Introduction
The relationship between infections and renal calculi has been known since the time of Hippocrates, but it was not until 1817 that Marcet identified the connection between urinary alkalinity, infection, phosphate calculi, and increased urinary ammonia. In 1901, Brown first suggested that urea splitting bacteria activity increased urinary ammonia production, which was the immediate cause of higher urinary alkalinity levels and phosphate (struvite) stone formation. The 1926 description of urease, the first enzyme ever isolated and purified, earned Sumner the Nobel Prize for Chemistry in 1946. Struvite was first discovered in bat droppings by Swedish geologist Georg Ulex who named it after his friend, Russian diplomat, and naturalist, Baron von Struve.
Struvite is a crystalline compound made up of magnesium ammonium phosphate (MgNH4PO4.6H20). Struvite stones are actually a mixture composed of three cations (calcium, magnesium, ammonium) and one anion (phosphate). They are therefore also known as triple phosphate stones even though pure struvite actually contains no calcium. The mixture is composed of struvite (MgNH4PO4.6H20) and calcium phosphate (Ca10.[PO4]6.CO3); hence carbonate ions are also usually found.[1] They can form in the kidney or bladder in patients with catheters or urinary stasis. For example, about 8% of patients with spinal cord lesions will form stones, and 98% of these will be struvite. If left untreated, renal struvite stones have a tendency to grow more rapidly compared to calcium-based stones and may eventually fill up the entire collecting system. This can lead to a staghorn stone or branched calculus formation. Among all stone formers, the percentage of struvite stones was found to be 5% to 15%.[2]
Struvite stones only form in alkaline environments (pH >7) and are always associated with urinary tract infections from urease-producing bacteria such as Proteus. Virtually all infectious urinary organisms make urease except E. coli, Citrobacter freundii, Streptococci, and Enterococci. It should be pointed out that staghorn stones, while predominantly made of struvite or triple phosphate, may also be composed of mixtures of calcium oxalate, calcium phosphate, uric acid, and cystine. However, for the purpose of this review, we shall focus exclusively on struvite staghorn calculi.
Staghorn stones are also defined as "partial" or "complete." A "partial" staghorn stone would include at least two calyces, while "complete" would indicate that at least 80% of the renal collecting system was involved.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Metabolic derangements and urinary infections are the two major causes of any renal stone formation. Struvite calculi are closely related to urinary tract infections (UTI); hence they are also known as infection stones. Infection or colonization by urease-producing organisms like Proteus, Klebsiella, Staphylococcus, Pseudomonas, Providentia, Serratia, and Morganella can cause struvite stones.[3] The urease-producing capacity varies between different organisms. For example, 100% of Proteus species produce urease while only 1.4% of Escherichia coli are thought to produce the enzyme. Therefore, Escherichia coli is considered the least likely cause of struvite stone formation.[4]
In general, patients with infection stones are less likely to have a concurrent metabolic abnormality contributing to nephrolithiasis than non-struvite stone formers.[5] However, we still recommend 24-hour urine testing in motivated struvite nephrolithiasis patients as a significant percentage, at least 50%, will demonstrate metabolic abnormalities when tested.[6][7][8] The most common metabolic defects found are hypercalciuria, hypocitraturia, and hypernatremia.[7]
Kang et al. reviewed 70 staghorn stone patients after percutaneous nephrolithotomy. After 3 years, those who had undergone a metabolic evaluation and took specific prophylactic therapy after surgery had a stone formation rate 98% less than the rate in those who did not have metabolic testing or specific preventive treatment.[9]
Epidemiology
Struvite stones occur much more commonly in females. This is explained by the much greater likelihood of UTIs in women and the close association of UTIs and struvite stone formation.[10] Among first-time struvite stone formers, the female to male ratio was found to be 3:1. Struvite stones were also common in patients with gross hematuria, advanced age, hypertension, fever, urinary tract infections, urinary diversion surgery, neurogenic bladder, indwelling catheters, medullary sponge kidney, distal tubular acidosis, diabetes, and low serum phosphorous levels. Besides patients with anatomic abnormalities that lead to urinary stasis, like congenital urinary malformations and obstruction of the ureteropelvic junction, a persistently hydronephrotic renal pelvis is also known to form struvite stones.[11][12] Triple phosphate stones are also common in patients who have undergone bladder augmentation surgery due to the prolonged urinary stasis that is found in these patients.[13]
In the United States, about 5% to 15% of all renal stones are composed of struvite, while worldwide, this percentage goes up to 30% of all nephrolithiasis cases.
