Introduction
Radiation proctitis refers to injury or damage to the rectum secondary to radiation therapy. It is postulated that almost half of all patients with pelvic malignancies undergo treatment that involves radiation.[1]
The clinical manifestation of radiation proctitis varies and is broadly categorized into acute and chronic phases. Acute radiation proctitis typically occurs during or immediately after the radiation treatment period, manifesting with symptoms such as diarrhea, rectal urgency, and mild rectal bleeding. On the other hand, chronic radiation proctitis develops more than 3 months after the completion of radiation therapy, and its symptoms can be more persistent and severe. Chronic proctitis may lead to complications like rectal strictures, ulcerations, and even fistulas, significantly impacting the patient's quality of life.
The increasing incidence of radiation proctitis is closely tied to advancements in cancer treatment, leading to improved overall survival rates. As patients with pelvic malignancies live longer, healthcare professionals are likely to encounter a growing number of individuals experiencing the long-term effects of radiation proctitis. Management strategies involve a multidisciplinary approach, combining the expertise of radiation oncologists, gastroenterologists, advanced care practitioners, nurses, pharmacists, and other healthcare professionals. Treatment may include medications to alleviate symptoms, dietary modifications, and, in severe cases, endoscopic or surgical interventions. Ongoing research continues to explore novel therapies and preventative measures, aiming to enhance the quality of care and patient outcomes for those affected by radiation proctitis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Radiation proctitis occurs as a result of radiation therapy for malignancy to organs in the pelvis, including the prostate, rectum, and reproductive organs. The degree of radiation proctitis varies, depending on the modality by which the radiation is delivered, the radiation dose, and the volume of irradiated tissue. Several specific risk factors have been identified, such as a dose of radiation to the rectum >45 Gy, a history of inflammatory bowel disease, HIV/AIDS, and a possible genetic predisposition.[2][3][4][5]
Epidemiology
It is difficult to assess the true incidence of radiation proctitis. There are several large series that estimate chronic radiation proctitis to be anywhere between 5% to 11% of patients treated with radiation therapy.[6] Almost 90% of patients who go on to experience chronic radiation proctitis will present in the first 2 years after treatment.[7][8]
The frequency of radiation proctitis exhibits variations across sexes and age groups, both in the United States and globally. While the prevalence rates can be influenced by the specific demographics of cancer incidence and treatment practices, some general patterns emerge. In the U.S., the occurrence of radiation proctitis is observed in both males and females undergoing pelvic radiation therapy for malignancies such as prostate, cervical, and rectal cancers. The incidence may differ based on the distribution of these cancers within each gender. Additionally, age plays a role, with older individuals more commonly affected due to the higher prevalence of cancer in this demographic.
Globally, the frequency of radiation proctitis is influenced by regional variations in cancer prevalence, treatment protocols, and healthcare infrastructure. In areas where pelvic malignancies are more prevalent or where radiation therapy is a common treatment modality, the incidence of radiation proctitis may be higher.
Pathophysiology
Radiation therapy works by damaging cells through the direct effect of ionizing radiation on DNA, lipids, and proteins.[9] Water makes up the majority of the cell, and ionizing radiation results in the creation of oxygen-free radicles. The direct effect of ionizing radiation also disrupts vital cellular proteins and DNA, causing cellular necrosis. Early radiation injury causes edema, mucosal hyperemia, and ulceration of the affected tissue.
The epithelium of the bowel is rapidly proliferating, and as such, it is more predisposed to the effects of radiation damage. Chronically, there is intimal proliferation and hyaline thickening of the media of arterioles.[10] Hypertrophy of rectal smooth muscle occurs, which can affect compliance and defecation as the rectum's ability to distend is diminished.[11] This is further worsened by fibrosis of the serosa.
History and Physical
Patients experiencing radiation proctitis may present with malabsorption, perforation, bowel obstruction, bleeding, and stricture formation. They can also present with fistulous disease. If the anal sphincter is directly involved in the radiation field, patients may present with fecal incontinence.
It is essential to consider a recurrent malignancy in patients who present several years after their radiation therapy with symptoms of malabsorption, abdominal pain, increased frequency of bowel movements, bleeding, etc. While these symptoms may be due to radiation proctitis, they may also signify a local recurrence.
