Introduction
Pediatric asthma is characterized by variable expiratory airway limitation and persistent respiratory symptoms, including wheezing, coughing, shortness of breath, and chest tightness. Asthma often starts in childhood, with nearly half of all infants wheezing in their first year, and most developing persistent asthma by age 6. A complex interplay between genetic predisposition and environmental factors underscores its prevalence and severity. Additionally, patients commonly experience airway hyperresponsiveness and inflammation. The severity ranges from intermittent symptoms to potentially life-threatening airway compromise, necessitating a comprehensive diagnostic approach.
Generally, 2 groups of children exhibit wheezing and asthma-like symptoms at a young age.[1][2] One group experiences sporadic symptoms, usually triggered by viral infections, and tends to overcome these episodes as they age. The other group tends to develop symptoms at a later age, often in combination with atopy, a family history of asthma, and an elevated risk of developing persistent asthma in the future.[3] Researchers have attempted to predict which children are prone to long-term asthma by categorizing epidemiologic phenotypes, identifying genetic risk factors, and creating predictive tools. However, the clinical utility of these measures is currently limited.[4]
Evaluation with spirometry, complemented by postbronchodilator response, is pivotal for establishing a definitive diagnosis. Spirometry aids in identifying various respiratory issues in children, including airway obstruction, restrictive lung disease, chest wall and respiratory muscle defects, diffusion defects, and respiratory muscle weakness. Although airborne agents typically dominate discussions of asthma triggers, a diverse range of stimuli, such as respiratory infections, allergen exposure, environmental irritants, physical activity, hormonal fluctuations, medications, and psychosocial factors, can precipitate or worsen symptoms. Treatment strategies revolve around educating patients and caregivers, regularly monitoring symptoms, and ensuring access to both fast-acting bronchodilators and appropriate controller medications tailored to disease severity.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Genetics
Intricate interactions between genetic and environmental factors influence the development of asthma. The genetic component of asthma is multifaceted, with various genes potentially contributing to the same asthma phenotype.[6] Additionally, in certain individuals, multiple genes may work together to create the asthma phenotype. Some genes directly affect asthma development, while others impact its severity or influence the patient's response to treatment. The interplay between genetic and environmental factors adds a layer of intricacy. Researchers propose that epigenetics, involving chemical modifications of DNA that regulate gene activity, is a mechanism through which the environment interacts with the genome, resulting in changes in gene expression.
Genetic studies have linked childhood-onset asthma to specific genetic markers near the ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) and gasdermin B (GSDMB) genes on chromosome 17q21.[7][8][9] Other genes associated with asthma are interleukin-33 (IL33), IL1R1, and interferon-inducible protein X (PYHIN1), mainly affecting individuals of African descent.[8] The EVE Consortium has also identified a genetic locus associated with thymic stromal lymphopoietin (TSLP)—a cytokine involved in asthma-related inflammation. Individuals with asthma frequently demonstrate elevated levels of TSLP expression in their airways compared to those without the condition.[8]
Other genetic loci implicated in asthma susceptibility include major histocompatibility complex, class II, DQ α1 (HLA-DQA1), Toll-like receptor 1 (TLR1), IL-6 receptor (IL6R), zona pellucida-binding protein 2 (ZPBP2), and gasdermin A (GSDMA).[10] Varying concordance rates among monozygotic twins indicate that exposure to environmental factors plays a crucial role in asthma development. Specific alleles may exert different effects based on environmental exposures. Due to this complex interaction, genetic testing for asthma currently lacks clinical utility.
Risk Factors
Risk factors for developing asthma span exposures across a patient's lifespan, including the perinatal period. However, the effects of mitigating these risks and their long-term impact on asthma development remain unclear. The most significant risk factor is atopy, characterized by a genetic propensity to produce specific immunoglobulin E (IgE) antibodies in response to common environmental allergens. Nearly one-third of children with atopy will develop asthma later in life.
Prenatal and Perinatal Factors
Prematurity is the most consistent and significant risk factor for asthma during the perinatal period, as preterm birth before 36 weeks is associated with increased asthma risk from childhood to adulthood due to impaired lung development. Maternal smoking during pregnancy is associated with reduced lung function in newborns and other adverse pregnancy outcomes, including premature delivery, which increases the likelihood of childhood asthma. Maternal age younger than 20 is correlated with higher rates of childhood asthma, whereas maternal age 30 or older is associated with lower rates.[11][12] Vitamin D deficiency during pregnancy may contribute to early-life wheezing and asthma due to the effects on immune function and fetal lung development. Although some studies show conflicting results, meta-analyses suggest that maternal vitamin D intake can protect against wheezing or asthma in offspring up to age 3.[13][14]
The Copenhagen Prospective Studies on Asthma in Childhood (COPSAC2010) reveals that children born to mothers with diets rich in omega-3 polyunsaturated fatty acids have a 17% chance of developing persistent wheeze or asthma in the first 3 years of life compared to a 24% chance in those with diets high in omega-6 polyunsaturated fatty acids.[13][15] Additionally, vitamins E and C and zinc may offer protective effects. Supplementing pregnant mothers with vitamin C at a dose of 500 mg/d seems to alleviate the detrimental effects of tobacco exposure, as offspring of supplemented mothers exhibit a wheezing incidence of 28% compared to 48% in those without vitamin C supplementation.[16][17]
Infancy, Childhood, and Adolescence
Risk factors during infancy and childhood include male sex until age 20 when the incidence equalizes, abnormal neonatal lung function, atopy, sensitization and exposure to common allergens, obesity, and early puberty.[18] Some studies indicate that the microbiome may also play a role. Exposure to certain bacteria and common allergens within the first year of life may lower the incidence of asthma, whereas exposure later increases the risk.[19]
Viral respiratory tract infections during infancy, especially those caused by respiratory syncytial virus and human rhinovirus, are predictors of subsequent asthma development in childhood and adulthood. In addition, it remains uncertain if the specific infections directly cause asthma or if wheezing during the infections predicts future asthma development. Additionally, early-life exposure to air pollution, such as products of combustion from gas-fired appliances and indoor fires, obesity, and early puberty, also increases the risk of developing asthma.
Some studies indicate a connection between maternal and infant use of acetaminophen, ibuprofen, and antibiotics and asthma. However, these studies did not account for confounding bias, necessitating further research. Active smoking and exposure to secondhand smoke are both risk factors for asthma development. This activity focuses on asthma in children aged 12 or younger. Please see StatPearls' companion resource, "Asthma in Adolescents and Adults," for additional information regarding the etiology of asthma in adolescents.
Epidemiology
Asthma leads to more school absences and hospitalizations than any other chronic illness and is the most common diagnosis upon admission in many children's hospitals in the United States. According to the United States Centers for Disease Control and Prevention (CDC), over 6 million (or 6.5%) children in the United States have asthma. The prevalence of asthma increases with age among children, ranging from 1.9% in children aged 0 to 4 to 7.7% in children and adolescents aged 5 to 14. Boys have a higher prevalence than girls aged 20 or younger, whereas, in adults, women are more affected than men.
Among infants, 20% experience wheezing with upper respiratory tract infections, but 60% will outgrow it by age 6. Black individuals have a higher prevalence of 10.1% compared to their White counterparts at 8.1%. Hispanic Americans generally have a lower prevalence of 6.4%, except for those from Puerto Rico, where the prevalence rises to 12.8%. Furthermore, underrepresented minorities and individuals living below the poverty line experience the highest incidence of asthma and asthma-related morbidity and mortality.