While it was previously thought that asymptomatic struvite stones were relatively harmless, more recent studies have demonstrated that 30% of patients treated conservatively without surgery ultimately died of sepsis or renal failure. Untreated, struvite stones have shown a 15-year overall survival rate of only 41%.[14]
While the majority of struvite stones are unilateral, up to 15% may be bilateral.[12]
Struvite stones also cause prolonged irritation, infection, and chronic inflammation, which can rarely result in squamous cell carcinoma of the renal pelvis or collecting system. These cancers, while rare, have an average 5-year survival rate of less than 10% and are considered quite dangerous.[15]
Pathophysiology
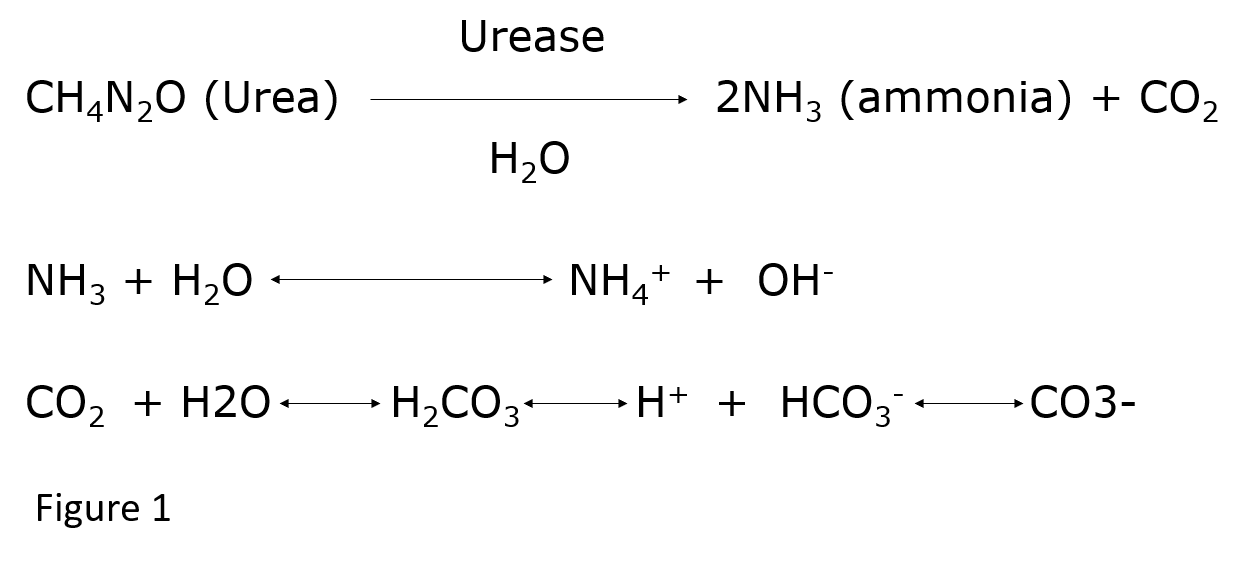
Urease produced by bacteria splits urinary urea into ammonia and carbon dioxide (CO2). Further hydrolysis leads to the production of two ammonium and one bicarbonate ion (Figure1) for each urea molecule.[1] This results in very alkaline urine not achievable under normal physiologic conditions. The high level of urinary alkalinity promotes supersaturation of magnesium ammonium phosphate and calcium phosphate (apatite).[5]
The highly alkaline urine also damages the glycosaminoglycans layer that normally protects the urothelial surface and cells from bacteria. This leads to the formation of bacterial biofilms in which precipitation of struvite and apatite crystals occur. As the bacteria secrete extracellular polysaccharides, the original nidus continues to grow, leading to the formation of a stone taking the shape of the renal pelvis, infundibula, and calyces, consisting of multiple layers of struvite stone material held together by biofilms.[4] Chemically, the critical conditions for struvite to form (other than the presence of an infection) include a urinary pH >7.2 and an increased level of urinary ammonia. The solubility of struvite significantly increases as the pH drops below 6.5.[16]
The most accurate way to identify the specific pathogen contributing to struvite stone formation is a stone culture. If this is not available or possible, a urine culture should be done from a specimen obtained directly from the kidney at the time of surgery.
Even if the current and most recent urine cultures are negative, at least one positive culture for a urea splitting organism can usually be found by checking earlier urine cultures up to 1 year prior to struvite stone surgery.[17]
Histopathology
Struvite stones are known to demonstrate "coffin-lid" crystals on urine microscopy. An in vitro experiment with urease-producing bacteria revealed that crystallization of struvite evolves from an initial arrow-head morphology to an X-shaped structure before finally exhibiting the "coffin-lid" crystalline morphology at the end of the third day.[18] Laboratory analysis of the spontaneously passed or extracted stone material will show magnesium ammonium phosphate crystals.
History and Physical
There are no symptoms that are specific to struvite stones. Patients commonly show general signs and symptoms of renal calculi like flank pain, dysuria, and hematuria, apart from urinary tract infections like fever and chills. Urinary pH will be persistently elevated (>7.2). Some patients can present with chronic pyelonephritis when an infected stone causes focal areas of renal parenchymal scarring.
Chronic flank pain and relapsing urinary tract infections (recurrent infections with the same organism) suggest the presence of a staghorn struvite stone. Patients may also be totally asymptomatic even when the stones involve the entire renal pelvis. Advanced cases in individuals with large struvite stones may demonstrate general symptoms such as fatigue, lethargy, malaise, intermittent fevers, loss of appetite, and weight loss.
Staghorn stones, even very large ones, do not typically cause the very severe type of renal colic that is commonly associated with classic acute ureteral obstruction from urinary calculi. This is because large staghorn stones may not cause acute urinary tract obstruction with ureteral and renal capsular stretching from dilation and hydronephrosis typical of most other symptomatic calculi.