In terms of physical examination, a focused abdominal examination and, most importantly, a digital rectal exam should be performed to identify any anorectal stenosis. The examination can be painful for patients and may not be able to be completed in the office setting, in which case an examination under anesthesia is indicated.
Evaluation
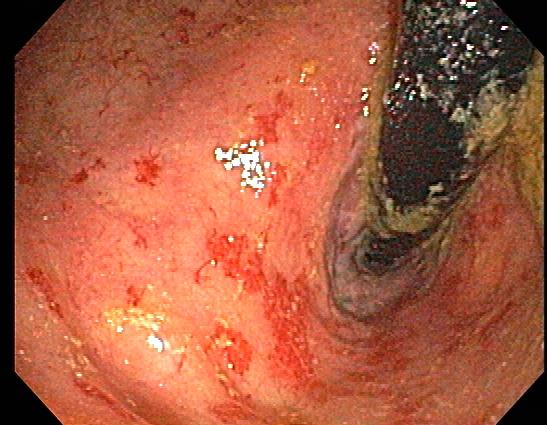
After completing a thorough history and physical examination, the next step is to perform either a rigid or flexible sigmoidoscopy. An experienced colorectal surgeon or gastroenterologist should perform this exam with minimal insufflation as the inflamed bowel is more susceptible to perforation, especially as it becomes fixated. The examination is likely to reveal a friable mucosa with a multitude of changes, including edema, oozing, and ulcerations.[12][13]
The mucosa may sometimes appear similar to that seen in inflammatory bowel disease with pseudopolyp formation. Areas of ulceration may require a biopsy, but this must be done cautiously (see Image. Colonoscopy of Radiation Proctitis, Moderate-to-Severe). There are likely to be multiple areas of strictures that can be indistinguishable from recurrent malignancy.[14]
Barium or water-soluble enema studies may also be performed, which can identify strictures, obstruction, shortening, and narrowing of the rectosigmoid area with loss of the normal curvature. It may also demonstrate decreased compliance of the rectum. The presacral space may appear to be increased due to rectal wall thickening.[15]
Once the diagnosis of radiation proctitis has been established, several grading systems exist to gauge severity, including RTOG/EORTC and LENT-SOMA.
Treatment / Management
Primary Prevention
Prophylactic medical therapies such as sucralfate, amifostine, and misoprostol have failed to demonstrate a reduction in the risk of proctitis in patients undergoing pelvic radiotherapy. However, data suggests that selenium supplementation taken during radiation treatments may reduce the risk of grade 2+ diarrhea. A small randomized trial of patients undergoing pelvic radiotherapy for uterine and cervical cancer taking 500 mg of selenium versus placebo demonstrated a significant reduction in the rate of grade 2+ diarrhea (20.5% vs 44.5%).[16] (A1)
Another avenue to minimize the risk of proctitis is the radiation planning and delivery process. Most of the data regarding late rectal complications are derived from the treatment of prostate cancer. There is a consistent dose-volume relationship with the risk of grade ≥2 rectal toxicity across several studies, specifically the volume of rectum receiving ≥60 Gy.[2] This emphasizes the importance of radiation delivery and the planning process in preventing late rectal toxicities. Several dose constraints have been put forward. For conventionally fractioned radiotherapy, the V50 <50%, V65 <25%, V70 <20%, and V75 <15% have been used, although they were derived from the 3D treatment era and easily met Intensity Modulated Radiotherapy (IMRT).[2] The QUANTEC dose constraints are a conservative starting point that, if adhered to, predict a grade ≥2 late rectal toxicity <15%.[2] Rectal constraints also exist for hypofractionated radiotherapy and stereotactic body radiotherapy (SBRT). (A1)
While adherence to established dose constraints is essential, how radiotherapy is delivered can also influence the risk of late rectal toxicity. When comparing 3D conformal treatment to IMRT when treating the pelvis, the incidence of late rectal toxicity appears to be lower with IMRT in the treatment of genitourinary and gynecologic malignancies. Late rectal toxicity in patients treated with 3D radiation for prostate cancer was 13% versus 5% in those treated with IMRT.[17] Postoperative endometrial and cervical cancer patients treated with IMRT also demonstrate lower rates of late gastrointestinal (GI) toxicity compared to 3D conformal (42% vs 21%).[18][19] Despite the claims that proton therapy will lead to even lower rates of toxicity, the data suggest that proton therapy, at best, has no effect and, at worst, may have higher rates of GI toxicity compared to IMRT for patients with prostate cancer.[20][21] (A1)
Improvements in daily image guidance also contribute to the reduction of late rectal toxicity as they allow for tumor and normal tissue motion to be taken into account, allowing for smaller planning target volumes (PTV) margins. Retrospective analysis of prostate cancer patients treated with IMRT and image guidance demonstrated a reduction in 3-year late rectal toxicity compared to those treated with IMRT without image guidance (4.1% vs 13.1%).[22]