According to the Global Burden of Disease report, asthma accounts for approximately 420,000 deaths per year worldwide. Similar trends are observed in the United States, where the mortality rate of asthma has consistently declined. Currently, the mortality rate stands at 9.86 per million, compared to 15.09 per million in 2001. However, mortality rates remain consistently higher for Black patients than their White counterparts. According to the CDC, from 1999 to 2016, the asthma death rates among adults aged 55 to 64 were 16.32 per 1 million persons, 9.95 per 1 million for females, 9.39 per 1 million for individuals who were not Hispanic or Latino, and 25.60 per 1 million for Black patients.[20][21][22][23]
Pathophysiology
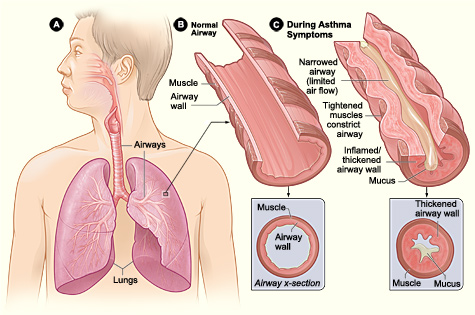
Asthma is a syndrome characterized by underlying mechanisms involving intricate interactions among inflammatory and resident airway cells. These mechanisms result in airway inflammation, intermittent airflow obstruction, and bronchial hyperresponsiveness (see Image. Pathophysiology of Asthma).
Airway Inflammation
The key to the development of clinical asthma lies in the activation of mast cells by cytokines and other mediators. Following initial allergen inhalation, affected patients exhibit an overexpression of the T-helper 2 subset (Th2) of lymphocytes relative to the Th1 type, leading to the production of specific IgE antibodies. The cytokines, including IL-4, IL-5, and IL-13, produced by Th2 lymphocytes promote IgE and eosinophilic responses in atopy. Once produced, these specific IgE antibodies bind to receptors on mast cells and basophils.
Additional allergen inhalation results in the cross-linking of allergen-specific IgE antibodies on the mast cell surface, causing rapid degranulation and release of histamine, prostaglandin D2 (PGD2), and cysteinyl leukotrienes such as LTC4, LTCD4, and LTCE4.[24][25] This process triggers contraction of the airway smooth muscle within minutes and may also stimulate reflex neural pathways. Subsequently, an influx of inflammatory cells, such as monocytes, dendritic cells, neutrophils, T lymphocytes, eosinophils, and basophils, may cause delayed bronchoconstriction 7 hours later.
Airflow Obstruction
Variable narrowing of the airway lumen throughout the tracheobronchial tree results in differing levels of airflow obstruction. Several factors contribute to this narrowing, including the contraction of airway smooth muscle, thickening of the airway wall due to edema, mucus plugging in the airways, and airway remodeling.[25]
The contraction and relaxation of airway smooth muscle, triggered by mediators released from inflammatory cells or through reflex neural pathways, causes acute limitation in airflow. Mast cells and eosinophil mediators, such as histamine and leukotrienes, are potent inducers of bronchoconstriction.
Another notable aspect of asthma is the heightened sensitivity of the bronchial passages, characterized by an excessive tightening of the airway smooth muscles in response to various physical, chemical, or environmental triggers. The precise mechanism leading to this hyperresponsiveness remains unclear. Some researchers propose alterations in breathing patterns, where smooth muscles contract excessively or lack relaxation normally associated with deep breaths. In addition, they also propose alterations in smooth muscle function or mass, enhanced sensitivity of neural pathways leading to bronchoconstriction, and exaggerated airway narrowing from smooth muscle contraction as a consequence of remodeling and structural abnormalities of the airway.[26][27][26]
Airway remodeling, characterized by thickening of the basement membrane, collagen deposition, and shedding of epithelial cells, can result in irreversible changes in the airways. This process accelerates the decline in lung function, especially in patients with severe and early-onset asthma.[28]
History and Physical
When initially diagnosing asthma, the history should concentrate on the presence and pattern of symptoms, any precipitating factors or conditions, and known asthma risk factors. For children with a confirmed diagnosis of asthma seeking follow-up care, the history should emphasize the presence, frequency, and severity of symptoms, recent emergency room visits or hospital admissions, the utilization of controller and rescue medications, and the assessment of proper inhaler techniques.
Cough and wheezing are frequently reported symptoms in children, with cough often being the sole presenting symptom.[29] Clinicians should consider asthma when evaluating a child with a cough, particularly if it primarily occurs at night or in response to specific triggers like cold air or exercise. Additionally, a persistent cough following a viral infection may suggest asthma. Poor school performance and excessive daytime fatigue may also indicate disrupted sleep due to nocturnal symptoms. Depending on the child's age, they may describe additional symptoms such as shortness of breath and chest tightness.
Physical examination may appear normal if the child is not currently symptomatic. However, additional examination findings may include nasal discharge, decreased air entry or wheezing, inflamed nasal mucosa, Dennie-Morgan lines, a transverse nasal crease, sinus tenderness, dark circles under the eyes, halitosis, eczema, atopic dermatitis, and nasal polyps. Nasal polyps can be associated with aspirin-exacerbated respiratory disease in adolescents and adults but should prompt an evaluation for cystic fibrosis in children. Features such as digital clubbing, a barrel chest, localized wheezing, urticarial rash, or stridor may suggest other diagnoses or comorbid conditions.[29][30]
During an acute exacerbation, potential symptoms include tachypnea, hypoxia, wheezing, a prolonged expiratory phase, and the use of accessory muscles, such as subcostal, intercostal, or supraclavicular retractions. Other signs may include nasal flaring, tripod positioning, inability to speak in complete sentences, or grunting. Notably, a child who initially exhibits significantly increased work of breathing but subsequently "tires out," appears to breathe at a normal rate, becomes lethargic, or no longer exhibits wheezing may be at risk of impending respiratory failure. Altered mental status, lethargy, unresponsiveness, cyanosis, or a "silent chest" are all signs indicating impending respiratory failure and arrest.
Evaluation
The presence of intermittent or chronic symptoms consistent with asthma, along with wheezing on physical examination, strongly suggests the diagnosis of asthma. Confirming the diagnosis involves excluding alternative diagnoses and demonstrating variable airflow limitation, typically observed on spirometry.
Spirometry
Spirometry is recommended by the National Asthma Education and Prevention Program (NAEPP) for patients aged 5 and older. This technique evaluates forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) by measuring a maximal inhalation followed by rapid and forceful exhalation into a spirometer. Asthma presents as an obstructive pattern on spirometry, indicated by an FEV1 reduced to less than 80% predicted and an FEV1/FVC ratio of less than 0.85 or 85%. FEV1 serves as a reliable indicator of future exacerbations. In children with a normal FEV1, a forced expiratory flow between 25% and 75% of vital capacity (FEF25%-75%) of less than 65% also correlates with reversible airflow.
