 80% of TOF cases are nonsyndromic, meaning that the patient has an isolated cardiac defect. Of these, 7% have a loss of function gene mutation, including NOTCH1, FLT4, and TBX1, a transcription factor involved in cardiac development that is affected in those with 22q11.2 microdeletion.[5] Mutations in certain transcription factors involved in cardiac morphogenesis (eg, NKX2.5, GATA-6, GATA-4, HAND1, HAND2, ZFPM2, and NF-ATC) have also been associated with nonsyndromic TOF.[6][7] In general, mutations in the NKX2.5 gene are commonly observed in familial CHD. Not surprisingly, CHDs reoccur in approximately 3% of affected families, though not necessarily TOF, when compared to the general population.[8]
The remaining 20% to 25% of patients with TOF have an associated syndrome or a chromosomal abnormality, with the most frequent being trisomy 21 (ie, Down syndrome) and the 22q11.2 deletion syndromes, which range from the more severe DiGeorge syndrome that is accompanied by dysmorphic facies, palatal abnormalities, immune deficiencies, hypocalcemia, and learning disabilities, to the less severe Sprintzen or velocardiofacial syndrome which does not have the immune deficiency and hypocalcemia. Because 22% to 48% of patients with a 22q11.2 microdeletion have an interrupted aortic arch, and 24% have a right-sided aortic arch, TOF is not infrequently associated with aortic arch malformations. When a patient with TOF presents with pulmonary valve atresia, the association with 22q11.2 microdeletion increases to 40%. Thus, genetic testing is currently recommended in fetuses with TOF to assess for the 22q11.2 microdeletion, especially since outcomes are worse than in those who do not carry the microdeletion.[8]
TOF can also occur in patients with known genetic mutations, including JAG1 and NOTCH2 genes (ie, Alagille syndrome), KMT2D and KDM6A genes (ie, Kabuki syndrome), CHD7 gene (ie, CHARGE syndrome), and the PTPN11, SOS1 or RAF1 genes (ie, Noonan syndrome). TOF may also be present in patients with unknown mutations (eg, VACTERL association and Goldenhar syndrome).[9]
Epidemiology
CHD is found in approximately 1% to 1.2% of live births.[10] TOF is the most common cyanotic CHD, with a nearly equal sex distribution, a prevalence of 1 out of 3,000 births, and an incidence of 5 to 7 out of 10,000 live births. TOF represents 5% to 7% of all CHD.[4][1][6][4] Medical and surgical advances have allowed an increased prevalence of CHD among older children and adults, with a current estimated adult CHD patient US population of approximately 1 million, of which an estimated 15% are TOF patients.[11] In contrast, without surgical intervention, survival decreases as the patient ages. Patients with unrepaired TOF have an estimated survival rate of 66% at 1 year of age, 40% at 3 years, 11% at 20 years, 6% at 30 years, and 3% at 40 years.[12]
Pathophysiology
The VSD in TOF is typically large or nonrestrictive, allowing pressure equalization within the ventricles. Therefore, whether the shunting is left to right or vice versa will depend on the degree of downstream pressure the blood flow encounters. With significant RVOTO, most of the blood will shunt toward the aorta, thereby bypassing the pulmonary bed with consequent cyanosis. Conversely, if the resistance across the RVOT is less than the systemic vascular resistance, most of the blood will be directed to the pulmonary vascular bed, and the patient will be minimally cyanotic or acyanotic (see Image. Tetralogy of Fallot).[13]
Different factors can contribute to RVOTO, including a stenotic pulmonary valve, a hypoplastic pulmonary valve annulus, the deviation of the infundibular septum that causes subvalvular obstruction, and the hypertrophy of the muscular bands of the RVOT. The obstruction across the RVOT can also be dynamic with sudden increases in cyanosis, a phenomenon known as “Tet or hypoxic spells.” The physiological process surrounding these hypercyanotic episodes is yet to be fully understood but appears to involve a decrease in systemic vascular resistance or an increase in pulmonary vascular resistance. The result is an increase in the right-to-left shunting across the ventricular septal defect, which produces marked desaturation. These episodes can be triggered by agitation, pain, anemia, fever, hypovolemia, or peripheral vasodilatation. These hypoxic spells occur in infants or children but do not occur in adults.[14][13]
Patients with TOF and pulmonary atresia are usually cyanotic in the newborn period, with worsening cyanosis as the ductus arteriosus closes. This becomes a lethal condition in the absence of sufficient blood flow from aortopulmonary collaterals. Patients with an absent pulmonary valve do not typically have cyanosis. However, these patients have aneurysmal branch pulmonary arteries that usually cause external compression of the airways, leading to tracheobronchomalacia with air trapping.[2] Approximately 10% to 35% of adult patients with TOF may have atrial arrhythmias (eg, reentrant atrial tachycardia and atrial fibrillation).[11] Ventricular arrhythmias can occur in up to 10% of patients, and sudden death is estimated to occur in 0.2% of patients each year of follow-up.[8] Life-threatening arrhythmias occur more frequently in those with moderate to severe pulmonary valve insufficiency with RV dilatation and fibrosis and in those with biventricular dysfunction with congestive heart failure. Therefore, life-long monitoring is recommended.[15] Progressive aortic root dilatation in repaired TOF with progressive aortic valve regurgitation is less common but warrants follow-up, as this may require aortic root replacement.[16]
History and Physical
Clinical Symptoms
Clinical presentation varies based on the severity of the RVOTO, most commonly presenting as a cyanotic neonate. Adult patients with TOF may have dyspnea and exercise intolerance. With the advent of fetal echocardiography, the diagnosis can be made prenatally, and in infants who present with severe RVOTO, prompt stabilization can avoid profound cyanosis and rapid deterioration.[17]
In those undiagnosed before birth, presentation depends on the degree of obstruction to the RV outflow. Those with moderate RVOTO may be asymptomatic at presentation, except for the presence of a loud murmur, and may be referred to as "pink Tets." Those with minimal RVOTO behave as those who have a large VSD and will present with symptoms of pulmonary overcirculation as the pulmonary vascular resistance falls after a few weeks of life. Because RVOTO usually increases with time, pink Tets can later develop cyanosis and hypercyanotic spells when crying or in hypovolemia.[17] Patients with TOF and absent pulmonary valves can present primarily with respiratory distress from tracheobronchial compression from the dilated pulmonary arteries; cyanosis in these patients is usually mild.[18]
"Tet spells" or hypercyanotic episodes can occur in infants or toddlers with unrepaired TOF, characterized by tachypnea and hyperpnea and a decrease in the intensity of the cardiac murmur. Some patients will be inconsolable in addition to worsening cyanosis. These episodes can either subside spontaneously or can cause syncope with or without cardiac arrest. Thus, rapid intervention should be provided with a referral for prompt primary repair or palliation. If the patient survives such episodes but remains unrepaired, "Tet spells" tend to become rare and may disappear by 4 to 5 years of age. Presently, patients with TOF presenting with cyanosis and severe, longstanding cyanosis are rare in developed countries with adequate pediatric cardiac services.[19][20]