Other preventative methods include introducing a physical space between the rectum and the target structure. Hyaluronic acid spacers involve the injection of a gel into the perirectal fat, thus enlarging the space between the anterior rectal wall and the posterior aspect of the prostate. Randomized multicenter data have demonstrated not only improved rectal V50-80 dosimetric parameters but also a reduction in late rectal toxicity with no patients experiencing >1 grade toxicity. There were no differences in acute rectal toxicity.[23](A1)
Medical Interventions
Acute Proctitis
If primary prevention fails, patients may develop acute radiation proctitis during treatment or within 6 months posttreatment. Acute proctitis is self-limited for most patients, and treatment is largely supportive. Unfortunately, many of these interventions lack evidence to support their use but are nevertheless utilized to address symptoms such as diarrhea, tenesmus, and rectal urgency. Patients who develop acute GI toxicity during treatment have a 42% ten-year likelihood of late GI toxicity compared to 9% in those who did not experience acute side effects.[17] Antimotility agents such as loperamide and diphenoxylate are used for patients experiencing diarrhea. Other symptoms, such as rectal urgency and tenesmus, have been treated with sodium butyrate, a short-chain fatty acid. A small randomized crossover trial of patients undergoing pelvic radiotherapy demonstrated a clear remission of symptoms with topical sodium butyrate compared to saline enemas.[24] Other therapies include mesalamine or corticosteroid enemas. In cases of extreme discomfort, treatment may be suspended to allow some tissue recovery and symptom resolution, but this delay may adversely affect the oncological outcome.(A1)
Chronic Proctitis
Patients who develop chronic proctitis over the ensuing months to years after treatment have several medical treatment options. Evidence for the various treatments ranges from anecdotal to small randomized prospective trials.
Some studies found sucralfate enemas helpful, with clinical improvement in 73% of patients who received pelvic radiotherapy and had continued tenesmus and rectal bleeding.[25] A prospective randomized, double-blind study comparing sucralfate enemas to rectal sulfasalazine and prednisone demonstrated better tolerance of sucralfate and superior clinical response. Ninety-two percent of patients had a significant reduction in rectal bleeding at the 4-month follow-up.[26] A possible mechanism of action is the repair of microvascular damage through the stimulation of angiogenesis and glutathione production.[27](A1)
Formalin (ie, formaldehyde 4% to 10%) has been studied and used to treat chronic radiation proctitis for over 20 years.[28] The advantages of formalin treatment are that it can be utilized in the clinical setting without general anesthesia and with only light sedation. The mechanism of action of formalin is the chemical cauterization of the ulcers and telangiectasias, which are the source of bleeding in chronic proctitis. The formalin can be applied with the direct application of a gauze that has been soaked in formalin and the direct application of it to the mucosa of the affected areas, usually under direct vision using rigid proctoscopy. The concentration of formalin is typically 4%, although some studies have utilized a 10% solution. A study from Poland showed that after the first application, 50% of the patients had complete resolution of their symptoms, and most patients required an average of 2 treatments.[29] (B3)
Glucocorticoid suppositories have also been utilized for patients with continued rectal bleeding. The anti-inflammatory effects are thought to lead to symptomatic improvement. However, an open randomized trial of mesalamine suppositories versus hydrocortisone foam demonstrated higher rates of hematochezia and mucus in the stool after 2 weeks of treatment with hydrocortisone foam.[30](A1)
The latest clinical consensus guidelines from the American Society of Colon and Rectal Surgery suggest that short-chain fatty acid enemas are not useful in treating chronic radiation proctitis.[31] Over the years, there have been investigations into other treatments, including ozone therapy, mesalamine, and metronidazole. However, no evidence exists to support their efficacy.[32] (A1)
In patients who are refractory to medical treatment, hyperbaric oxygen therapy (HBO) has reasonable evidence to support its use for radiation proctitis.[33][34] A randomized trial of 2.0 atm vs 1.1 atm demonstrated a significant improvement in healing responses and late effects normal tissue-subjective, objective, management, and analytic (LENT-SOMA) scores.[35] A Cochrane review of HBO therapy concluded it is a safe and likely efficacious intervention for patients with refractory radiation proctitis.[36](A1)