Evaluation
A urinalysis and urine culture should be done. Urinalysis will typically show an alkaline pH (>7.2) and crystals with a ''coffin-lid'' morphology. Urine culture usually reveals the growth of urease-forming organisms. Non-contrast Computed Tomography (CT) scan is the gold standard for the diagnosis of any renal stone and is particularly useful for staghorn stones. A contrast-enhanced CT can be added to better evaluate the internal renal anatomy, such as the calyces and infundibulae. When a CT scan is not available, ultrasonography with a plain radiograph of the abdomen and pelvis can be used. Struvite stones are usually radio-opaque due to their calcium content. A typical struvite stone will demonstrate a density reading on CT of 900 Hounsfield units or less.[19][20]
A large struvite stone may be seen as a staghorn or branched calculus filling the collecting system in the plain radiograph. Intravenous pyelography is less commonly used for diagnosis, while magnetic resonance imaging does provide much assistance in visualizing staghorn stones and is usually not recommended. Spontaneous passage of a struvite stone is rare, but occasionally, a small piece may break off and be passed or found in the bladder. Analysis of any passed or removed stone material is routinely performed as a part of good medical management.[21]
Treatment / Management
Dietary manipulation has generally not been highly successful in dissolving struvite stones in humans. The suggested diet would need to be low calcium and low phosphorus with the possible addition of aluminum hydroxide (to help reduce phosphorus absorption) and a urinary acidifier, such as ammonium chloride. Estrogen supplementation has also been recommended for appropriate individuals. Such a diet has serious potential risks of inducing hypercalciuria and aluminum toxicity, although a similar diet has been shown to be reasonably successful in cats who often make struvite urinary calculi.[22] Still, there are some studies suggesting that a modified diet can be effective in reducing the risk of staghorn stones in humans.[23]
L-methionine has been suggested to increase urinary acidification and possibly reduce the risk of struvite stones. In one study, the addition of 1500 mg of L-methionine daily decreased the urinary supersaturation ratio for struvite by 34% in healthy volunteers and significantly lowered their urinary pH.[24] Another small study showed that long-term use of L-methionine supplementation substantially reduced the expected high recurrence rate of struvite stones.[25] At this point, the information available on L-methionine supplementation is interesting but insufficient to recommend its routine use in struvite stone formers until more definitive studies have been done.
There are limited data to guide the non-surgical treatment of staghorn calculi other than culture-specific antibiotics. All patients suspected to have infection stones should be started on initial antibiotics regimens like cefepime, amoxicillin-clavulanate, or ciprofloxacin. Culture-specific antibiotics should be substituted when the data is available. Suppressive antibiotic therapy alone can significantly reduce the risk of urinary tract infections (UTIs), pyelonephritis, and sepsis while limiting further stone growth. While unlikely to dissolve the stone completely, partial dissolution from antibiotic therapy alone has been reported.[26][27] Therefore, antibiotic therapy should be viewed as a prophylactic treatment to inhibit serious infections and reduce stone growth.(B3)
Medical management of struvite stones only provides a relatively modest benefit and is rarely successful alone. However, a recent meta-analysis has revealed that conservative management of staghorn calculus might not be as unreasonable as previously thought.[7][28] Still, definitive medical management of struvite stones should be reserved only for patients who are too sick for surgery, refuse surgical stone removal, or after maximal surgical extraction has been accomplished. Long-term antibiotic prophylaxis may be appropriate for some of these patients.[29]
Acetohydroxamic acid (AHA) is a urease inhibitor that has been shown to decrease struvite stone growth and recurrence rates.[30][31] AHA has no direct acidifying effect, and it is not an antibiotic. It should be used together with culture-specific antibiotics and is often synergistic with them. It has a high renal clearance and can easily pass through bacterial cell walls. AHA will decrease urinary alkalinity and lower urinary ammonia levels through its effect on blocking the hydrolysis of urea. The usual dose is 250-500 mg TID. It can be safely used in patients with mild renal failure at a reduced rate but should not be used where serum creatinine levels are above 2.5 mg/dL due to increased toxicity and reduced efficacy.[32](A1)
The American Urological Association guidelines recommend using AHA only after all surgical options are exhausted for residual or recurrent struvite stones. The use of AHA has been limited by significant side effects like nausea, vomiting, tremulousness, edema, diarrhea, headache, loss of taste, rash, hallucinations, phlebitis, deep vein thrombosis, anemia, reticulocytosis, and hemolytic anemia. About 20% of patients treated with AHA will develop a significant side effect from the medication and discontinue use. The use of oral ammonium chloride for urinary acidification is theoretically attractive but has been shown to have only limited long-term clinical usefulness and risks serious side effects such as metabolic acidosis.[29]
There is no question that the optimal treatment for struvite stones is the complete surgical removal of all stone material. Struvite calculi tend to grow rapidly so that any remaining stone fragments can serve as a nidus for new stone formation. Recurrence rates are notoriously high at about 85% if residual fragments are left behind. Even with complete stone extraction, recurrence rates for struvite stones are reported as 10%. Antibiotics alone are relatively ineffective in controlling urinary infections if infected struvite stone fragments remain.[33][34](B2)