The clinical manifestations of patients with TOF and absent pulmonary valves depend on the severity of respiratory distress, ranging from those with no respiratory distress to those with severe respiratory distress due to trachea and main stem bronchi compression by the massive pulmonary artery branches.[21] The degree of obstructive airway disease among these patients varies markedly. Some patients will improve with prone positioning. Otherwise, intubation and positive pressure ventilation are required.[18] Patients with TOF and absent pulmonary valves usually have to-and-fro systolic and diastolic murmurs and a single-second heart sound. As newborns, these patients are typically cyanotic; however, the cyanosis improves by the first week of life. Due to a lesser degree of RVOTO, as pulmonary vascular resistance naturally decreases during the first days of life, these patients may exhibit signs and symptoms of pulmonary overcirculation with tachypnea, excessive diaphoresis with feeds, and failure to thrive.[22]
Physical Exam Findings
Patients with TOF may have a typical first heart sound with a single-second heart sound because the pulmonic component is usually inaudible. The greater the degree of obstruction, the more prominent the murmur, usually described as crescendo-decrescendo with a harsh systolic ejection quality and best heard at the left mid to upper sternal border with posterior radiation. Sometimes, the murmur can have a regurgitant quality, and an early systolic click may be auscultated along the left sternal border attributed to the flow through a dilated ascending aorta. A prominent ventricular impulse and a systolic thrill may be palpable. A continuous murmur can be heard over the back and axillary regions in patients with pulmonary atresia and aortopulmonary collaterals.[13]
Adult patients with TOF may have findings that include severe or free pulmonary insufficiency with RV dilatation, severe tricuspid valve regurgitation, and RV dyssynchrony from right bundle branch block (RBBB) with prolonged QRS duration from TOF repair. These factors increase the incidence of atrial and ventricular arrhythmias, which are a major risk for mortality.[23] Those rarely encountered patients who did not undergo surgical repair may have complications of chronic hypoxemia such as hyperviscosity, abnormal hemostasis, stroke, cerebral abscess, and endocarditis.[13][24]
Evaluation
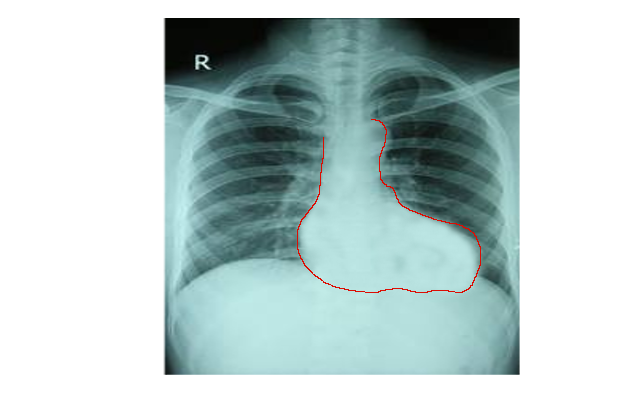
Chest radiography, electrocardiogram, and an echocardiogram are the primary imaging studies utilized to diagnose TOF. Typical findings on chest radiography include a normal-size heart silhouette with an upturned apex and a concave or "boot-shaped" main pulmonary artery segment (see Image. Chest X-ray of Tetralogy of Fallot). Right axis deviation, prominent R or qR waves in V1, and upright T waves in V1, characteristic of right atrial enlargement and right ventricular hypertrophy, are common on the electrocardiogram.
The echocardiogram is the gold standard for the diagnosis of TOF. This noninvasive modality is used at the patient's bedside to accurately assess the anatomy and severity of the RVOTO, the location and number of VSDs, associated anomalies, aortic arch, and coronary artery variants since RVOT crossing of coronary arteries will complicate the surgical approach. The major limitation of the transesophageal echocardiogram in patients with TOF is visualizing the distal pulmonary arteries.[19]
Cardiac magnetic resonance imaging (MRI) or cardiac computed tomography (CT) can be used, particularly in adults with repaired TOF. These noninvasive imaging modalities have made diagnostic cardiac catheterization no longer a routine for patients with classic TOF anatomy. However, cardiac catheterization can still be used to assess the level and degree of RVOTO, pulmonary stenosis or hypoplasia, distal branch pulmonary artery anatomy, and coronary artery anatomy. It can delineate aortopulmonary collaterals when present and show accessory septal defects best seen with a left ventricular injection. Cardiac catheterization can also be used in selected patients for ductal and RVOT stenting. Knowing that catheter manipulation can trigger a hypercyanotic or "tet spell" and should thus be used when strictly necessary is essential.[19]
Treatment / Management
Neonates with severe RVOTO who present with profound hypoxemia and cyanosis will require prostaglandin therapy to maintain ductal patency and adequate pulmonary blood flow before a transcatheter intervention or surgical repair. Tet spells require a rapid and aggressive approach, including knee-chest positioning to increase systemic vascular resistance and "force" more blood into the pulmonary artery, oxygen therapy to cause pulmonary vasodilation, and decrease pulmonary vascular resistance. Patients may require intravenous fluid boluses to improve RV filling and increase pulmonary blood flow. They may also need intravenous beta-blockers to decrease heart rate to aid RV filling further and, sometimes, intravenous phenylephrine to increase systemic afterload. Morphine can also be given as a sedative during a tet spell, alleviating pain and anxiety and helping decrease heart and respiratory rates. A tet spell, left untreated, may lead to loss of consciousness and, ultimately, death.[25][26][27] (B3)
Without surgical treatment, the survival rate of patients with TOF without additional cardiac defects is approximately 66% at 1 year of age, 40% at 3 years, 11% at 20 years, 6% at 30 years, and 3% at 40 years.[13] Surgical or transcatheter palliation and surgical repair aim to provide adequate and stable pulmonary blood flow. The timing of TOF repair is still in debate, with some centers advocating neonatal repair with better results than systemic-to-pulmonary artery shunting, deferring the complete surgical repair to after the first 3 months of life. The technique involves placing a "modified" Blalock-Taussig shunt graft between the subclavian artery and the ipsilateral branch pulmonary artery.[8]
Reasons for an initial surgical palliative shunting include technical difficulties in the systemic-to-pulmonary shunt placement due to the smaller pulmonary arteries of the newborn, decreased postoperative mortality after the neonatal period, unfavorable coronary artery anatomy with a large branch crossing the RVOT that will require a right ventricle to pulmonary artery conduit, which is safer to operate in the larger heart of an older infant, and the surgeon's preference.[19][28] Stenting of the ductus arteriosus with or without RVOT stenting with pulmonary artery banding is a less invasive alternative to surgical palliation, as it does not require cardiopulmonary bypass with the additional advantage of not requiring a sternotomy or thoracotomy.[29] (B2)
When possible, earlier techniques of transannular patching and ventriculotomy are being replaced by right atrial atriotomy and operation through the tricuspid valve, limiting the transannular incision.[1] During the VSD closure, care is taken to avoid injuring the His bundle, which would produce an atrioventricular conduction block. Reduced RV compliance may require leaving or creating a minor atrial septal defect to allow right atrial decompression at the expense of residual cyanosis.[19]
An RV to pulmonary artery conduit is used in patients with TOF and pulmonary atresia in the presence of a markedly hypoplastic or atretic RVOT or a sizeable conal branch coronary artery crossing the RVOT. Pulmonary artery conduits are also used in those requiring reoperation for severe pulmonary insufficiency or recurrent stenosis. These conduits include pulmonary or aortic homografts, heterografts (ie, bovine jugular vein grafts), or autologous pericardial valved conduits. However, they have a limited lifespan, requiring reoperation within a few years.[30]