Several studies have demonstrated that endoscopic argon beam plasma coagulation can reduce bleeding by approximately 79% to 100%.[37] This treatment is not without adverse effects. Complications include rectal ulcerations and fistula formation. While endoscopic argon beam plasma coagulation treatment has been substantiated, other endoscopic treatments, such as bipolar electrocoagulation, radio-frequency ablation, and Nd-YAG laser, have not been sufficiently studied.[32](A1)
Patients may develop strictures and present with obstructive symptoms that may not respond to stool softeners. Endoscopic Savary-Gilliard dilatation may be used in patients with short strictures that are not responsive to medical therapy. In patients with longer strictures, surgery may be preferable.
Surgery is reserved for patients who do not show improvement in their symptoms following the above medical and endoscopic interventions. It is also used for some of the more severe complications that are associated with radiation proctitis, including strictures that may lead to large bowel obstruction, fistulas, or even perforation. Studies have estimated that only 10% of patients with radiation proctitis will ultimately require operative intervention.[38] In very severe cases, a proctectomy may be necessary. However, studies have demonstrated that diversion in the form of an ileostomy or colostomy may improve quality of life, and no further surgical procedures may be needed.[39](B2)
Differential Diagnosis
The differential diagnosis of radiation proctitis involves careful consideration of various conditions that may present with similar symptoms or findings. Clinicians must distinguish radiation proctitis from other causes of rectal inflammation, bleeding, and GI distress.
Radiation proctitis must be distinguished from other etiologies of infectious and noninfectious dysentery, including:
- Diverticulitis
- Crohn disease
- Irritable bowel disease
- Infectious colitis
- Recurrence of malignancy
Given the potential overlap in clinical presentations, a meticulous evaluation, including endoscopic procedures, histological examination, and imaging studies, is essential to ensure an accurate and timely diagnosis of radiation proctitis and appropriate differentiation from other potential etiologies.
Prognosis
The prognosis depends on the severity of the individual patient's disease. Up to 30% of patients with severe symptoms may have a significant decrease in health-related quality of life.[40] Patients with radiation proctitis are also at risk of developing a secondary malignancy, of which the majority are colon or rectal cancers.[41]
In many cases, acute radiation proctitis tends to be self-limiting, with symptoms resolving once radiation therapy concludes. However, the prognosis becomes more nuanced when the condition progresses to its chronic phase, occurring more than 3 months posttreatment. Chronic radiation proctitis can lead to persistent symptoms, impacting the patient's quality of life. While some individuals may experience manageable symptoms with conservative management, others may face more challenging complications, such as rectal strictures or fistulas, influencing long-term outcomes. Early detection, proactive monitoring, and a tailored treatment approach can significantly improve the prognosis by addressing symptoms promptly and mitigating the risk of severe complications.
Complications
The complications of radiation proctitis can significantly impact the quality of life of individuals undergoing pelvic radiation therapy and include the following:
- Colitis
- Bowel perforation
- Sepsis
- Fistula formation
- Radiation associated malignancies
In its acute phase, patients may experience symptoms such as diarrhea, rectal bleeding, and abdominal pain. However, the transition to chronic radiation proctitis, occurring more than 3 months posttreatment, can lead to persistent and potentially severe complications. Chronic symptoms may include rectal strictures, fecal urgency, and incontinence, posing substantial challenges to daily activities. In some cases, radiation proctitis can progress to more severe outcomes, such as fistulas or perforations, necessitating surgical intervention. The complexity of managing these complications underscores the importance of proactive monitoring, early intervention, and a multidisciplinary approach to provide comprehensive care for individuals affected by radiation proctitis.