All children should undergo spirometry before and after bronchodilator administration, as some may exhibit a significant response to bronchodilators despite having a normal FEV1. An improvement of FEV1 of 12% or more from baseline after administration of a short-acting bronchodilator indicates significant reversibility, although researchers established this value in adults. Some authors suggest an increase in FEV1 of 8% or more may be better for children.[31][32]
Diagnosing asthma for children aged 5 or younger can be challenging. Clinicians must rely on a pattern of symptoms indicative of asthma in addition to family history and physical examination findings. According to the Global Initiative for Asthma (GINA), symptoms consistent with asthma in young children include:
- Recurrent, persistent cough that gets worse at night.
- Cough that worsens with exposure to triggers such as laughing, crying, exercise, or tobacco smoke exposure.
- Decreased level of activity compared to other children.
- Personal or family history of allergic or atopic disease.
- Improvement in symptoms with a 2- to 3-month trial of inhaled corticosteroids (ICS) or worsening after stopping a trial of controller medication.
- Reversal of symptoms within the timeframe that albuterol should be effective.
Allergy Testing
Selective allergy testing can help develop avoidance strategies for children exposed to furry animals, molds, cockroaches, or dust mites. Although outdoor allergens are rare triggers in infants and young children, they may affect older children. Food allergy testing is unnecessary unless the child has a clear history of gastrointestinal symptoms, shortness of breath, asthma, or urticaria temporally linked to ingesting specific foods.
Bronchoprovocation Testing
During bronchoprovocation testing, clinicians induce bronchoconstriction using inhaled methacholine, cold air, or exercise. This testing may be beneficial for children suspected of having exercise-induced asthma, those with suspected asthma but normal spirometry, or patients presenting with atypical symptoms or an isolated cough. Patients receive increasing doses of the provocative agent, followed by spirometry to create a dose-response curve. A decrease in FEV1 of 20% or more from baseline with the standard dose of methacholine or 15% or more with the standard dose of hypertonic saline, mannitol, or hyperventilation indicates a positive test.
Exhaled Nitric Oxide
Eosinophilic airway inflammation causes an upregulation of nitric oxide synthase in the respiratory mucosa and increased nitric oxide levels in the exhaled breath. The fractional exhaled nitric oxide (FENO) levels in some patients with asthma are higher than those without asthma. FENO is a noninvasive biomarker that indicates the presence of asthma and eosinophilic airway inflammation. A FENO of less than 25 ppb in adults and less than 20 ppb in children aged 12 or younger implies the absence of eosinophilic airway inflammation. A FENO greater than 35 ppb in children suggests eosinophilic airway inflammation. The exact role of FENO measurement in diagnosing and managing asthma is undefined.
Peak Flow
Peak expiratory flow measurement should not be used as the sole diagnostic tool for asthma in children. Although it can aid in monitoring asthma severity, serial spirometry is the preferred method for assessment.
Additional Testing
A chest radiograph is unnecessary unless the child fails to respond to initial therapy or there is suspicion of an alternative underlying pathology. A modified barium swallow is necessary for suspicion of aspiration or swallowing abnormalities. Clinicians should consider a sweat chloride test in children with recurrent respiratory complaints or pneumonia, frequent foul-smelling stool, malabsorption, and failure to thrive.
Acute Exacerbation
Children presenting with an acute exacerbation should undergo a rapid assessment, including a complete set of vital signs and oxygen saturation. Essential observations are level of consciousness, anxiety, agitation, breathlessness, wheezing, air entry, accessory muscle use, and retractions.[33] In the hospital setting, severity scores such as the Pediatric Respiratory Assessment Measure (PRAM) help predict initial exacerbation severity, assess response to treatment, and help determine if hospitalization is necessary.[34]
Clinicians may also assess severity using peak flow measurement, although this method is less common in children. Children aged 6 or younger may struggle to perform these measurements accurately, and very ill children may be unable to provide 3 readings. Asthma exacerbations are diagnosed clinically and do not require routine laboratory or imaging studies.
A chest radiograph is warranted in cases of asymmetric lung findings, chest pain, unexplained fever, worsening symptoms despite treatment, and when the patient is critically ill. Common findings on chest radiographs in acute asthma exacerbations include hyperinflated lungs and interstitial prominence. Focal consolidation on a chest radiograph suggests the presence of pneumonia.
Additional laboratory studies, such as arterial blood gas analysis, may be warranted in patients with worsening symptoms despite treatment or in critically ill patients. However, these tests should not delay the initiation of bronchodilator therapy.
Treatment / Management
The initial treatment of pediatric asthma is determined by assessing the intensity and severity of symptoms and the likelihood of future exacerbations. In children aged 5 or younger, the risk of developing persistent asthma is also considered. This assessment involves evaluating the frequency of daytime and nocturnal symptoms, using short-acting β-agonists (SABA) for symptom management, assessing the impact of symptoms on daily activities, and conducting spirometry tests in children aged 5 and older. In addition, the number of exacerbations requiring glucocorticoids in the previous year helps determine the risk of future exacerbations.
Rather than evaluating severity, healthcare professionals assess the level of symptom control in patients already receiving controller therapy. Experts advise including spirometry alongside assessing symptoms and medication usage to gauge asthma control effectively. Clinicians can consider measuring FENO if uncertainty exists regarding diagnosis or level of management.
Non-Pharmacological Management
Non-pharmacological management of asthma involves various patient education strategies. According to the National Asthma Education and Prevention Program-Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, personalized one-on-one education from the patient's primary clinician proves highly effective. Studies indicate that such education reduces asthma exacerbations and hospitalizations. Healthcare professionals should provide culturally specific asthma education that explains asthma and its symptoms, individual triggers, and avoidance strategies. Avoiding environmental triggers that could trigger asthma, such as firsthand or secondhand tobacco smoke, food or medication triggers, as well as pollutants and irritants, is vital.[35]
Patients and caregivers must grasp the correct inhaler technique and distinguish between rescue, controller, and combination medications. Clinicians should actively explore potential barriers to medication adherence and work collaboratively with patients to resolve any concerns or obstacles, thereby enhancing medication compliance.
Pharmacological Management
The following is a general overview of the stepwise process outlined by GINA for choosing pharmacological therapy in children aged 5 and younger. Tables 1 and 2 provide comprehensive details on the NAEPP and GINA guidelines, including alternative therapeutic options. Variations in the guidelines notably commence from Step 3 onward. Table 3 provides a detailed description of the NAEPP and GINA guidelines for the stepwise management of asthma for children aged 5 to 11.
- Step 1: Every child experiencing wheezing should be able to use an SABA. The only exception is infants aged 1 or younger who present with wheezing caused by bronchiolitis. If patients require an SABA more than twice a week for 1 month, they should progress to step 2.
- Step 2: Healthcare professionals should initiate a daily low-dose ICS along with SABA as needed, maintaining this regimen for at least 3 months. If symptoms remain poorly controlled, clinicians must verify the absence of an alternative diagnosis, ensure compliance with prescribed medication, assess inhaler technique, and investigate exposure to tobacco smoke or environmental allergens.
- Step 3: Doubling the initial dose of the ICS for a period of 3 months is recommended. If asthma symptoms persist despite this adjustment, referral to an asthma specialist is warranted.
- Step 4: Treatment options at this stage may involve further increasing the ICS dosage, adding a leukotriene receptor antagonist (LTRA), combining a long-acting β-agonist (LABA) with ICS, or introducing a low-dose oral corticosteroid (OCS) until symptom improvement is observed.