Extracorporeal shockwave lithotripsy: Shock-wave lithotripsy (SWL) should be considered only when the stone size is less than 2.5 cm with a non-dilated collecting system, or in the pediatric population.[35] When SWL is used, either a double J stent or percutaneous nephrostomy tube should be placed before treatment initiation to ensure adequate drainage. A percutaneous nephrostomy is necessary for adequate renal drainage in up to 40% of cases.[36] At least 50% of staghorn stone patients treated with SWL will require at least one more surgical treatment to become stone-free. Patients with wide infundibulae that allow easier transit of small stones are better candidates for SWL treatment than similar patients with narrow infundibulae, which will limit or restrict the passage of small stone fragments. Patients should be informed that multiple procedures may be needed to make them stone-free. Overall stone-free rates for staghorn patients treated with SWL alone is about 60%, but in those with smaller stones, this rate goes up to 90%.(A1)
Percutaneous nephrolithotomy: Percutaneous nephrolithotomy (PCNL) is generally considered the gold standard for the surgical treatment of most large staghorn calculi. The procedure begins with the creation of a percutaneous tract from the skin to the renal collecting system. This allows for the passage of instruments, including rigid and flexible scopes, lasers, ultrasonic and pneumatic lithotrites, graspers, guide wires, etc. Ultrasound or fluoroscopy is used to guide the initial placement of the tract, which is typically 24Fr to 30 Fr in size. Multiple tracts may be necessary for larger, complex, branched stones. A prone flexed prone or supine position may be used instead of the traditional flat prone position.[37][38] The stone-free rate after PCNL alone is about 80%.
Rarely, if PCNL is not feasible due to anatomic abnormalities like pelvic kidney, spinal deformities, or retro-renal colon, open surgery like atrophic nephrolithotomy might be considered. Surgery should also be considered in the morbidly obese patient, in whom endoscopy and fluoroscopy are difficult. The acute complications rate is lowest for PCNL and highest for open surgery and SWL. Some of the common acute complications associated with stone removal procedures are perforation of the renal pelvis, perinephric hematoma, significant blood loss, hydrothorax, pneumothorax, sepsis, renal impairment, and wound infection, among others. Long-term complications include stone recurrence, renal impairment, and growth of residual stone fragments. Antibiotics should be continued postoperatively. However, there are no current guidelines regarding specific regimen, dose, or duration.
When the stone is big, and/or fragments are not accessible by PCNL, a combination of PCNL and SWL can be used.[39][40] Ureteroscopy can also be a useful adjunct to other surgical procedures. In such circumstances, usually, a PCNL is the final procedure done to ensure the removal of all residual stones. Combination therapy is reasonable to ensure complete stone removal. Using PCNL followed by SWL and then another PCNL constitutes the "sandwich" technique and is one of the recommended methods for dealing with particularly large stones.[41] The general principle in the surgical management of struvite, staghorn stones is eliminating all the struvite stone material. To help achieve this, postoperative antibiotics and AHA can be helpful.
Tranexamic acid is a reversible blocker of lysine sites on plasminogen molecules. It is normally used for hemorrhage control in patients with nose bleeds and heavy menstrual periods. Several studies have suggested that the administration of tranexamic acid (1 gram at induction followed by 500 mg every 8 hours x 3) can help reduce blood loss associated with percutaneous nephrolithotomy, and we now recommend it except in patients with renal failure where it is contraindicated.[12][42][43][44] (A1)
Various nomograms have been developed to help urologists predict surgical outcomes after PCNL. Of the three nomograms currently available (CROES, Guy's, and STONE), the STONE nomogram achieved the highest predictive accuracy, especially when combined with the presence of any pre-existing urinary tract infections and the number of involved calyces.[45][46][47](A1)
Checking for stone fragments after PCNL with CT or by ultrasound together with plain radiographs (KUB) is critical in identifying residual stone fragments after surgery.[12] Another approach is to use intraoperative flexible nephroscopy together with high-resolution fluoroscopy. This technique has been shown to find 72% to 78% of all remaining stone fragments, which can then be removed or treated later with a separate procedure.[48]
Dissolving struvite stones: The use of ion exchange resins and acidifying solutions for direct irrigation of staghorn stones is theoretically an attractive option for chemo-dissolution of struvite calculi. By chemically exchanging magnesium in the irrigation fluid for calcium in the stones within an acidic environment, the stone material becomes more soluble. While there is evidence that this technique can work to dissolve struvite stones, conditions must be optimal to avoid serious side effects. The urine must be kept sterile, serum phosphate and magnesium must be checked regularly, and the renal pelvic pressure must be kept low to avoid serious hypermagnesemia. Antibiotics are recommended before, during, and after therapy.[49] Patients with renal failure are particularly prone to hypermagnesemia. Due to the difficulties involved and the strict criteria, chemo-dissolution of struvite stones is performed relatively infrequently.[49][50][51]
Open surgery: Traditional open surgery for staghorn stones is rarely performed currently, although it was the gold standard years ago. Today, it is only performed when the intrarenal anatomy does not allow any other approach or the intention is to remove the kidney.