Deterrence and Patient Education
Deterrence and patient education play pivotal roles in mitigating the impact of radiation proctitis. Through comprehensive patient education initiatives, healthcare professionals can empower individuals undergoing pelvic radiation therapy with the knowledge to recognize early signs and symptoms. Educating patients on lifestyle modifications, dietary adjustments, and self-care practices can contribute to deterrence by minimizing risk factors and promoting overall rectal health.
Patients should be educated that eating foods high in fiber can soften stools naturally and improve some symptoms. The use of fiber supplements, including psyllium, can help improve symptoms. Patients should also attempt to avoid caffeine, smoking, complex sugars, and alcohol, as these can worsen diarrhea and lead to worsening pain and bleeding.
Informing patients about the importance of adherence to treatment regimens and regular follow-up appointments can aid in the early identification and intervention of any emerging complications. Patients should be educated that if the bleeding is excessive or they experience symptoms of dizziness and weakness, they should seek immediate medical attention.
Deterrence is not only about preventing the occurrence of radiation proctitis but also about equipping patients with the tools to actively participate in their care, fostering a collaborative approach between healthcare professionals and patients to optimize outcomes and enhance overall well-being during and after radiation therapy.
Enhancing Healthcare Team Outcomes
Healthcare professionals involved in managing radiation proctitis need specialized skills in evaluating, diagnosing, and treating this condition. Physicians and advanced care practitioners should possess clinical expertise in radiation oncology and gastroenterology, while nurses and pharmacists must be adept in administering and monitoring treatment regimens. Advanced procedural skills may be required for certain interventions.
Developing a cohesive strategy involves aligning clinicians with evidence-based practices for radiation proctitis. Establishing standardized screening, treatment, and follow-up protocols ensures a consistent approach across disciplines. Individualized treatment plans are likely required depending on the context of the patient, and discussion with at least 1 colleague in an interprofessional team setting regarding the management is recommended.
Collaboration, shared decision-making, and communication are key elements for a good patient outcome. The multidisciplinary collaboration among healthcare professionals remains pivotal in optimizing the prognosis of patients with radiation proctitis, ensuring comprehensive care that aligns with each patient's unique needs.
Strategic planning should also include ongoing education to keep the healthcare team abreast of the latest advancements in the field. The interprofessional care provided to the patient must use an integrated care pathway combined with an evidence-based approach to planning and evaluating all joint activities.
A comprehensive and patient-centered approach to radiation proctitis requires a blend of specialized skills, a well-defined strategy, clearly outlined responsibilities, effective interprofessional communication, and meticulous care coordination. By addressing these elements, healthcare professionals can enhance patient outcomes, safety, and overall team performance in the management of radiation proctitis.
Media
(Click Image to Enlarge)
References
Ballas LK, Elkin EB, Schrag D, Minsky BD, Bach PB. Radiation therapy facilities in the United States. International journal of radiation oncology, biology, physics. 2006 Nov 15:66(4):1204-11 [PubMed PMID: 17145535]
Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. International journal of radiation oncology, biology, physics. 2010 Mar 1:76(3 Suppl):S123-9. doi: 10.1016/j.ijrobp.2009.03.078. Epub [PubMed PMID: 20171506]
Level 1 (high-level) evidenceKerns SL, Fachal L, Dorling L, Barnett GC, Baran A, Peterson DR, Hollenberg M, Hao K, Narzo AD, Ahsen ME, Pandey G, Bentzen SM, Janelsins M, Elliott RM, Pharoah PDP, Burnet NG, Dearnaley DP, Gulliford SL, Hall E, Sydes MR, Aguado-Barrera ME, Gómez-Caamaño A, Carballo AM, Peleteiro P, Lobato-Busto R, Stock R, Stone NN, Ostrer H, Usmani N, Singhal S, Tsuji H, Imai T, Saito S, Eeles R, DeRuyck K, Parliament M, Dunning AM, Vega A, Rosenstein BS, West CML. Radiogenomics Consortium Genome-Wide Association Study Meta-Analysis of Late Toxicity After Prostate Cancer Radiotherapy. Journal of the National Cancer Institute. 2020 Feb 1:112(2):179-190. doi: 10.1093/jnci/djz075. Epub [PubMed PMID: 31095341]
Level 1 (high-level) evidenceWillett CG, Ooi CJ, Zietman AL, Menon V, Goldberg S, Sands BE, Podolsky DK. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. International journal of radiation oncology, biology, physics. 2000 Mar 1:46(4):995-8 [PubMed PMID: 10705022]
Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. International journal of radiation oncology, biology, physics. 1999 Apr 1:44(1):127-31 [PubMed PMID: 10219805]
Level 2 (mid-level) evidenceOtchy DP, Nelson H. Radiation injuries of the colon and rectum. The Surgical clinics of North America. 1993 Oct:73(5):1017-35 [PubMed PMID: 8378826]
Gilinsky NH, Burns DG, Barbezat GO, Levin W, Myers HS, Marks IN. The natural history of radiation-induced proctosigmoiditis: an analysis of 88 patients. The Quarterly journal of medicine. 1983 Winter:52(205):40-53 [PubMed PMID: 6603628]
Level 2 (mid-level) evidencePalmer JA, Bush RS. Radiation injuries to the bowel associated with the treatment of carcinoma of the cervix. Surgery. 1976 Oct:80(4):458-64 [PubMed PMID: 968730]
Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nature reviews. Gastroenterology & hepatology. 2014 Aug:11(8):470-9. doi: 10.1038/nrgastro.2014.46. Epub 2014 Apr 1 [PubMed PMID: 24686268]
Level 3 (low-level) evidenceVillasanta U. Complications of radiotherapy for carcinoma of the uterine cervix. American journal of obstetrics and gynecology. 1972 Nov 15:114(6):717-26 [PubMed PMID: 4633571]
Varma JS, Smith AN, Busuttil A. Correlation of clinical and manometric abnormalities of rectal function following chronic radiation injury. The British journal of surgery. 1985 Nov:72(11):875-8 [PubMed PMID: 4063752]
Sharma B, Gupta M, Sharma R, Gupta A, Sharma N, Sharma M, Sharma V, Vats S, Gupta M, Seam RK. Four percent formalin application for the management of radiation proctitis in carcinoma cervix patients: An effective, safe, and economical practice. Journal of cancer research and therapeutics. 2019 Jan-Mar:15(1):92-95. doi: 10.4103/jcrt.JCRT_393_17. Epub [PubMed PMID: 30880761]
Kapp KS, Stuecklschweiger GF, Kapp DS, Poschauko J, Pickel H, Hackl A. Carcinoma of the cervix: analysis of complications after primary external beam radiation and Ir-192 HDR brachytherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1997 Feb:42(2):143-53 [PubMed PMID: 9106923]
Ali F, Hu KY. Evaluation and Management of Chronic Radiation Proctitis. Diseases of the colon and rectum. 2020 Mar:63(3):285-287. doi: 10.1097/DCR.0000000000001592. Epub [PubMed PMID: 32032142]
Mendelson RM, Nolan DJ. The radiological features of chronic radiation enteritis. Clinical radiology. 1985 Mar:36(2):141-8 [PubMed PMID: 4064491]
Muecke R, Schomburg L, Glatzel M, Berndt-Skorka R, Baaske D, Reichl B, Buentzel J, Kundt G, Prott FJ, Devries A, Stoll G, Kisters K, Bruns F, Schaefer U, Willich N, Micke O, German Working Group Trace Elements and Electrolytes in Oncology-AKTE. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. International journal of radiation oncology, biology, physics. 2010 Nov 1:78(3):828-35. doi: 10.1016/j.ijrobp.2009.08.013. Epub 2010 Feb 3 [PubMed PMID: 20133068]