Table 1. National Asthma Education and Prevention Program: Expert Panel Working Group. Initial Asthma Therapy in Infants and Children Aged 4 or Younger, With Recurrent Wheezing
| Steps | Asthma Symptoms | Therapies |
|
Step 1, Intermittent |
Daytime symptoms ≤2 days per week No nocturnal awakenings No interference with activities Exacerbations treated with OCS ≤1 per year |
SABA, as needed A short course of daily ICS beginning at the start of a respiratory tract infection |
|
Step 2, Mild persistent |
Daytime symptoms >2 but <7 days per week Nocturnal awakenings up to 1 to 2 nights per month Minor interference with activities Exacerbations treated with OCS ≥2 episodes in 6 months or ≥4 episodes of wheezing lasting >1 day per week And risk factors for persistent asthma* |
Preferred: Low-dose ICS daily and SABA as needed Or, Alternative: Daily LTRA and SABA as needed |
|
Step 3, Moderate persistent |
Daily asthma symptoms Nocturnal awakenings 3 to 4 nights per month Need daily relief inhaler Some activity limitation Exacerbations treated with OCS ≥2 times in 6 months or ≥4 episodes of wheezing lasting >1 day per year and risk factors for persistent asthma* |
Preferred: Low-dose ICS-LABA and SABA as needed Or, Daily low-dose ICS plus LTRA and SABA as needed Or, Daily medium-dose ICS and SABA as needed |
|
Step 4, Severe persistent |
Symptoms all day Nightly awakenings >1 per week Need for SABA several times daily Extreme limitation in activity Exacerbations treated with OCS ≥2 times in 6 months or ≥4 episodes of wheezing lasting >1 day per year and risk factors for persistent asthma* |
Preferred: Medium-dose ICS-LABA and SABA as needed Alternative: Daily medium-dose ICS plus LTRA and SABA as needed |
|
Step 5 |
Poorly controlled severe asthma |
Preferred: Daily high-dose ICS-LABA plus SABA as needed Alternative: Daily high-dose ICS and SABA as needed, plus LTRA |
|
Step 6 |
Preferred: Daily high-dose ICS-LABA plus SABA as needed and OCS Alternative: Daily high-dose ICS and SABA as needed, plus LTRA and OCS |
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β-agonist.
*Risk factors for future asthma exacerbations include uncontrolled asthma symptoms, experiencing one or more severe exacerbations in the last year, exposure to tobacco smoke or other known triggers, outdoor pollution, psychological and socioeconomic stressors affecting the child and their family, and outdoor pollution.
Table 2. Global Initiative for Asthma. Initial Asthma Therapy in Infants and Children Aged 5 or Younger, With Recurrent Wheezing
| Steps | Asthma Symptoms | Therapies |
| Step 1 |
Wheezing primarily with viral infections and minimal to no symptoms between infections
|
Preferred: SABA as needed Or, A short course of daily ICS beginning at the start of a respiratory tract infection
|
| Step 2 |
Asthma symptoms that require reliever inhaler ≥2 times per week for 1 month or ≥3 exacerbations per year Or, Treated with SABA every 6 to 8 weeks, but the diagnosis of asthma is uncertain
|
Preferred: Low-dose ICS and SABA as needed Or, Daily LTRA and SABA as needed Or, A short course of daily ICS beginning at the start of a respiratory tract infection
|
| Step 3 | Symptoms are not well controlled on daily ICS
|
Preferred: Daily dose of ICS and SABA should be doubled as needed or Daily low-dose ICS plus LTRA and SABA as needed
|
| Step 4 | Symptoms are not well controlled on a double dose of ICS |
Preferred: Double dose of ICS and SABA should be continued, as needed, and patients should refer to an asthma specialist Or, LTRA should be added Or, The frequency of ICS should be increased
|
| Step 5 | Severe asthma is not well controlled |
Preferred: Daily high-dose ICS-LABA and SABA as needed Alternative: Daily high-dose ICS and LTRA plus SABA as needed
|
| Step 6 |
Preferred: Daily high-dose ICS-LABA and SABA as needed and OCS Alternative: Daily high-dose ICS and LTRA plus SABA, as needed, and OCS
|
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β-agonist.
Table 3. Management of Asthma in Children Aged 5 Through 11
| NAEPP (Ages 5-11) | Asthma Symptoms | Therapies |
GINA (Ages 6-11) |
Asthma Symptoms | Therapies |
| Step 1 |
Intermittent: Daytime symptoms ≤2 days per week Nocturnal awakenings ≤2 per month No interference with activities Normal FEV1 and FEV1/FVC Exacerbations ≤1 per year |
SABA as needed | Step 1 | Symptoms <2 times per month |
Preferred: SABA as needed Or, A short course of daily ICS beginning at the start of a respiratory tract infection
|
| Step 2 |
Mild persistent: Daytime symptoms >2 but <7 days per week Nocturnal awakenings 3 to 4 nights per month Minor interference with activities Normal FEV1 and FEV1/FVC Exacerbations ≥2 per year |
Preferred: Daily low-dose ICS and SABA as needed Alternative: Daily LTRA and SABA as needed |
Step 2 | Symptoms or need for reliever inhaler ≥2 times per month |
Preferred: Low-dose ICS and SABA as needed or Daily LTRA and SABA as needed or A short course of daily ICS beginning at the start of a respiratory tract infection
|
| Step 3 |
Moderate persistent: Daily symptoms Nocturnal awakenings >1 per week but not daily Daily use of SABA Some activity limitation FEV1 60% to 80% predicted; FEV1/FVC below normal Exacerbations ≥2 per year |
Preferred: Daily and low-dose ICS-formoterol as needed Alternative: Daily medium-dose ICS and SABA as needed Or, Daily low-dose ICS-LABA or low-dose ICS plus LTRA and SABA as needed |
Step 3 | Symptoms most days or nocturnal symptoms ≥1 time per month |
Preferred: Daily dose of ICS and SABA should be doubled as needed Or, Daily low-dose ICS plus LTRA and SABA as needed |
| Step 4 | Severe persistent:
Symptoms throughout the day and need for SABA several times a day Nocturnal awakenings most nights Extreme activity limitation FEV1 <60% predicted and FEV1/FVC below normal Exacerbations ≥2 per year |
Preferred: Medium-dose ICS-formoterol daily and as needed Alternative: Daily medium-dose ICS-LABA and SABA as needed Or, Daily medium-dose ICS plus LTRA and SABA as needed |
Step 4 | Severe asthma |
Preferred: Double dose of ICS and SABA should be continued, as needed, and patients are advised to refer to an asthma specialist Or, LTRA should be added Or, The frequency of ICS should be increased |
| Step 5 | Severe asthma is not well controlled |
Preferred: Daily high-dose ICS-LABA and SABA as needed Alternative: Daily high-dose ICS plus LTRA and SABA as needed Add-on therapy: A biological agent such as omalizumab is an additional option for patients aged 6 or older |
Step 5 | Severe asthma is not well controlled |
Preferred: Daily high-dose ICS-LABA and SABA as needed Alternative: Daily high-dose ICS and LTRA plus SABA as needed
|
| Step 6 | Severe asthma is not well controlled |
Preferred: Daily high-dose ICS-LABA plus OCS and SABA as needed Alternative: Daily high-dose ICS plus LTRA and OCS with a SABA as needed Add-on therapy: A biological agent like omalizumab is an additional option for patients aged 6 or older |
Step 6 | Severe asthma is not well controlled |
Preferred: Daily high-dose ICS-LABA and SABA, as needed, and OCS Alternative: Daily high-dose ICS and LTRA plus SABA, as needed, and OCS |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; LABA, long-acting β-agonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β-agonist.