Patients with TOF and pulmonary atresia with major aortopulmonary collaterals (MAPCAs) might require prostaglandin E1 in the early neonatal period if these collaterals are not sufficiently developed. These patients can undergo early complete repair with segmental pulmonary artery reconstruction in the neonatal period incorporating all lung segments, or they can undergo a staged repair with initial unifocalization of the MAPCAs followed by intracardiac TOF repair at 4 to 7 months of age.[31]
Patients with TOF and absent pulmonary valves require plication and reduction of the size of the pulmonary arteries with or without suspension of the left pulmonary artery to the anterior chest wall to relieve bronchial compression, in addition to the intracardiac patch closure of the VSD. Another surgical technique is to transect the ascending aorta and anteriorly move the right pulmonary artery. Some undergo placement of a valved RV to pulmonary artery conduit to place a functioning pulmonary valve. Airway stenting is rarely indicated and reserved for those with severe airway obstruction despite surgery.[32] Early extubation will improve cardiac preload and RV filling, improving cardiac output.[19]
Differential Diagnosis
“Tet spells” can present with respiratory distress and worsening cyanosis. This clinical scenario can also be observed with other cardiac malformations with right-to-left shunting, including complete transposition of the great arteries (d-TGA) with pulmonary stenosis, double outlet right ventricle with severe pulmonary stenosis, tricuspid atresia, and Ebstein anomaly. Isolated severe pulmonic or aortic stenosis can produce a similar clinical picture. Viral or bacterial infections such as bronchiolitis or pneumonia can also be included as a differential diagnosis and pneumothorax.
Prognosis
Although current surgical mortality can be as low as 2%, with 20-year survival exceeding 90%, most patients will develop residual hemodynamic and electrophysiologic abnormalities, especially after the third decade of life.[33] Pulmonary regurgitation, which results from the reconstruction of the RVOT, produces RV dilatation, dysfunction, and diffuse interstitial fibrosis that perpetuates the dysfunction.[34][35][36][37][34] The RV dysfunction affects the left ventricle via abnormal ventricular interaction from shared myofibers, creating electromechanical dyssynchrony.[38][39]
Conventional practice has been that a QRS duration greater than 180 msec, sustained ventricular tachycardia, and a history of syncope are associated with exercise intolerance, cardiac failure, and death in these patients.[23][34][40] However, a very recent study in adults with repaired TOF observed that the highest mortality risk appears to be related to any of the following:
- Age younger than 50 years
- Findings on cardiac magnetic resonance imaging (MRI)
- Extensive RV and LV late gadolinium enhancement, which confirms the presence of extensive collagen deposition or fibrosis
- RV ejection fraction of ≤35%
- LV ejection fraction ≤35%
- Peak oxygen uptake ≤17 mL/kg/m2
- B-type natriuretic peptide ≥127 ng/L
- Sustained atrial arrhythmias [41]
Atrial tachyarrhythmias, usually intra-atrial reentrant tachycardia and cavotricuspid isthmus-dependent atrial flutter, can be observed with advancing age after TOF repair and comprise up to 20% of the arrhythmias observed in these patients. Large right atrial size, sinus node dysfunction, and at least moderate tricuspid regurgitation are associated with atrial arrhythmias. Atrial fibrillation is more commonly associated with older patient age, decreased left ventricular function, left atrial dilatation, hypertension, and multiple cardiac surgeries.[42] Atrial arrhythmias have been recently recognized to be associated with severe adverse events in adult patients who had TOF repair.[43]
The prognosis of these patients is also affected by the need for pulmonary valve replacement due to progressive pulmonary insufficiency, which places them at risk of bacterial endocarditis.[24] Very recently, a multinational, multicenter study demonstrated for the first time that pulmonary valve replacement (PVR) in patients with repaired TOF is associated with a lower probability of sustained ventricular tachycardia (VT) and death, especially in those patients with more severe RV volume overload from pulmonary insufficiency, who are at high risk of developing mechanoelectrical cardiomyopathy. The study, however, did not determine the best timing for PVR or whether surgery or transcatheter pulmonary valve placement yields better results.[44]
Complications
Short Term Complications
Palliative surgery to place a modified Blalock-Taussig shunt poses the risk of recurrent laryngeal and phrenic nerve damage. In addition, shunt size might create inadequate or excessive pulmonary blood flow, leading to either pulmonary overcirculation/congestive heart failure (CHF) and failure to thrive or cyanosis, respectively.[19] Shunt thrombosis can be life-threatening and will require urgent reoperation, interventional catheterization for direct thrombolysis, or, if all fail, extracorporeal membrane oxygenation (ECMO).[45]
Complications within the immediate postoperative period after complete TOF repair include pericardial and pleural effusion requiring drainage, chylothorax, bleeding requiring reoperation, superficial wound infection, moderate pulmonary regurgitation in up to 14% of patients, residual ventricular septal defects with persistent hypoxemia, and RVOTO.[46]
Junctional ectopic tachycardia (JET) can occur in approximately 15% to 20% of patients in the immediate postoperative period, causing a low cardiac output with profound hypotension and hemodynamic instability. Treatment may include correction of any electrolyte imbalance, cooling the patient (hypothermia), decreasing vasoactive drugs such as dopamine, atrial pacing over the ventricular rate to restore atrioventricular synchrony, increasing sedation to control pain (ie, dexmedetomidine infusion), magnesium sulfate, and antiarrhythmics such as amiodarone, procainamide, or esmolol. Nonresponders may require ECMO until the arrhythmia subsides and cardiac output can be maintained.[47][48][47] Significant predictors for the development of postoperative JET include a patient younger than 6 months of age and postoperative use of inotropes such as dopamine.[49]
Patients undergoing a transatrial-transpulmonary approach appear to have fewer tachyarrhythmias and virtually no permanent pacemaker requirement for postoperative conduction abnormalities in the early postoperative period compared to those receiving a right ventriculotomy.[46] Damage to the conduction system can occur, with RBBB occurring frequently, bifascicular block in 8% to 12% of patients, and complete atrioventricular block in 3% to 5%. Poor RV compliance is another postoperative complication, resulting from the combination of RV hypertrophy and diastolic dysfunction with uncoordinated RV contraction from damage to the conduction system and the creation of a transannular patch with resection of contractile muscle fibers that a non-contractile patch on the RV free wall has replaced. Management of RV dysfunction in the immediate postoperative period may require beta-agonist avoidance, beta-blocker use, and monitoring fluid balance.[19]
Arrhythmias can occur after TOF repair, including ventricular tachycardia, atrial fibrillation or flutter, and intra-atrial reentrant tachycardia. Risk factors for ventricular arrhythmias and sudden cardiac death include older age at repair, male gender, transient complete heart block beyond postoperative day 3, and QRS duration greater than 180 milliseconds.[50] Prematurity is associated with higher postoperative mortality risk.[46] The incidence of postoperative moderate to severe pulmonary regurgitation has decreased due to valve-sparing techniques.[46]
Long-Term Complications