Level 1 (high-level) evidenceZelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, Amols HI. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. International journal of radiation oncology, biology, physics. 2008 Mar 15:70(4):1124-9. doi: 10.1016/j.ijrobp.2007.11.044. Epub [PubMed PMID: 18313526]
Chopra S, Gupta S, Kannan S, Dora T, Engineer R, Mangaj A, Maheshwari A, Shylasree TS, Ghosh J, Paul SN, Phurailatpam R, Charnalia M, Alone M, Swamidas J, Mahantshetty U, Deodhar K, Kerkar R, Shrivastava SK. Late Toxicity After Adjuvant Conventional Radiation Versus Image-Guided Intensity-Modulated Radiotherapy for Cervical Cancer (PARCER): A Randomized Controlled Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2021 Nov 20:39(33):3682-3692. doi: 10.1200/JCO.20.02530. Epub 2021 Sep 10 [PubMed PMID: 34506246]
Level 1 (high-level) evidenceKlopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, Gifford K, Gaffney DK, Small W Jr, Thompson S, Doncals DE, Cantuaria GHC, Yaremko BP, Chang A, Kundapur V, Mohan DS, Haas ML, Kim YB, Ferguson CL, Pugh SL, Kachnic LA, Bruner DW. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Aug 20:36(24):2538-2544. doi: 10.1200/JCO.2017.77.4273. Epub 2018 Jul 10 [PubMed PMID: 29989857]
Pan HY, Jiang J, Hoffman KE, Tang C, Choi SL, Nguyen QN, Frank SJ, Anscher MS, Shih YT, Smith BD. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men With Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Jun 20:36(18):1823-1830. doi: 10.1200/JCO.2017.75.5371. Epub 2018 Mar 21 [PubMed PMID: 29561693]
Level 2 (mid-level) evidenceFang P, Mick R, Deville C, Both S, Bekelman JE, Christodouleas JP, Guzzo TJ, Tochner Z, Hahn SM, Vapiwala N. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer. 2015 Apr 1:121(7):1118-27. doi: 10.1002/cncr.29148. Epub 2014 Nov 25 [PubMed PMID: 25423899]
Level 2 (mid-level) evidenceDelobel JB, Gnep K, Ospina JD, Beckendorf V, Chira C, Zhu J, Bossi A, Messai T, Acosta O, Castelli J, de Crevoisier R. Nomogram to predict rectal toxicity following prostate cancer radiotherapy. PloS one. 2017:12(6):e0179845. doi: 10.1371/journal.pone.0179845. Epub 2017 Jun 22 [PubMed PMID: 28640871]
Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, Kos M, Ellis R, Logsdon M, Zimberg S, Forsythe K, Zhang H, Soffen E, Francke P, Mantz C, Rossi P, DeWeese T, Hamstra DA, Bosch W, Gay H, Michalski J. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. International journal of radiation oncology, biology, physics. 2015 Aug 1:92(5):971-977. doi: 10.1016/j.ijrobp.2015.04.030. Epub 2015 Apr 23 [PubMed PMID: 26054865]
Level 1 (high-level) evidenceVernia P, Fracasso PL, Casale V, Villotti G, Marcheggiano A, Stigliano V, Pinnaro P, Bagnardi V, Caprilli R. Topical butyrate for acute radiation proctitis: randomised, crossover trial. Lancet (London, England). 2000 Oct 7:356(9237):1232-5 [PubMed PMID: 11072942]
Level 1 (high-level) evidenceMcElvanna K, Wilson A, Irwin T. Sucralfate paste enema: a new method of topical treatment for haemorrhagic radiation proctitis. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2014 Apr:16(4):281-4. doi: 10.1111/codi.12507. Epub [PubMed PMID: 24299100]
Level 2 (mid-level) evidenceKochhar R, Patel F, Dhar A, Sharma SC, Ayyagari S, Aggarwal R, Goenka MK, Gupta BD, Mehta SK. Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Digestive diseases and sciences. 1991 Jan:36(1):103-7 [PubMed PMID: 1670631]
Level 1 (high-level) evidenceSandor Z, Nagata M, Kusstatscher S, Szabo S. Stimulation of mucosal glutathione and angiogenesis: new mechanisms of gastroprotection and ulcer healing by sucralfate. Scandinavian journal of gastroenterology. Supplement. 1995:210():19-21 [PubMed PMID: 8578199]