Children aged 5 or younger should use a pressurized metered dose inhaler (MDI) with a valved spacer. Depending on their age, a face mask may also be helpful. Typically, an average child takes 5 to 10 breaths to empty a spacer. Nebulizers are the only alternative for young children. Routine follow-up every 1 to 3 months is necessary to ensure adequate symptom management. Upon reevaluation, patients who experience poor asthma symptom control, exacerbations requiring systemic glucocorticoids, or are at high risk of an exacerbation at their current step of therapy escalate to the next level of treatment. After maintaining good control for 3 to 6 months, clinicians can attempt stepwise therapy reduction based on GINA or NAEPP guidelines.
Asthma action plans are necessary for all patients with asthma. These plans are individualized and developed in collaboration with each patient and caregiver. They include detailed directions for managing asthma during periods of wellness, at the onset of symptoms, and during acute exacerbations requiring medical evaluation.[36] The NAEPP provides sample asthma action plans for children aged 0 to 5, patients aged 5 or older, and for use in school settings. Please refer to the Deterrence and Patient Education section of this activity for a link to a printable action plan. Clinicians develop an asthma action plan based on symptoms or peak flow readings and divide it into 3 zones—green, yellow, and red. Patients in the green zone are asymptomatic, with peak flows at 80% or better than their personal best. They feel good and continue to take their long-term control medication. Peak flow readings in the yellow zone are 50% to 79% of the patient's personal best, and symptoms such as cough, wheezing, and shortness of breath begin to interfere with activity levels. In the red zone, patients experience peak flow readings below 50% of their best, accompanied by severe shortness of breath, and an inability to perform everyday activities.
Biological Agents
Biological agents are possible for children aged 6 and older who have not responded to traditional therapies. The candidates for omalizumab, a monoclonal antibody against IgE, are children with sensitization to at least 1 perennial aeroallergen and moderate-to-severe asthma. Dupilumab, another monoclonal antibody targeting the IL-4 receptor and inhibiting IL-4 and IL-13 signaling, is used in children aged 6 and older as additional maintenance therapy. Mepolizumab, which targets the IL-5 receptor, is utilized in children with severe eosinophilic asthma.
Acute Exacerbation
Patients experiencing an acute asthma exacerbation may manage symptoms at home or require urgent medical care, depending on severity and risk factors for fatal asthma. Urgent medical attention is needed for those with marked shortness of breath, inability to speak more than short phrases, use of accessory muscles, mental status changes, or at high risk of fatal exacerbation, which requires urgent medical attention. Risk factors that predict a fatal asthma exacerbation include:
- Previous life-threatening exacerbation
- Exacerbation despite OCS use
- Multiple emergency room visits (3 or more) or hospitalizations (more than 1) in the last year
- Use of more than 1 SABA MDI per month
- Food allergies
- Chronic heart or lung disease
- Medication nonadherence
- Psychosocial stresssors
Home or Office Care
At the onset of an exacerbation, the patient should receive rescue medication with a repeat dose 20 minutes after the initial dose. Typical formulations include:
- Albuterol MDI of 2 to 4 puffs or nebulization solution dose of 1.25 to 2.5 mg for children aged 4 or younger and 2.5 to 5 mg if the children are aged 4 to 11 when using a SABA.
- Budesonide-formoterol of 1 puff or up to a maximum dosage of 8 puffs per day in children aged 4 and older with moderate-to-severe persistent asthma.
Children aged 6 to 11 with severe symptoms or those with mild-to-moderate symptoms and are at high risk of a fatal exacerbation should also receive OCS soon after beginning SABAs. However, guidelines do not support caregiver administration of OCS to children aged 5 and younger.
Patients who respond well to inhaling their SABA and do not experience recurrence of symptoms within 4 hours can stay at home and continue the SABA every 4 to 6 hours as needed. However, patients who do not fully respond should receive a third dose of rescue medication and contact their clinician for further instructions or proceed to the emergency department.
Patients presenting to the office may receive up to 3 doses of a higher dose of SABA, such as 4 to 8 puffs of an albuterol MDI or 2.5 mg to 5 mg via nebulizer over 1 hour. Patients with moderate-to-severe symptoms can also receive inhaled ipratropium with the dosage based on age and weight. Patients who do not respond to 3 doses or who continue to require oxygen after the first or second bronchodilator dose should be transferred to the emergency department. If not already administered, OCS should be given.
Emergency Room Care
In the emergency room, the severity of an asthma exacerbation is generally determined by clinical assessment, aided by scales such as the Pulmonary Index Score and the Pediatric Respiratory Assessment Measure.
All patients should receive oxygen to maintain saturations above 92%.[37] Within the first hour, patients then receive 3 treatments of an inhaled SABA like albuterol via a nebulizer or MDI, followed by repeat dosing every 1 to 4 hours. Nebulized treatments are administered individually or continuously. Research comparing the efficacy of an MDI combined with a valved-holding chamber to nebulizer delivery reveals that administration via MDI is at least as effective as small-volume nebulizers.[38][39][40] The advantages of nebulizer use in children are the ability to administer humidified oxygen and ipratropium simultaneously. The NAEPP dosing recommendations for albuterol MDI in the emergency room for acute asthma exacerbations are 4 to 8 puffs.(A1)
One potential dosing strategy includes:
- Body weight 5 to 10 kg: 4 puffs
- Body weight 10 to 20 kg: 6 puffs
- Body weight more than 20 kg: 8 puffs
The individualized dose of nebulized albuterol is 0.15 mg/kg, with a minimum of 2.5 mg and a maximum of 5 mg. The dosing for continuous albuterol nebulizer treatment varies. One protocol includes:
- Body weight 5 to 10 kg: 5 to 7.5 mg/h
- Body weight 10 to 20 kg: 10 to 12.5 mg/h
- Body weight more than 20 kg: 15 to 20 mg/h
In addition to a SABA, patients with moderate-to-severe asthma exacerbations receive inhaled ipratropium, a short-acting muscarinic antagonist (SAMA), at a dosage of 250 µg for children with a body weight of less than 20 kg and 500 µg for those with a body weight of more than 20 kg by nebulization or 4 to 8 puffs by MDI, every 20 minutes for 3 doses. Treatment with 2 to 3 doses of ipratropium combined with a SABA reduces hospitalization rates when compared to children who receive SABA therapy alone.[41](A1)
Magnesium Sulfate
NAEPP guidelines suggest that children aged 4 or older who present with a severe asthma exacerbation or those who do not respond to SABAs, ipratropium, and glucocorticoids receive magnesium sulfate (MgSO4) 25 to 75 mg/kg with a maximum of 2 g intravenously (IV) over 20 minutes.[42][43] A meta-analysis reveals that the addition of MgSO4 to the treatment regimen for children with severe exacerbations reduces hospital admissions.[44][45] However, it is noteworthy that kidney failure is a relative contraindication to the use of MgSO4. (A1)
Parenteral β-Agonists
Subcutaneous and intramuscular β-agonists, such as epinephrine and terbutaline, are possible treatment options for children presenting with severe symptoms, extremely poor air flow, or those unable to cooperate with nebulizer treatments. In severe cases, clinicians may administer these agents rapidly alongside nebulized albuterol.