Adult patients with CHD are increasing in prevalence by an approximate estimate of 5% per year, surpassing the pediatric population. Thus, long-term complications from the surgical repair or CHD must be acknowledged. The progressive nature of pulmonary regurgitation despite valve-sparing techniques is well recognized, with a cumulative incidence of 40% at 35 years of age after TOF repair.[51][46][51] The most common indication for reoperation is pulmonary insufficiency. Long-term consequences include RV volume overload from pulmonary insufficiency and right and left ventricular dysfunction.[39] Pulmonary valve replacement can be achieved surgically or by a transcatheter approach.[52]
Other long-term complications include RV aneurysm from the outflow patch or the ventriculotomy, distal pulmonary artery obstruction, ventricular hypertrophy, and aortic root dilation and insufficiency. Patients can have exercise intolerance, which can be aggravated by concomitant ventricular dyssynchrony and signs and symptoms of CHF.[23] An aortic root dilatation >55 cm with aortic insufficiency warrants aortic root surgery.[20] Patients with TOF, repaired or unrepaired, are at risk of endocarditis and should receive SBE prophylaxis before any dental or elective surgical procedure.[13]
Leading causes of mortality in patients with repaired TOF include late arrhythmias (ventricular and atrial), heart failure, and complications from reoperations. Forty to 50% of adults with repaired TOF can have ventricular arrhythmias, including sustained ventricular tachycardia with syncope. The risk of sudden death ranges between 6% to 9% 30 years after the surgical procedure. Some factors associated with this risk are QRS duration greater than 180 milliseconds, older age at repair, especially older than 3 years, significant pulmonary valve or tricuspid valve regurgitation, history of syncope, multifocal premature ventricular contractions, and presence of ventricular tachycardia.[11]
Atrial flutter and fibrillation become increasingly prevalent with advancing age in the TOF population, causing substantial morbidity.[13] Larger right atrial size and at least moderate tricuspid regurgitation are associated with such atrial tachyarrhythmias. Additionally, atrial tachyarrhythmias frequently occur concomitantly with sinus node dysfunction and may perpetuate each other.[42] Those with RV to pulmonary artery conduits who reach adulthood are also at increased risk of death or sustained ventricular tachycardia.[33] Among adults with CHD, acquired cardiac disease secondary to metabolic syndrome, hypertension, obesity, and diabetes type 2 are leading causes of morbidity and mortality.[53]
Patients with CHD, in general, are known to be at risk for neurodevelopmental disability from underlying syndromes, genetic or developmental disorders, and medical and surgical therapies. These patients usually present mild cognitive impairment, impaired communication skills and social interaction, inattention, impulsivity, and impaired executive function. Tutoring, special education, and physical, occupational, and speech therapy are frequently required, with a significant proportion of patients needing these services into adulthood. These issues may limit their educational achievements, employability, insurability, and quality of life.[54]
Pregnancy Complications
Women who had correction of TOF are expected to have similar outcomes as the general obstetric population. Pregnancy complications are related to the degree of pulmonary hypertension, the severity of pulmonary regurgitation, and the magnitude of right and left ventricular dysfunction. Maternal cardiovascular events can include arrhythmias requiring treatment (eg, atrial flutter or fibrillation, ventricular tachycardia, cardiac failure during the third trimester, and pulmonary embolism). Offspring complications have been described, such as small for gestational age, prematurity, intrauterine demise, and increased incidence of CHD such as atrial septal defect, TOF, and atrioventricular septal defect.[55]
Patients with moderate RV hypertension and those who had a palliative shunt are at an increased risk for cardiac events during pregnancy and miscarriage. Pregnancy in the setting of unrepaired TOF carries the risk of progressive RV dilatation and congestive heart failure, atrial and ventricular arrhythmias, postpartum hemorrhage, and progressive aortic root dilatation. Maternal causes of death in this patient population include pulmonary hemorrhage, thromboembolism, and brain abscess. Obstetric complications include prematurity, low birth weight, and increased risk of miscarriage.[56]
Mortality in Unrepaired Tetralogy of Fallot
The most frequent causes of mortality in patients with no surgical intervention include hypoxic spells (68%), cerebrovascular accidents (17%), and brain abscesses (13%). Within the first year of life, 25% of infants with severe RVOTO die if left untreated, 40% by 3 years of age, 70% by 10 years of age, and 95% by 40 years of age.[57][58]
Deterrence and Patient Education
Due to advances in surgical correction, TOF is not an uncommon diagnosis among the adult congenital heart disease population. As these patients age, they may require reoperation or transcatheter pulmonary valve replacement, arrhythmia treatment, and lifestyle modification to prevent cardiovascular disease secondary to dyslipidemia and diabetes.[15][59][52]
Most patients ' exercise capacity, measured as peak oxygen uptake and heart rate, is limited after TOF repair. However, patients can participate in all forms of physical activity with individualized guidelines. However, restriction from competitive sports is recommended for TOF patients with severe ventricular dysfunction (ie, EF <40%), severe outflow tract obstruction, or recurrent atrial or ventricular arrhythmias, with the possible exception of low-intensity activities.[20][60]
Pregnancy is generally well tolerated in women with TOF with good underlying hemodynamics. However, in women with important RVOT obstruction, severe pulmonary and or tricuspid regurgitation, as well as right and left ventricular dysfunction, the increased volume load during pregnancy can precipitate RV failure and arrhythmias.[61] There is also a higher incidence of cesarean section, miscarriage, preterm births (14%), and small gestational-age newborns (10%). Therefore, these patients are usually regarded as low to moderate risk. In addition, the chances of producing a fetus with a congenital heart defect are higher than among the general population.[62][63][62]
Enhancing Healthcare Team Outcomes
The diagnosis and management of TOF require an interprofessional team, including pediatric cardiologist specialists (ie, imaging, interventionist, and electrophysiologist), neonatologists, intensivists, pediatric cardiac surgeons, respiratory therapists, nurses, geneticists, nutritionists, physical and occupational therapists, psychologists or family therapists.[54]
In general, patients with TOF require several surgeries. Pulmonary valve replacement via surgical or transcatheter approach will eventually be necessary during early adulthood. These patients require lifelong subacute bacterial endocarditis prophylaxis.[64] Most patients will also require services to help with their neurodevelopmental disabilities, which are being increasingly recognized in patients with CHD. Should a woman with TOF reach childbearing age and decide to become pregnant, a professional team including obstetricians, high-risk perinatologists, adult congenital cardiologists, adult congenital interventionists, and electrophysiologists, as well as pediatric cardiac surgeons, will be required to assist throughout the pregnancy, peripartum and postpartum period.[65]
Media
(Click Image to Enlarge)

Tetralogy of Fallot. The diagram shows a healthy heart on the left and a heart with the 4 anatomic malformations characteristic of the Tetralogy of Fallot on the right.