Level 3 (low-level) evidenceIsenberg GA, Goldstein SD, Resnik AM. Formalin therapy for radiation proctitis. JAMA. 1994 Dec 21:272(23):1822 [PubMed PMID: 7990213]
Level 3 (low-level) evidenceDziki Ł, Kujawski R, Mik M, Berut M, Dziki A, Trzciński R. Formalin therapy for hemorrhagic radiation proctitis. Pharmacological reports : PR. 2015 Oct:67(5):896-900. doi: 10.1016/j.pharep.2015.03.006. Epub 2015 Mar 21 [PubMed PMID: 26398382]
Lucidarme D, Marteau P, Foucault M, Vautrin B, Filoche B. Efficacy and tolerance of mesalazine suppositories vs. hydrocortisone foam in proctitis. Alimentary pharmacology & therapeutics. 1997 Apr:11(2):335-40 [PubMed PMID: 9146772]
Level 1 (high-level) evidenceal-Sabbagh R, Sinicrope FA, Sellin JH, Shen Y, Roubein L. Evaluation of short-chain fatty acid enemas: treatment of radiation proctitis. The American journal of gastroenterology. 1996 Sep:91(9):1814-6 [PubMed PMID: 8792704]
Level 3 (low-level) evidencePaquette IM, Vogel JD, Abbas MA, Feingold DL, Steele SR, Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis. Diseases of the colon and rectum. 2018 Oct:61(10):1135-1140. doi: 10.1097/DCR.0000000000001209. Epub [PubMed PMID: 30192320]
Level 1 (high-level) evidenceOscarsson N, Arnell P, Lodding P, Ricksten SE, Seeman-Lodding H. Hyperbaric oxygen treatment in radiation-induced cystitis and proctitis: a prospective cohort study on patient-perceived quality of recovery. International journal of radiation oncology, biology, physics. 2013 Nov 15:87(4):670-5. doi: 10.1016/j.ijrobp.2013.07.039. Epub 2013 Sep 11 [PubMed PMID: 24035333]
Level 2 (mid-level) evidenceHoggan BL, Cameron AL. Systematic review of hyperbaric oxygen therapy for the treatment of non-neurological soft tissue radiation-related injuries. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014 Jun:22(6):1715-26. doi: 10.1007/s00520-014-2198-z. Epub 2014 Mar 29 [PubMed PMID: 24794980]
Level 1 (high-level) evidenceClarke RE, Tenorio LM, Hussey JR, Toklu AS, Cone DL, Hinojosa JG, Desai SP, Dominguez Parra L, Rodrigues SD, Long RJ, Walker MB. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. International journal of radiation oncology, biology, physics. 2008 Sep 1:72(1):134-143. doi: 10.1016/j.ijrobp.2007.12.048. Epub 2008 Mar 14 [PubMed PMID: 18342453]
Level 1 (high-level) evidenceBennett MH, Feldmeier J, Hampson NB, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. The Cochrane database of systematic reviews. 2016 Apr 28:4(4):CD005005. doi: 10.1002/14651858.CD005005.pub4. Epub 2016 Apr 28 [PubMed PMID: 27123955]
Level 1 (high-level) evidenceSwan MP, Moore GT, Sievert W, Devonshire DA. Efficacy and safety of single-session argon plasma coagulation in the management of chronic radiation proctitis. Gastrointestinal endoscopy. 2010 Jul:72(1):150-4. doi: 10.1016/j.gie.2010.01.065. Epub 2010 May 20 [PubMed PMID: 20493484]
Jao SW, Beart RW Jr, Gunderson LL. Surgical treatment of radiation injuries of the colon and rectum. American journal of surgery. 1986 Feb:151(2):272-7 [PubMed PMID: 3946764]
Pricolo VE, Shellito PC. Surgery for radiation injury to the large intestine. Variables influencing outcome. Diseases of the colon and rectum. 1994 Jul:37(7):675-84 [PubMed PMID: 8026234]
Level 2 (mid-level) evidenceLev EL, Eller LS, Gejerman G, Lane P, Owen SV, White M, Nganga N. Quality of life of men treated with brachytherapies for prostate cancer. Health and quality of life outcomes. 2004 Jun 15:2():28 [PubMed PMID: 15198803]
Level 2 (mid-level) evidenceLiauw SL, Sylvester JE, Morris CG, Blasko JC, Grimm PD. Second malignancies after prostate brachytherapy: incidence of bladder and colorectal cancers in patients with 15 years of potential follow-up. International journal of radiation oncology, biology, physics. 2006 Nov 1:66(3):669-73 [PubMed PMID: 16887293]