Glucocorticoids
As with outpatient therapy, clinicians should administer glucocorticoids promptly to children with moderate-to-severe asthma exacerbations, as their early administration reduces hospital admission rates. Oral and IV glucocorticoids have equivalent effects when given in comparable doses.[46] OCS are equally efficacious and preferred due to their less invasive nature. Dexamethasone, at 0.6 mg/kg/d for 2 days, is often preferred in the emergency department due to its long half-life and equivalent efficacy to prednisone.[47][48] When administering prednisone or prednisolone, the dosage is 1 to 2 mg/kg/d with a maximum dosage of 20 mg/d in children aged 0 to 2, 30 mg/d in children aged 3 to 5, and 40 mg/d in children aged 6 to 11 for 3 to 5 days. A shorter course and lower dose of OCS are equally as effective as a higher dose and longer course. IV steroids are necessary for patients with impending or actual respiratory arrest or those who are intolerant of oral glucocorticoids. (A1)
Impending Respiratory Failure
Clinicians should promptly notify the intensive care unit team or anesthesiology if children continue to decompensate or exhibit cyanosis, inability to sustain respiratory effort, decreased mental status, oxygen saturation below 90%, or respiratory acidosis. IV terbutaline and a trial of noninvasive positive pressure ventilation, including continuous positive airway pressure or bilevel positive airway support, may be beneficial in such cases.
Indications for endotracheal intubation include:
- Respiratory or cardiac arrest
- Hypoxemia despite high concentrations of oxygen or noninvasive positive pressure ventilation
- Severe increased work of breathing
- Altered mental status
Differential Diagnosis
The following list includes the differential diagnoses for asthma in children aged 12 or younger:
Upper Airway Diseases
- Allergic rhinitis and sinusitis [49]
Large Airway Obstruction
- Foreign body aspiration
- Vascular ring or laryngeal webs
- Laryngomalacia
- Tracheomalacia
- Lymphadenopathy
- Mass
- Epiglottitis
- Vocal cord dysfunction [49]
Small Airway Obstruction
- Bronchiolitis or wheezing associated with respiratory infections
- Cystic fibrosis
- Primary ciliary dyskinesia
- Bronchopulmonary dysplasia [49]
Other Causes
- Congestive heart failure
- Gastroesophageal reflux disease
- Anaphylaxis
- Angioedema
- Chronic obstructive pulmonary disease (more likely in adults)
- Pulmonary embolism
- Recurrent aspiration
- Immunodeficiency
- Pulmonary edema
- Cardiomegaly
- Atypical infection with Mycoplasma pneumonia [49]
Prognosis
Childhood asthma patterns are strong predictors of long-term outcomes. Episodic asthma tends to result in better adult outcomes, whereas persistent childhood asthma often leads to ongoing symptoms and modest lung function impairment in adulthood. Research suggests that 30% to 70% of children with asthma experience significant improvement or become symptom-free by early adulthood.[50] However, nearly 75% of those with asthma and wheezing during adolescence continue to experience symptoms into adulthood. Persistent asthma is associated with factors such as atopy, low lung function, and increased airway hyperresponsiveness, with sensitization and exposure to indoor allergens posing a 3-fold higher risk.
Effective asthma management is crucial for long-term prognosis. The goals of asthma management include reducing the risk of future exacerbations, preventing hindered lung development in children, preserving lung function, and minimizing adverse medication effects. Factors such as a history of exacerbations within the past year, poor adherence to asthma medication, improper inhaler technique, reduced lung function, smoking or vaping, elevated FENO levels, and blood eosinophilia all contribute to an elevated risk of exacerbations and poorer prognosis.
Complications
Complications associated with asthma can stem from the condition itself or from medications and therapeutic interventions. The following lists outline potential complications of asthma:
Complications of Asthma
- Pneumonia
- Interference with school and sports
- Lung remodeling
- Poor sleep and fatigue
- Death
Complications due to Endotracheal Intubation
- Hypotension
- Pneumothorax (also a complication of asthma)
- Myopathy
- Pneumomediastinum (also a complication of asthma)
- Pneumoperitoneum
- Subcutaneous emphysema
- Aspiration
- Subglottic stenosis
- Infection
- Gastrointestinal bleeding due to stress ulcers
Complications due to Medications
- Potential neuropsychiatric symptoms such as agitation, depression, insomnia, and suicidal thoughts or actions associated with montelukast, which is a LTRA
- Dysphonia and oral candidiasis resulting from ICS
- Rare occurrences of adrenal insufficiency attributed to ICS [51]
- Slight reduction in linear growth velocity due to ICS use [52]
- Glaucoma, cataracts, adrenal insufficiency, and hyperglycemia due to OCS
- Decreased serum potassium, phosphate, and magnesium, and increase in serum glucose associated with albuterol [53]
- Stress-induced or takotsubo cardiomyopathy associated with the treatment of status asthmaticus [54]
Consultations
According to the guidelines from the NAEPP and GINA, challenges in confirming an asthma diagnosis or uncertainties regarding a prior diagnosis warrant consideration for evaluation by a pulmonology or allergy specialist. This is particularly important if signs or symptoms suggest an alternative or exacerbating condition. Referral is also recommended for children with a history of severe asthma exacerbations, such as ICU admissions or requiring mechanical ventilation, as well as for those experiencing frequent hospitalizations or needing 2 or more courses of oral glucocorticoids within a year.
Specialist input is advisable when asthma control remains inadequate despite active therapy and appropriate monitoring, when children aged 5 or older require step 3 or 4 level therapy, or when children younger than 5 necessitate step 2 or higher therapy. Additional circumstances warranting specialist involvement include cases requiring further diagnostic tests, such as allergy skin testing, or consideration of allergen immunotherapy or biologic therapy. Ensuring timely specialist involvement in these scenarios can optimize asthma management and enhance patient outcomes.
Deterrence and Patient Education
In pediatric asthma management, patient education is pivotal in deterring exacerbations and promoting optimal disease control. Central to this approach is educating both caregivers and children about asthma triggers, recognizing symptoms, and the importance of following prescribed treatment plans. Caregivers and patients should understand how to recognize early warning signs of exacerbations and be instructed on when and how to seek prompt medical assistance. Furthermore, teaching proper usage of inhalers, spacer devices, and monitoring techniques such as peak flow measurements builds confidence in managing asthma at home. In addition, it is essential to discuss the potential adverse effects of asthma medications with caregivers, empowering them to identify adverse reactions and make informed decisions about their child's healthcare.
Furthermore, emphasizing the importance of avoiding tobacco smoke, allergens, and environmental pollutants can significantly mitigate asthma exacerbations. Developing an asthma action plan tailored to the child's needs and level of asthma control provides a structured approach for managing exacerbations and adjusting treatment as necessary. Please refer to the following link for an asthma action plan download from the CDC, "Asthma Action Plan." Scheduled follow-up visits with healthcare professionals enable continual evaluation of symptom management and medication performance and the opportunity to address any concerns or apprehensions. By integrating comprehensive patient education, medication management, and personalized asthma action plans, clinicians can empower families to proactively manage pediatric asthma and improve long-term outcomes.