Mariana Ruiz, Public Domain, via Wikimedia Commons.
References
Barron DJ. Tetralogy of Fallot: controversies in early management. World journal for pediatric & congenital heart surgery. 2013 Apr:4(2):186-91. doi: 10.1177/2150135112471352. Epub [PubMed PMID: 23799733]
Bailliard F, Anderson RH. Tetralogy of Fallot. Orphanet journal of rare diseases. 2009 Jan 13:4():2. doi: 10.1186/1750-1172-4-2. Epub 2009 Jan 13 [PubMed PMID: 19144126]
Karl TR, Stocker C. Tetralogy of Fallot and Its Variants. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016 Aug:17(8 Suppl 1):S330-6. doi: 10.1097/PCC.0000000000000831. Epub [PubMed PMID: 27490619]
Bittel DC, Butler MG, Kibiryeva N, Marshall JA, Chen J, Lofland GK, O'Brien JE Jr. Gene expression in cardiac tissues from infants with idiopathic conotruncal defects. BMC medical genomics. 2011 Jan 5:4():1. doi: 10.1186/1755-8794-4-1. Epub 2011 Jan 5 [PubMed PMID: 21208432]
Level 2 (mid-level) evidenceMatos-Nieves A, Yasuhara J, Garg V. Another Notch in the Genetic Puzzle of Tetralogy of Fallot. Circulation research. 2019 Feb 15:124(4):462-464. doi: 10.1161/CIRCRESAHA.118.314520. Epub [PubMed PMID: 30763217]
Page DJ, Miossec MJ, Williams SG, Monaghan RM, Fotiou E, Cordell HJ, Sutcliffe L, Topf A, Bourgey M, Bourque G, Eveleigh R, Dunwoodie SL, Winlaw DS, Bhattacharya S, Breckpot J, Devriendt K, Gewillig M, Brook JD, Setchfield KJ, Bu'Lock FA, O'Sullivan J, Stuart G, Bezzina CR, Mulder BJM, Postma AV, Bentham JR, Baron M, Bhaskar SS, Black GC, Newman WG, Hentges KE, Lathrop GM, Santibanez-Koref M, Keavney BD. Whole Exome Sequencing Reveals the Major Genetic Contributors to Nonsyndromic Tetralogy of Fallot. Circulation research. 2019 Feb 15:124(4):553-563. doi: 10.1161/CIRCRESAHA.118.313250. Epub [PubMed PMID: 30582441]
Chung IM, Rajakumar G. Genetics of Congenital Heart Defects: The NKX2-5 Gene, a Key Player. Genes. 2016 Jan 23:7(2):. doi: 10.3390/genes7020006. Epub 2016 Jan 23 [PubMed PMID: 26805889]
Villafañe J, Feinstein JA, Jenkins KJ, Vincent RN, Walsh EP, Dubin AM, Geva T, Towbin JA, Cohen MS, Fraser C, Dearani J, Rosenthal D, Kaufman B, Graham TP Jr, Adult Congenital and Pediatric Cardiology Section, American College of Cardiology. Hot topics in tetralogy of Fallot. Journal of the American College of Cardiology. 2013 Dec 10:62(23):2155-66. doi: 10.1016/j.jacc.2013.07.100. Epub 2013 Sep 27 [PubMed PMID: 24076489]
Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL, American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007 Jun 12:115(23):3015-38 [PubMed PMID: 17519398]
Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, Mital S, Priest JR, Pu WT, Roberts A, Ware SM, Gelb BD, Russell MW, American Heart Association Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Genomic and Precision Medicine. Genetic Basis for Congenital Heart Disease: Revisited: A Scientific Statement From the American Heart Association. Circulation. 2018 Nov 20:138(21):e653-e711. doi: 10.1161/CIR.0000000000000606. Epub [PubMed PMID: 30571578]
Wu MH, Lu CW, Chen HC, Kao FY, Huang SK. Adult Congenital Heart Disease in a Nationwide Population 2000-2014: Epidemiological Trends, Arrhythmia, and Standardized Mortality Ratio. Journal of the American Heart Association. 2018 Feb 8:7(4):. doi: 10.1161/JAHA.117.007907. Epub 2018 Feb 8 [PubMed PMID: 29437602]
Level 2 (mid-level) evidenceBertranou EG, Blackstone EH, Hazelrig JB, Turner ME, Kirklin JW. Life expectancy without surgery in tetralogy of Fallot. The American journal of cardiology. 1978 Sep:42(3):458-66 [PubMed PMID: 685856]
Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. The New England journal of medicine. 2000 Feb 3:342(5):334-42 [PubMed PMID: 10655533]
O'Brien P, Marshall AC. Cardiology patient page. Tetralogy of Fallot. Circulation. 2014 Jul 22:130(4):e26-9. doi: 10.1161/CIRCULATIONAHA.113.005547. Epub [PubMed PMID: 25047589]
Diller GP, Kempny A, Liodakis E, Alonso-Gonzalez R, Inuzuka R, Uebing A, Orwat S, Dimopoulos K, Swan L, Li W, Gatzoulis MA, Baumgartner H. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation. 2012 May 22:125(20):2440-6. doi: 10.1161/CIRCULATIONAHA.111.086983. Epub 2012 Apr 11 [PubMed PMID: 22496160]
Mongeon FP, Gurvitz MZ, Broberg CS, Aboulhosn J, Opotowsky AR, Kay JD, Valente AM, Earing MG, Lui GK, Fernandes SM, Gersony DR, Cook SC, Ting JG, Nickolaus MJ, Landzberg MJ, Khairy P, Alliance for Adult Research in Congenital Cardiology (AARCC). Aortic root dilatation in adults with surgically repaired tetralogy of fallot: a multicenter cross-sectional study. Circulation. 2013 Jan 15:127(2):172-9. doi: 10.1161/CIRCULATIONAHA.112.129585. Epub 2012 Dec 6 [PubMed PMID: 23224208]
Level 2 (mid-level) evidencevan der Ven JPG, van den Bosch E, Bogers AJCC, Helbing WA. Current outcomes and treatment of tetralogy of Fallot. F1000Research. 2019:8():. pii: F1000 Faculty Rev-1530. doi: 10.12688/f1000research.17174.1. Epub 2019 Aug 29 [PubMed PMID: 31508203]