Followup imaging and periodic urine cultures after struvite stone surgery is recommended at 6 to 12 months, although this can be done more often at 3 to 6 months in patients who've demonstrated rapid or frequent stone recurrences.[12]
Summary of Current AUA/Endourological Society Guidelines for the Management of Struvite/Staghorn Stones (Modified)[52][53]
- A non-contrast computed tomography (CT) scan should be obtained prior to performing percutaneous nephrolithotomy (PCNL) and to assist in selecting the best surgical option between shockwave lithotripsy (SWL) and ureteroscopy.
- If a significant loss of renal function is suspected in one or both kidneys, a functional imaging study (DPTA or MAG-3) renal nuclear scan may be obtained.
- A urinalysis should be done prior to urological instrumentation, and urine culture should be obtained in patients with struvite/staghorn stones or whenever there are laboratory or clinical signs of a possible infection.
- Preoperative laboratories for urologic procedures like PCNL, where there is a significant risk of bleeding or in patients known to have anemia, thrombocytopenia, or infection, should include a CBC and platelet count.
- Serum creatinine and electrolytes should be checked if there is any history or suspicion of renal failure.
- Additional imaging with contrast may be obtained in patients with complex stones or unusual anatomy if better visualization of the renal collecting system and/or ureter is needed.
- PCNL is the recommended initial treatment for staghorn patients who are symptomatic with stones >20 mm.
- PCNL is also recommended as the initial treatment for lower pole stones >10 mm as PCNL has a higher stone-free rate, although it is more invasive with higher morbidity than SWL.
- SWL should generally not be offered as the preferred initial therapy to staghorn patients with stones >20 mm or with lower pole stones >10 mm as PCNL has a higher stone-free rate but is more invasive with higher morbidity.
- If an involved kidney has a minimal or negligible function, a nephrectomy should be considered.
- Stone treatment may be considered in patients with flank pain and non-obstructing, calyceal stones where there is no other obvious cause of the pain.
- Standard PCNL should routinely include flexible nephroscopy.
- Ureteroscopy and PCNL should only use normal saline for irrigation.
- Patients with a staghorn or large renal stones who are not candidates for PCNL for some reason may be offered ureteroscopy as a staged procedure.
- After SWL, alpha-blocker medications can be used to facilitate the passage of stone fragments.
- Stone patients with functional or anatomic ureteral restrictions or any untreated obstructions of the ureter distal to the stones should not undergo SWL surgery.
- All urinary stone material collected should be sent for chemical analysis.
- Staghorn stones should undergo complete removal whenever possible and safe to do so.
- Endoscopic procedures should be offered to patients as needed to make them totally stone-free, particularly when residual infected stone fragments remain.
- Robotic, laparoscopic and open surgery for stones should be considered as the recommended initial treatment only in cases of significant anatomical abnormalities, particularly complex or large calculi, and those patients where urinary tract reconstruction will be performed concomitantly.
- Most endoscopic procedures should be performed with a safety guidewire in place.
- Antibiotics should be used before stone surgical procedures. Antibiotic selection should be based on prior urine cultures, the local antibiogram and comply with the latest Best Practice Policy Statement.
- When performing an endoscopic stone intervention, purulent urine may be encountered. When this happens, the procedure should be stopped immediately, and appropriate urinary drainage established via nephrostomy or double J stenting. A urine sample should be sent for culture, and antibiotics should be continued. Antibiotics should be changed as appropriate based on the culture results when they are available.
- Ureteroscopy is the preferred modality for treating most stone patients with uncorrected bleeding disorders and for those who are cannot safely stop their anticoagulation therapy.
- Endoscopic therapy is recommended if initial therapy with SWL fails.
Differential Diagnosis
Blood clots can mimic flank pain caused by any renal stone in the urinary tract, pyelonephritis, ectopic pregnancy, rupture or torsion of an ovarian cyst, aortic aneurysm, biliary colic, cholecystitis, appendicitis, acute mesenteric ischemia, ureteropelvic junction obstruction, and dysmenorrhea.
Suitable imaging techniques like CT scans can differentiate renal stones from these entities. Hematuria will be absent if the cause of abdominal pain is gastrointestinal. The location, appearance, and density may help identify stone composition in a CT scan. For example, a large staghorn-shaped calculus in the renal pelvis favors struvite stone. Like cystine and uric acid stones, struvite stones are radiopaque in nature, but they are not as dense as calcium stones.[54] Laboratory analysis of the passed stone helps differentiate the various types of stones.
Prognosis
Staghorn calculi cause significant morbidity and mortality. The overall rate of renal deterioration with or without definitive treatment was as high as 28%.[55] Patients with complete staghorn calculi, urinary diversions, solitary kidneys, and those with a prior history of renal stones, hypertension, and neurogenic bladder were more likely to have poor renal outcomes.
Historically, during a 10-year follow-up, the mortality rate of staghorn calculus was found to be 7.2% in patients who underwent stone-removal procedures compared to 28% in those who were managed conservatively.[56] However, more recent studies have shown a very low overall mortality rate of <1% for PCNL and SWL; even this low rate is typically associated with pre-existing cardiac dysfunction.