Pearls and Other Issues
- Not all cases of wheezing indicate asthma. Clinicians must remember that wheezing has a broad differential.
- Clinicians should be cautious with patients exhibiting significant respiratory distress despite apparently normal blood gas levels, as well as those who appear lethargic or altered, as they may be approaching respiratory failure and subsequent cardiac arrest.
- Patients who necessitate continuous nebulized treatments or interventions beyond standard medications, such as albuterol, ipratropium, and steroids, may be experiencing status asthmatics. This requires hospital admission for comprehensive evaluation and treatment.
- Importantly, clinicians should investigate common asthma triggers, such as upper respiratory tract infections, allergens, and exercise, as well as less common triggers, such as gastroesophageal reflux, medications, and psychological distress
Enhancing Healthcare Team Outcomes
Pediatric asthma is a prevalent and multifaceted respiratory condition most often characterized by recurrent wheezing and coughing. These symptoms result from variable expiratory airflow limitation, airway hyperresponsiveness, and inflammation. Although asthma can occur at any stage of life, it frequently initiates during childhood, and the pattern of asthma during childhood is highly indicative of long-term outcomes. A substantial portion of cases that persist into adolescence continue into adulthood, especially when accompanied by atopy, low lung function, and elevated airway hyperresponsiveness.
For optimal management, healthcare professionals need to integrate comprehensive patient education, evidence-based medication administration following a stepwise therapeutic approach tailored to symptom severity, and personalized asthma action plans. Regular follow-up appointments with healthcare professionals are vital for continuous assessment of asthma control and medication effectiveness, as well as for addressing any concerns or questions. Effective interprofessional communication is crucial for keeping all team members informed about acute exacerbations, emergency department visits, hospital admissions, and medication changes, ensuring seamless care coordination across different healthcare settings.
A collaborative approach among healthcare professionals is crucial for enhancing patient-centered care, ensuring patient safety, and reducing morbidity and mortality. Physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals each contribute unique skills and expertise to the care team. By leveraging these skills collaboratively, the team can develop an individualized comprehensive strategy for pediatric asthma care. Such a collaborative and multidisciplinary approach will optimize pediatric asthma care, providing safe, effective, patient-centered treatment while enhancing team performance and improving patient outcomes.[55][56][57]
Media
(Click Image to Enlarge)
References
Vonk JM, Postma DS, Boezen HM, Grol MH, Schouten JP, Koëter GH, Gerritsen J. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004 Nov:59(11):925-9 [PubMed PMID: 15516465]
Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet (London, England). 2008 Sep 20:372(9643):1058-64. doi: 10.1016/S0140-6736(08)61447-6. Epub [PubMed PMID: 18805334]
Matricardi PM, Illi S, Grüber C, Keil T, Nickel R, Wahn U, Lau S. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. The European respiratory journal. 2008 Sep:32(3):585-92. doi: 10.1183/09031936.00066307. Epub 2008 May 14 [PubMed PMID: 18480107]
Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. The Journal of allergy and clinical immunology. 2010 Aug:126(2):187-97; quiz 198-9. doi: 10.1016/j.jaci.2010.07.011. Epub [PubMed PMID: 20688204]
Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, DiMango E, Dixon AE, Elward KS, Hartert T, Krishnan JA, Lemanske RF Jr, Ouellette DR, Pace WD, Schatz M, Skolnik NS, Stout JW, Teach SJ, Umscheid CA, Walsh CG. 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. The Journal of allergy and clinical immunology. 2020 Dec:146(6):1217-1270. doi: 10.1016/j.jaci.2020.10.003. Epub [PubMed PMID: 33280709]
Meyers DA. Approaches to genetic studies of asthma. American journal of respiratory and critical care medicine. 1994 Nov:150(5 Pt 2):S91-3 [PubMed PMID: 7952602]
Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, Heinzmann A, Simma B, Frischer T, Willis-Owen SA, Wong KC, Illig T, Vogelberg C, Weiland SK, von Mutius E, Abecasis GR, Farrall M, Gut IG, Lathrop GM, Cookson WO. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007 Jul 26:448(7152):470-3 [PubMed PMID: 17611496]
Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, Himes BE, Levin AM, Mathias RA, Hancock DB, Baurley JW, Eng C, Stern DA, Celedón JC, Rafaels N, Capurso D, Conti DV, Roth LA, Soto-Quiros M, Togias A, Li X, Myers RA, Romieu I, Van Den Berg DJ, Hu D, Hansel NN, Hernandez RD, Israel E, Salam MT, Galanter J, Avila PC, Avila L, Rodriquez-Santana JR, Chapela R, Rodriguez-Cintron W, Diette GB, Adkinson NF, Abel RA, Ross KD, Shi M, Faruque MU, Dunston GM, Watson HR, Mantese VJ, Ezurum SC, Liang L, Ruczinski I, Ford JG, Huntsman S, Chung KF, Vora H, Li X, Calhoun WJ, Castro M, Sienra-Monge JJ, del Rio-Navarro B, Deichmann KA, Heinzmann A, Wenzel SE, Busse WW, Gern JE, Lemanske RF Jr, Beaty TH, Bleecker ER, Raby BA, Meyers DA, London SJ, Mexico City Childhood Asthma Study (MCAAS), Gilliland FD, Children's Health Study (CHS) and HARBORS study, Burchard EG, Genetics of Asthma in Latino Americans (GALA) Study, Study of Genes-Environment and Admixture in Latino Americans (GALA2) and Study of African Americans, Asthma, Genes & Environments (SAGE), Martinez FD, Childhood Asthma Research and Education (CARE) Network, Weiss ST, Childhood Asthma Management Program (CAMP), Williams LK, Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-Ethnicity (SAPPHIRE), Barnes KC, Genetic Research on Asthma in African Diaspora (GRAAD) Study, Ober C, Nicolae DL. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nature genetics. 2011 Jul 31:43(9):887-92. doi: 10.1038/ng.888. Epub 2011 Jul 31 [PubMed PMID: 21804549]