Salazar AM, Newth CC, Khemani RG, Jürg H, Ross PA. Pulmonary function testing in infants with tetralogy of Fallot and absent pulmonary valve syndrome. Annals of pediatric cardiology. 2015 May-Aug:8(2):108-12. doi: 10.4103/0974-2069.154152. Epub [PubMed PMID: 26085760]
Wise-Faberowski L, Asija R, McElhinney DB. Tetralogy of Fallot: Everything you wanted to know but were afraid to ask. Paediatric anaesthesia. 2019 May:29(5):475-482. doi: 10.1111/pan.13569. Epub 2019 Apr 15 [PubMed PMID: 30592107]
Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet (London, England). 2009 Oct 24:374(9699):1462-71. doi: 10.1016/S0140-6736(09)60657-7. Epub 2009 Aug 14 [PubMed PMID: 19683809]
Chelliah A, Moon-Grady AJ, Peyvandi S, Chiu JS, Bost JE, Schidlow D, Carroll SJ, Davey B, Divanovic A, Hornberger L, Howley LW, Kavanaugh-McHugh A, Kovalchin JP, Levasseur SM, Lindblade CL, Morris SA, Ngwezi D, Pruetz JD, Puchalski MD, Rychik J, Samai C, Tacy TA, Tworetzky W, Vernon MM, Yeh J, Donofrio MT. Contemporary Outcomes in Tetralogy of Fallot With Absent Pulmonary Valve After Fetal Diagnosis. Journal of the American Heart Association. 2021 Jun 15:10(12):e019713. doi: 10.1161/JAHA.120.019713. Epub 2021 Jun 8 [PubMed PMID: 34098741]
Recker F, Weber EC, Strizek B, Geipel A, Berg C, Gembruch U. Management and outcome of prenatal absent pulmonary valve syndrome. Archives of gynecology and obstetrics. 2022 Nov:306(5):1449-1454. doi: 10.1007/s00404-022-06397-4. Epub 2022 Jan 18 [PubMed PMID: 35043273]
Lumens J, Fan CS, Walmsley J, Yim D, Manlhiot C, Dragulescu A, Grosse-Wortmann L, Mertens L, Prinzen FW, Delhaas T, Friedberg MK. Relative Impact of Right Ventricular Electromechanical Dyssynchrony Versus Pulmonary Regurgitation on Right Ventricular Dysfunction and Exercise Intolerance in Patients After Repair of Tetralogy of Fallot. Journal of the American Heart Association. 2019 Jan 22:8(2):e010903. doi: 10.1161/JAHA.118.010903. Epub [PubMed PMID: 30651018]
Havers-Borgersen E, Butt JH, Smerup M, Gislason GH, Torp-Pedersen C, Gröning M, Schmidt MR, Søndergaard L, Køber L, Fosbøl EL. Incidence of Infective Endocarditis Among Patients With Tetralogy of Fallot. Journal of the American Heart Association. 2021 Nov 16:10(22):e022445. doi: 10.1161/JAHA.121.022445. Epub 2021 Nov 3 [PubMed PMID: 34730003]
Sun HY, Proudfoot JA, McCandless RT. Prenatal detection of critical cardiac outflow tract anomalies remains suboptimal despite revised obstetrical imaging guidelines. Congenital heart disease. 2018 Sep:13(5):748-756. doi: 10.1111/chd.12648. Epub 2018 Jul 18 [PubMed PMID: 30022603]
Mawad W, Mertens LL. Recent Advances and Trends in Pediatric Cardiac Imaging. Current treatment options in cardiovascular medicine. 2018 Feb 21:20(1):9. doi: 10.1007/s11936-018-0599-x. Epub 2018 Feb 21 [PubMed PMID: 29468314]
Level 3 (low-level) evidenceRefaat MM, Ballout J, Mansour M. Ablation of Atrial Fibrillation in Patients with Congenital Heart Disease. Arrhythmia & electrophysiology review. 2017 Dec:6(4):191-194. doi: 10.15420/2017.2017.15.1. Epub [PubMed PMID: 29326834]
Al Habib HF, Jacobs JP, Mavroudis C, Tchervenkov CI, O'Brien SM, Mohammadi S, Jacobs ML. Contemporary patterns of management of tetralogy of Fallot: data from the Society of Thoracic Surgeons Database. The Annals of thoracic surgery. 2010 Sep:90(3):813-9; discussion 819-20. doi: 10.1016/j.athoracsur.2010.03.110. Epub [PubMed PMID: 20732501]
Level 2 (mid-level) evidenceSandoval JP, Chaturvedi RR, Benson L, Morgan G, Van Arsdell G, Honjo O, Caldarone C, Lee KJ. Right Ventricular Outflow Tract Stenting in Tetralogy of Fallot Infants With Risk Factors for Early Primary Repair. Circulation. Cardiovascular interventions. 2016 Dec:9(12):. pii: e003979. Epub [PubMed PMID: 27965298]
Balzer D. Pulmonary Valve Replacement for Tetralogy of Fallot. Methodist DeBakey cardiovascular journal. 2019 Apr-Jun:15(2):122-132. doi: 10.14797/mdcj-15-2-122. Epub [PubMed PMID: 31384375]
Bauser-Heaton H, Borquez A, Han B, Ladd M, Asija R, Downey L, Koth A, Algaze CA, Wise-Faberowski L, Perry SB, Shin A, Peng LF, Hanley FL, McElhinney DB. Programmatic Approach to Management of Tetralogy of Fallot With Major Aortopulmonary Collateral Arteries: A 15-Year Experience With 458 Patients. Circulation. Cardiovascular interventions. 2017 Apr:10(4):. pii: e004952. doi: 10.1161/CIRCINTERVENTIONS.116.004952. Epub [PubMed PMID: 28356265]
Jonas RA. Surgical Management of Absent Pulmonary Valve Syndrome. World journal for pediatric & congenital heart surgery. 2016 Sep:7(5):600-4. doi: 10.1177/2150135116651838. Epub [PubMed PMID: 27587495]
Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, Landzberg MJ, Mulder B, Powell AJ, Wald R, Geva T. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart (British Cardiac Society). 2014 Feb:100(3):247-53. doi: 10.1136/heartjnl-2013-304958. Epub 2013 Oct 31 [PubMed PMID: 24179163]
Level 2 (mid-level) evidenceGeva T. Tetralogy of Fallot repair: ready for a new paradigm. The Journal of thoracic and cardiovascular surgery. 2012 Jun:143(6):1305-6. doi: 10.1016/j.jtcvs.2012.01.076. Epub 2012 Feb 18 [PubMed PMID: 22342479]