Complications
The complications of struvite and triple phosphate renal calculi are complete and persistent obstruction of the kidney causing permanent renal damage, pyonephrosis, sepsis, chronic pyelonephritis, and renal failure especially if present bilaterally.[55]
Complications of treatment for these stones (ESWL and/or PCNL) include pyelonephritis, perinephric abscess, bleeding, injury to adjacent organs, perforation of the renal pelvis, dilutional hyponatremia, hypothermia, renal failure, retroperitoneal hematomas, and stricture formation.
Deterrence and Patient Education
Patient education about struvite and renal calculi is needed to prevent associated complications. Any person who forms or passes a renal stone should undergo imaging and laboratory analysis of the stone. Besides, patients who have recurrent UTIs should also be made aware that they might be harboring struvite and triple phosphate renal calculi in their kidneys or collecting system. In such cases, treatment of the infection with antibiotics alone is not enough; complete surgical removal of the stone is needed to prevent long-term renal damage and decrease the risk of recurrent UTIs.
Enhancing Healthcare Team Outcomes
Struvite and triple phosphate renal calculi are strongly associated with urinary tract infections, unlike calcium-based and uric acid calculi, formed as a result of underlying metabolic derangements. Proper identification of the chemical nature of these stones is important to provide the appropriate treatment. This might be difficult for general practitioners, Emergency Room personnel, and primary care providers. Timely referral to a urologist is important to prevent short and long-term complications. Definitive diagnosis and treatment often require close coordination and communication between radiologists, laboratory personal, nephrologists, Emergency Room personnel, infectious disease specialists, and urologists.
All staghorn calculus should be considered infected, and the patient should be started on broad-spectrum antibiotics after sending out urine cultures. Further management, including either a stone removal procedure or conservative management, is determined by the urologist based on the patient's comorbidities, BMI, stone size and shape, renal function, symptom status, and exact anatomical location of the calculus.
The laboratory performs analysis and culture of the extracted or spontaneously passed the stone. Repeat imaging and urine cultures are needed 3 months after definitive treatment to confirm stone-free status versus recurrence or residual fragments. Based on the culture and sensitivity report, low-dose long-term antibiotics can be considered for chronic suppression or prophylaxis. Acetohydroxamic acid (AHA) can be used as a urease inhibitor to help prevent stone growth and recurrence, especially in patients with anatomical abnormalities of the urinary tract.[Level 1][35]
Media
(Click Image to Enlarge)
References
Griffith DP, Struvite stones. Kidney international. 1978 May; [PubMed PMID: 351265]
Moe OW, Kidney stones: pathophysiology and medical management. Lancet (London, England). 2006 Jan 28; [PubMed PMID: 16443041]
Bichler KH,Eipper E,Naber K,Braun V,Zimmermann R,Lahme S, Urinary infection stones. International journal of antimicrobial agents. 2002 Jun; [PubMed PMID: 12135839]
Flannigan R,Choy WH,Chew B,Lange D, Renal struvite stones--pathogenesis, microbiology, and management strategies. Nature reviews. Urology. 2014 Jun; [PubMed PMID: 24818849]
Lingeman JE,Siegel YI,Steele B, Metabolic evaluation of infected renal lithiasis: clinical relevance. Journal of endourology. 1995 Feb; [PubMed PMID: 7780431]
Iqbal MW,Shin RH,Youssef RF,Kaplan AG,Cabrera FJ,Hanna J,Scales CD Jr,Ferrandino MN,Preminger GM,Lipkin ME, Should metabolic evaluation be performed in patients with struvite stones? Urolithiasis. 2017 Apr [PubMed PMID: 27240693]
Terry RS,Preminger GM, Metabolic evaluation and medical management of staghorn calculi. Asian journal of urology. 2020 Apr [PubMed PMID: 32257805]
Iqbal MW,Youssef RF,Neisius A,Kuntz N,Hanna J,Ferrandino MN,Preminger GM,Lipkin ME, Contemporary Management of Struvite Stones Using Combined Endourologic and Medical Treatment: Predictors of Unfavorable Clinical Outcome. Journal of endourology. 2016 Jul [PubMed PMID: 24251429]
Level 2 (mid-level) evidenceAbnormal haemoglobins, thalassaemias, and hereditary ovalocytosis in the Papuan Gulf area., Booth K,Garo N,, Papua and New Guinea medical journal, 1978 Jun [PubMed PMID: 17437820]
Level 2 (mid-level) evidenceKristensen C,Parks JH,Lindheimer M,Coe FL, Reduced glomerular filtration rate and hypercalciuria in primary struvite nephrolithiasis. Kidney international. 1987 Nov; [PubMed PMID: 3430961]
Parmar MS, Kidney stones. BMJ (Clinical research ed.). 2004 Jun 12; [PubMed PMID: 15191979]
Cytogenetic studies in Papua New Guinea., Woodliff HJ,Swann S,, Papua and New Guinea medical journal, 1978 Jun [PubMed PMID: 32213203]
Kaefer M,Hendren WH,Bauer SB,Goldenblatt P,Peters CA,Atala A,Retik AB, Reservoir calculi: a comparison of reservoirs constructed from stomach and other enteric segments. The Journal of urology. 1998 Dec; [PubMed PMID: 9817364]
Level 2 (mid-level) evidenceTreatment of paraquat poisoning., Howard JK,, Papua and New Guinea medical journal, 1978 Jun [PubMed PMID: 18111841]
Jongyotha K,Sriphrapradang C, Squamous Cell Carcinoma of the Renal Pelvis as a Result of Long-Standing Staghorn Calculi. Case reports in oncology. 2015 Sep-Dec [PubMed PMID: 26557077]
Level 3 (low-level) evidenceJacobs D,Heimbach D,Hesse A, Chemolysis of struvite stones by acidification of artificial urine--an in vitro study. Scandinavian journal of urology and nephrology. 2001 Oct [PubMed PMID: 11771859]
Parkhomenko E,De Fazio A,Tran T,Thai J,Blum K,Gupta M, A Multi-Institutional Study of Struvite Stones: Patterns of Infection and Colonization. Journal of endourology. 2017 May [PubMed PMID: 28355093]
Manzoor MAP,Singh B,Agrawal AK,Arun AB,Mujeeburahiman M,Rekha PD, Morphological and micro-tomographic study on evolution of struvite in synthetic urine infected with bacteria and investigation of its pathological biomineralization. PloS one. 2018; [PubMed PMID: 30106992]
[Diagnosis of premature contacts]., Komári J,, ZWR, 1978 Aug 25 [PubMed PMID: 20952016]
[Close relationship between social origin and dental health]., Nikolitsch JM,, Zahnarztliche Mitteilungen, 1978 Aug 16 [PubMed PMID: 23321831]
Level 2 (mid-level) evidenceHall PM, Nephrolithiasis: treatment, causes, and prevention. Cleveland Clinic journal of medicine. 2009 Oct; [PubMed PMID: 19797458]
[Dentists are losing their reputation as anxiety symbols]., Laux G,, Zahnarztliche Mitteilungen, 1978 Sep 1 [PubMed PMID: 32705911]
Lavengood RW Jr,Marshall VF, The prevention of renal phosphatic calculi in the presence of infection by the Shorr regimen. The Journal of urology. 1972 Sep [PubMed PMID: 5052239]
Siener R,Struwe F,Hesse A, Effect of L-Methionine on the Risk of Phosphate Stone Formation. Urology. 2016 Dec [PubMed PMID: 27521063]
Jarrar K,Boedeker RH,Weidner W, Struvite stones: long term follow up under metaphylaxis. Annales d'urologie. 1996 [PubMed PMID: 8766146]
[Importance, duties and function of continuing education while at work]., Zukunft D,, Zahntechnik; Zeitschrift fur Theorie und Praxis der wissenschaftlichen Zahntechnik, 1977 Jan-Feb [PubMed PMID: 458940]
Alsawi M,Amer T,Mariappan M,Nalagatla S,Ramsay A,Aboumarzouk O, Conservative management of staghorn stones. Annals of the Royal College of Surgeons of England. 2020 Apr; [PubMed PMID: 31918554]
[Structure and function of central laboratories of dental technology]., Stiebing W,, Zahntechnik; Zeitschrift fur Theorie und Praxis der wissenschaftlichen Zahntechnik, 1977 Jan-Feb [PubMed PMID: 29658394]
Griffith DP,Gleeson MJ,Lee H,Longuet R,Deman E,Earle N, Randomized, double-blind trial of Lithostat (acetohydroxamic acid) in the palliative treatment of infection-induced urinary calculi. European urology. 1991; [PubMed PMID: 1726639]
Level 1 (high-level) evidenceGriffith DP,Khonsari F,Skurnick JH,James KE, A randomized trial of acetohydroxamic acid for the treatment and prevention of infection-induced urinary stones in spinal cord injury patients. The Journal of urology. 1988 Aug; [PubMed PMID: 3294442]
Level 1 (high-level) evidenceSchwartz BF,Stoller ML, Nonsurgical management of infection-related renal calculi. The Urologic clinics of North America. 1999 Nov [PubMed PMID: 10584617]
Martínez-Piñeiro JA,de Iriarte EG,Armero AH, The problem of recurrences and infection after surgical removal of staghorn calculi. European urology. 1982 [PubMed PMID: 7037422]
Beck EM,Riehle RA Jr, The fate of residual fragments after extracorporeal shock wave lithotripsy monotherapy of infection stones. The Journal of urology. 1991 Jan [PubMed PMID: 1984100]
Level 2 (mid-level) evidencePreminger GM,Assimos DG,Lingeman JE,Nakada SY,Pearle MS,Wolf JS Jr, Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. The Journal of urology. 2005 Jun; [PubMed PMID: 15879803]