Yim D, Riesenkampff E, Caro-Dominguez P, Yoo SJ, Seed M, Grosse-Wortmann L. Assessment of Diffuse Ventricular Myocardial Fibrosis Using Native T1 in Children With Repaired Tetralogy of Fallot. Circulation. Cardiovascular imaging. 2017 Mar:10(3):. pii: e005695. doi: 10.1161/CIRCIMAGING.116.005695. Epub [PubMed PMID: 28292861]
Tretter JT, Redington AN. Risk Factors and Biomarkers of Poor Outcomes: Time to Throw Out Right Ventricular Volumes in Repaired Tetralogy of Fallot? Lessons From the INDICATOR Cohort. Circulation. 2018 Nov 6:138(19):2116-2118. doi: 10.1161/CIRCULATIONAHA.118.037000. Epub [PubMed PMID: 30474414]
Chen CA, Dusenbery SM, Valente AM, Powell AJ, Geva T. Myocardial ECV Fraction Assessed by CMR Is Associated With Type of Hemodynamic Load and Arrhythmia in Repaired Tetralogy of Fallot. JACC. Cardiovascular imaging. 2016 Jan:9(1):1-10. doi: 10.1016/j.jcmg.2015.09.011. Epub 2015 Dec 9 [PubMed PMID: 26684969]
Hui W, Slorach C, Dragulescu A, Mertens L, Bijnens B, Friedberg MK. Mechanisms of right ventricular electromechanical dyssynchrony and mechanical inefficiency in children after repair of tetralogy of fallot. Circulation. Cardiovascular imaging. 2014 Jul:7(4):610-8. doi: 10.1161/CIRCIMAGING.113.001483. Epub 2014 May 1 [PubMed PMID: 24785673]
Andrade AC, Jerosch-Herold M, Wegner P, Gabbert DD, Voges I, Pham M, Shah R, Hedderich J, Kramer HH, Rickers C. Determinants of Left Ventricular Dysfunction and Remodeling in Patients With Corrected Tetralogy of Fallot. Journal of the American Heart Association. 2019 Sep 3:8(17):e009618. doi: 10.1161/JAHA.118.009618. Epub 2019 Aug 31 [PubMed PMID: 31474177]
Moon TJ, Choueiter N, Geva T, Valente AM, Gauvreau K, Harrild DM. Relation of biventricular strain and dyssynchrony in repaired tetralogy of fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. The American journal of cardiology. 2015 Mar 1:115(5):676-80. doi: 10.1016/j.amjcard.2014.12.024. Epub 2014 Dec 18 [PubMed PMID: 25727084]
Level 2 (mid-level) evidenceGhonim S, Gatzoulis MA, Ernst S, Li W, Moon JC, Smith GC, Heng EL, Keegan J, Ho SY, McCarthy KP, Shore DF, Uebing A, Kempny A, Alpendurada F, Diller GP, Dimopoulos K, Pennell DJ, Babu-Narayan SV. Predicting Survival in Repaired Tetralogy of Fallot: A Lesion-Specific and Personalized Approach. JACC. Cardiovascular imaging. 2022 Feb:15(2):257-268. doi: 10.1016/j.jcmg.2021.07.026. Epub 2021 Oct 13 [PubMed PMID: 34656466]
Krieger EV, Zeppenfeld K, DeWitt ES, Duarte VE, Egbe AC, Haeffele C, Lin KY, Robinson MR, Sillman C, Upadhyay S, American Heart Association Adults With Congenital Heart Disease Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young and Council on Clinical Cardiology. Arrhythmias in Repaired Tetralogy of Fallot: A Scientific Statement From the American Heart Association. Circulation. Arrhythmia and electrophysiology. 2022 Nov:15(11):e000084. doi: 10.1161/HAE.0000000000000084. Epub 2022 Oct 20 [PubMed PMID: 36263773]
Dennis M, Moore B, Kotchetkova I, Pressley L, Cordina R, Celermajer DS. Adults with repaired tetralogy: low mortality but high morbidity up to middle age. Open heart. 2017:4(1):e000564. doi: 10.1136/openhrt-2016-000564. Epub 2017 Mar 1 [PubMed PMID: 28698799]
Bokma JP, Geva T, Sleeper LA, Lee JH, Lu M, Sompolinsky T, Babu-Narayan SV, Wald RM, Mulder BJM, Valente AM. Improved Outcomes After Pulmonary Valve Replacement in Repaired Tetralogy of Fallot. Journal of the American College of Cardiology. 2023 May 30:81(21):2075-2085. doi: 10.1016/j.jacc.2023.02.052. Epub [PubMed PMID: 37225360]
Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, Feltes TF, Foster E, Hinoki K, Ichord RN, Kreutzer J, McCrindle BW, Newburger JW, Tabbutt S, Todd JL, Webb CL, American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Stroke Council. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2013 Dec 17:128(24):2622-703. doi: 10.1161/01.cir.0000436140.77832.7a. Epub 2013 Nov 13 [PubMed PMID: 24226806]
Mouws EMJP, de Groot NMS, van de Woestijne PC, de Jong PL, Helbing WA, van Beynum IM, Bogers AJJC. Tetralogy of Fallot in the Current Era. Seminars in thoracic and cardiovascular surgery. 2019 Autumn:31(3):496-504. doi: 10.1053/j.semtcvs.2018.10.015. Epub 2018 Nov 2 [PubMed PMID: 30395964]
He D, Sznycer-Taub N, Cheng Y, McCarter R, Jonas RA, Hanumanthaiah S, Moak JP. Magnesium Lowers the Incidence of Postoperative Junctional Ectopic Tachycardia in Congenital Heart Surgical Patients: Is There a Relationship to Surgical Procedure Complexity? Pediatric cardiology. 2015 Aug:36(6):1179-85. doi: 10.1007/s00246-015-1141-5. Epub 2015 Mar 12 [PubMed PMID: 25762470]
Level 2 (mid-level) evidenceSahu MK, Das A, Siddharth B, Talwar S, Singh SP, Abraham A, Choudhury A. Arrhythmias in Children in Early Postoperative Period After Cardiac Surgery. World journal for pediatric & congenital heart surgery. 2018 Jan:9(1):38-46. doi: 10.1177/2150135117737687. Epub [PubMed PMID: 29310559]