Level 1 (high-level) evidence[Present state and development of stomatologic-orthodontic technic]., Schmeil F,, Zahntechnik; Zeitschrift fur Theorie und Praxis der wissenschaftlichen Zahntechnik, 1977 Jan-Feb [PubMed PMID: 9048858]
Parietal cell vagotomy. Methodological, physiological and pathological aspects., Poppen B,, Acta chirurgica Scandinavica. Supplementum, 1978 [PubMed PMID: 21571342]
[Erythroleukemia and sarcolysine]., De Bock RF,Peetermans ME,, Acta clinica Belgica, 1978 [PubMed PMID: 21599527]
Lam HS,Lingeman JE,Mosbaugh PG,Steele RE,Knapp PM,Scott JW,Newman DM, Evolution of the technique of combination therapy for staghorn calculi: a decreasing role for extracorporeal shock wave lithotripsy. The Journal of urology. 1992 Sep; [PubMed PMID: 1507330]
Larrea Masvidal E,García Serrano C,Castillo Rodríguez M,Hernández Silverio D,Casals Armada J,Valdés Gómez C,Báez Hernández D, [Percutaneous nephrolitholapaxy combined with extracorporeal shockwave lithotripsy in the treatment of staghorn lithiasis]. Archivos espanoles de urologia. 1990 May [PubMed PMID: 2383049]
Streem SB,Yost A,Dolmatch B, Combination "sandwich" therapy for extensive renal calculi in 100 consecutive patients: immediate, long-term and stratified results from a 10-year experience. The Journal of urology. 1997 Aug [PubMed PMID: 9224299]
Mohammadi M,Nouri-Mahdavi K,Barzegar A, Effects of Tranexamic Acid on Bleeding and Hemoglobin Levels in Patients with Staghorn Calculi Undergoing Percutaneous Nephrolithotomy: Randomized Controlled Trial. Iranian journal of medical sciences. 2019 Nov [PubMed PMID: 31875080]
Level 1 (high-level) evidenceSafe and effective use of transcutaneous blood gas monitors., Indyk L,, Acta anaesthesiologica Scandinavica. Supplementum, 1978 [PubMed PMID: 23165402]
Transcutaneous oxygen tension in imminent foot gangrene., Tønnesen KH,, Acta anaesthesiologica Scandinavica. Supplementum, 1978 [PubMed PMID: 23123376]
Level 1 (high-level) evidenceAn improved sensor and a method for transcutaneous CO2 monitoring., Beran AV,Shigezawa GY,Yeung HN,Huxtable RF,, Acta anaesthesiologica Scandinavica. Supplementum, 1978 [PubMed PMID: 26659063]
Sfoungaristos S,Gofrit ON,Yutkin V,Landau EH,Pode D,Duvdevani M, External Validation of CROES Nephrolithometry as a Preoperative Predictive System for Percutaneous Nephrolithotomy Outcomes. The Journal of urology. 2016 Feb [PubMed PMID: 26316372]
Level 1 (high-level) evidenceChoi SW,Bae WJ,Ha US,Hong SH,Lee JY,Kim SW,Cho HJ, Prognostic Impact of Stone-Scoring Systems After Percutaneous Nephrolithotomy for Staghorn Calculi: A Single Center's Experience Over 10 Years. Journal of endourology. 2016 Sep [PubMed PMID: 27368353]
Portis AJ,Laliberte MA,Drake S,Holtz C,Rosenberg MS,Bretzke CA, Intraoperative fragment detection during percutaneous nephrolithotomy: evaluation of high magnification rotational fluoroscopy combined with aggressive nephroscopy. The Journal of urology. 2006 Jan [PubMed PMID: 16406897]
Reliability of cutaneous oxygen measurement by skin sensors with large-size cathodes., Eberhard P,Mindt W,, Acta anaesthesiologica Scandinavica. Supplementum, 1978 [PubMed PMID: 10778477]
Pfister RC,Dretler SP, Percutaneous chemolysis of renal calculi. Urologic radiology. 1984 [PubMed PMID: 6330953]
Dretler SP,Pfister RC, Primary dissolution therapy of struvite calculi. The Journal of urology. 1984 May [PubMed PMID: 6708214]
Assimos D,Krambeck A,Miller NL,Monga M,Murad MH,Nelson CP,Pace KT,Pais VM Jr,Pearle MS,Preminger GM,Razvi H,Shah O,Matlaga BR, Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART II. The Journal of urology. 2016 Oct [PubMed PMID: 27238615]
Assimos D,Krambeck A,Miller NL,Monga M,Murad MH,Nelson CP,Pace KT,Pais VM Jr,Pearle MS,Preminger GM,Razvi H,Shah O,Matlaga BR, Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. The Journal of urology. 2016 Oct [PubMed PMID: 27238616]
Williams JC Jr,Paterson RF,Kopecky KK,Lingeman JE,McAteer JA, High resolution detection of internal structure of renal calculi by helical computerized tomography. The Journal of urology. 2002 Jan; [PubMed PMID: 11743350]
Teichman JM,Long RD,Hulbert JC, Long-term renal fate and prognosis after staghorn calculus management. The Journal of urology. 1995 May; [PubMed PMID: 7714951]
Level 2 (mid-level) evidenceBlandy JP,Singh M, The case for a more aggressive approach to staghorn stones. The Journal of urology. 1976 May; [PubMed PMID: 1271539]
Level 3 (low-level) evidence