Hoffman TM, Bush DM, Wernovsky G, Cohen MI, Wieand TS, Gaynor JW, Spray TL, Rhodes LA. Postoperative junctional ectopic tachycardia in children: incidence, risk factors, and treatment. The Annals of thoracic surgery. 2002 Nov:74(5):1607-11 [PubMed PMID: 12440616]
Gatzoulis MA, Till JA, Redington AN. Depolarization-repolarization inhomogeneity after repair of tetralogy of Fallot. The substrate for malignant ventricular tachycardia? Circulation. 1997 Jan 21:95(2):401-4 [PubMed PMID: 9008456]
Cuypers JA, Menting ME, Konings EE, Opić P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Boersma E, Bogers AJ, Roos-Hesselink JW. Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation. 2014 Nov 25:130(22):1944-53. doi: 10.1161/CIRCULATIONAHA.114.009454. Epub 2014 Oct 23 [PubMed PMID: 25341442]
Level 2 (mid-level) evidenceCabalka AK, Asnes JD, Balzer DT, Cheatham JP, Gillespie MJ, Jones TK, Justino H, Kim DW, Lung TH, Turner DR, McElhinney DB. Transcatheter pulmonary valve replacement using the melody valve for treatment of dysfunctional surgical bioprostheses: A multicenter study. The Journal of thoracic and cardiovascular surgery. 2018 Apr:155(4):1712-1724.e1. doi: 10.1016/j.jtcvs.2017.10.143. Epub 2017 Dec 6 [PubMed PMID: 29395214]
Level 2 (mid-level) evidenceNiwa K. Metabolic syndrome and coronary artery disease in adults with congenital heart disease. Cardiovascular diagnosis and therapy. 2021 Apr:11(2):563-576. doi: 10.21037/cdt-20-781. Epub [PubMed PMID: 33968634]
Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH Jr, Li J, Smith SE, Bellinger DC, Mahle WT, American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012 Aug 28:126(9):1143-72 [PubMed PMID: 22851541]
Balci A, Drenthen W, Mulder BJ, Roos-Hesselink JW, Voors AA, Vliegen HW, Moons P, Sollie KM, van Dijk AP, van Veldhuisen DJ, Pieper PG. Pregnancy in women with corrected tetralogy of Fallot: occurrence and predictors of adverse events. American heart journal. 2011 Feb:161(2):307-13. doi: 10.1016/j.ahj.2010.10.027. Epub 2011 Jan 15 [PubMed PMID: 21315213]
Level 2 (mid-level) evidenceWang K, Xin J, Wang X, Yu H, Liu X. Pregnancy outcomes among 31 patients with tetralogy of Fallot, a retrospective study. BMC pregnancy and childbirth. 2019 Dec 10:19(1):486. doi: 10.1186/s12884-019-2630-y. Epub 2019 Dec 10 [PubMed PMID: 31823779]
Level 2 (mid-level) evidencePhillips S, Pirics M. Congenital Heart Disease and Reproductive Risk: An Overview for Obstetricians, Cardiologists, and Primary Care Providers. Methodist DeBakey cardiovascular journal. 2017 Oct-Dec:13(4):238-242. doi: 10.14797/mdcj-13-4-238. Epub [PubMed PMID: 29744016]
Level 3 (low-level) evidenceTownsley MM, Windsor J, Briston D, Alegria J, Ramakrishna H. Tetralogy of Fallot: Perioperative Management and Analysis of Outcomes. Journal of cardiothoracic and vascular anesthesia. 2019 Feb:33(2):556-565. doi: 10.1053/j.jvca.2018.03.035. Epub 2018 Mar 24 [PubMed PMID: 29706570]
Morray BH, McElhinney DB, Boudjemline Y, Gewillig M, Kim DW, Grant EK, Bocks ML, Martin MH, Armstrong AK, Berman D, Danon S, Hoyer M, Delaney JW, Justino H, Qureshi AM, Meadows JJ, Jones TK. Multicenter Experience Evaluating Transcatheter Pulmonary Valve Replacement in Bovine Jugular Vein (Contegra) Right Ventricle to Pulmonary Artery Conduits. Circulation. Cardiovascular interventions. 2017 Jun:10(6):. pii: e004914. doi: 10.1161/CIRCINTERVENTIONS.116.004914. Epub [PubMed PMID: 28600328]
Van Hare GF, Ackerman MJ, Evangelista JA, Kovacs RJ, Myerburg RJ, Shafer KM, Warnes CA, Washington RL, American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 4: Congenital Heart Disease: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation. 2015 Dec 1:132(22):e281-91. doi: 10.1161/CIR.0000000000000240. Epub 2015 Nov 2 [PubMed PMID: 26621645]
Quattrone A, Lie OH, Nestaas E, de Lange C, Try K, Lindberg HL, Skulstad H, Erikssen G, Edvardsen T, Haugaa K, Estensen ME. Impact of pregnancy and risk factors for ventricular arrhythmias in women with tetralogy of Fallot. Open heart. 2021 Jan:8(1):. doi: 10.1136/openhrt-2020-001400. Epub [PubMed PMID: 33414183]
Pedersen LM, Pedersen TA, Ravn HB, Hjortdal VE. Outcomes of pregnancy in women with tetralogy of Fallot. Cardiology in the young. 2008 Aug:18(4):423-9. doi: 10.1017/S1047951108002345. Epub 2008 Jun 18 [PubMed PMID: 18559134]
Level 2 (mid-level) evidenceVeldtman GR, Connolly HM, Grogan M, Ammash NM, Warnes CA. Outcomes of pregnancy in women with tetralogy of Fallot. Journal of the American College of Cardiology. 2004 Jul 7:44(1):174-80 [PubMed PMID: 15234429]
Diller GP, Baumgartner H. Endocarditis in adults with congenital heart disease: new answers-new questions. European heart journal. 2017 Jul 7:38(26):2057-2059. doi: 10.1093/eurheartj/ehx044. Epub [PubMed PMID: 28329113]
Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, Mital S, Rose C, Silversides C, Stout K, American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017 Feb 21:135(8):e50-e87. doi: 10.1161/CIR.0000000000000458. Epub 2017 Jan 12 [PubMed PMID: 28082385]
Level 2 (mid-level) evidence