Level 1 (high-level) evidenceStein MM, Thompson EE, Schoettler N, Helling BA, Magnaye KM, Stanhope C, Igartua C, Morin A, Washington C 3rd, Nicolae D, Bønnelykke K, Ober C. A decade of research on the 17q12-21 asthma locus: Piecing together the puzzle. The Journal of allergy and clinical immunology. 2018 Sep:142(3):749-764.e3. doi: 10.1016/j.jaci.2017.12.974. Epub 2018 Jan 4 [PubMed PMID: 29307657]
Demenais F, Margaritte-Jeannin P, Barnes KC, Cookson WOC, Altmüller J, Ang W, Barr RG, Beaty TH, Becker AB, Beilby J, Bisgaard H, Bjornsdottir US, Bleecker E, Bønnelykke K, Boomsma DI, Bouzigon E, Brightling CE, Brossard M, Brusselle GG, Burchard E, Burkart KM, Bush A, Chan-Yeung M, Chung KF, Couto Alves A, Curtin JA, Custovic A, Daley D, de Jongste JC, Del-Rio-Navarro BE, Donohue KM, Duijts L, Eng C, Eriksson JG, Farrall M, Fedorova Y, Feenstra B, Ferreira MA, Australian Asthma Genetics Consortium (AAGC) collaborators, Freidin MB, Gajdos Z, Gauderman J, Gehring U, Geller F, Genuneit J, Gharib SA, Gilliland F, Granell R, Graves PE, Gudbjartsson DF, Haahtela T, Heckbert SR, Heederik D, Heinrich J, Heliövaara M, Henderson J, Himes BE, Hirose H, Hirschhorn JN, Hofman A, Holt P, Hottenga J, Hudson TJ, Hui J, Imboden M, Ivanov V, Jaddoe VWV, James A, Janson C, Jarvelin MR, Jarvis D, Jones G, Jonsdottir I, Jousilahti P, Kabesch M, Kähönen M, Kantor DB, Karunas AS, Khusnutdinova E, Koppelman GH, Kozyrskyj AL, Kreiner E, Kubo M, Kumar R, Kumar A, Kuokkanen M, Lahousse L, Laitinen T, Laprise C, Lathrop M, Lau S, Lee YA, Lehtimäki T, Letort S, Levin AM, Li G, Liang L, Loehr LR, London SJ, Loth DW, Manichaikul A, Marenholz I, Martinez FJ, Matheson MC, Mathias RA, Matsumoto K, Mbarek H, McArdle WL, Melbye M, Melén E, Meyers D, Michel S, Mohamdi H, Musk AW, Myers RA, Nieuwenhuis MAE, Noguchi E, O'Connor GT, Ogorodova LM, Palmer CD, Palotie A, Park JE, Pennell CE, Pershagen G, Polonikov A, Postma DS, Probst-Hensch N, Puzyrev VP, Raby BA, Raitakari OT, Ramasamy A, Rich SS, Robertson CF, Romieu I, Salam MT, Salomaa V, Schlünssen V, Scott R, Selivanova PA, Sigsgaard T, Simpson A, Siroux V, Smith LJ, Solodilova M, Standl M, Stefansson K, Strachan DP, Stricker BH, Takahashi A, Thompson PJ, Thorleifsson G, Thorsteinsdottir U, Tiesler CMT, Torgerson DG, Tsunoda T, Uitterlinden AG, van der Valk RJP, Vaysse A, Vedantam S, von Berg A, von Mutius E, Vonk JM, Waage J, Wareham NJ, Weiss ST, White WB, Wickman M, Widén E, Willemsen G, Williams LK, Wouters IM, Yang JJ, Zhao JH, Moffatt MF, Ober C, Nicolae DL. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nature genetics. 2018 Jan:50(1):42-53. doi: 10.1038/s41588-017-0014-7. Epub 2017 Dec 22 [PubMed PMID: 29273806]
Gómez Real F, Burgess JA, Villani S, Dratva J, Heinrich J, Janson C, Jarvis D, Koplin J, Leynaert B, Lodge C, Lærum BN, Matheson MC, Norbäck D, Omenaas ER, Skulstad SM, Sunyer J, Dharmage SC, Svanes C. Maternal age at delivery, lung function and asthma in offspring: a population-based survey. The European respiratory journal. 2018 Jun:51(6):. pii: 1601611. doi: 10.1183/13993003.01611-2016. Epub 2018 Jun 7 [PubMed PMID: 29880541]
Level 3 (low-level) evidenceLaerum BN, Svanes C, Wentzel-Larsen T, Gulsvik A, Torén K, Norrman E, Gíslason T, Janson C, Omenaas E. Young maternal age at delivery is associated with asthma in adult offspring. Respiratory medicine. 2007 Jul:101(7):1431-8 [PubMed PMID: 17350816]
Venter C, Agostoni C, Arshad SH, Ben-Abdallah M, Du Toit G, Fleischer DM, Greenhawt M, Glueck DH, Groetch M, Lunjani N, Maslin K, Maiorella A, Meyer R, Antonella M, Netting MJ, Ibeabughichi Nwaru B, Palmer DJ, Palumbo MP, Roberts G, Roduit C, Smith P, Untersmayr E, Vanderlinden LA, O'Mahony L. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2020 Nov:31(8):889-912. doi: 10.1111/pai.13303. Epub 2020 Aug 6 [PubMed PMID: 32524677]
Level 1 (high-level) evidenceWolsk HM, Chawes BL, Litonjua AA, Hollis BW, Waage J, Stokholm J, Bønnelykke K, Bisgaard H, Weiss ST. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PloS one. 2017:12(10):e0186657. doi: 10.1371/journal.pone.0186657. Epub 2017 Oct 27 [PubMed PMID: 29077711]
Level 1 (high-level) evidenceBisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S, Følsgaard NV, Fink NR, Thorsen J, Pedersen AG, Waage J, Rasmussen MA, Stark KD, Olsen SF, Bønnelykke K. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. The New England journal of medicine. 2016 Dec 29:375(26):2530-9. doi: 10.1056/NEJMoa1503734. Epub [PubMed PMID: 28029926]
McEvoy CT, Shorey-Kendrick LE, Milner K, Schilling D, Tiller C, Vuylsteke B, Scherman A, Jackson K, Haas DM, Harris J, Schuff R, Park BS, Vu A, Kraemer DF, Mitchell J, Metz J, Gonzales D, Bunten C, Spindel ER, Tepper RS, Morris CD. Oral Vitamin C (500 mg/d) to Pregnant Smokers Improves Infant Airway Function at 3 Months (VCSIP). A Randomized Trial. American journal of respiratory and critical care medicine. 2019 May 1:199(9):1139-1147. doi: 10.1164/rccm.201805-1011OC. Epub [PubMed PMID: 30522343]
Level 1 (high-level) evidenceMcEvoy CT, Shorey-Kendrick LE, Milner K, Schilling D, Tiller C, Vuylsteke B, Scherman A, Jackson K, Haas DM, Harris J, Park BS, Vu A, Kraemer DF, Gonzales D, Bunten C, Spindel ER, Morris CD, Tepper RS. Vitamin C to Pregnant Smokers Persistently Improves Infant Airway Function to 12 Months of Age: A Randomised Trial. The European respiratory journal. 2020 Jul 2:():. doi: 10.1183/13993003.02208-2019. Epub 2020 Jul 2 [PubMed PMID: 32616589]
Level 1 (high-level) evidenceMacsali F, Real FG, Plana E, Sunyer J, Anto J, Dratva J, Janson C, Jarvis D, Omenaas ER, Zemp E, Wjst M, Leynaert B, Svanes C. Early age at menarche, lung function, and adult asthma. American journal of respiratory and critical care medicine. 2011 Jan 1:183(1):8-14. doi: 10.1164/rccm.200912-1886OC. Epub 2010 Aug 23 [PubMed PMID: 20732985]
Kirjavainen PV, Karvonen AM, Adams RI, Täubel M, Roponen M, Tuoresmäki P, Loss G, Jayaprakash B, Depner M, Ege MJ, Renz H, Pfefferle PI, Schaub B, Lauener R, Hyvärinen A, Knight R, Heederik DJJ, von Mutius E, Pekkanen J. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nature medicine. 2019 Jul:25(7):1089-1095. doi: 10.1038/s41591-019-0469-4. Epub 2019 Jun 17 [PubMed PMID: 31209334]
Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2009 Oct 27:181(9):E181-90. doi: 10.1503/cmaj.080612. Epub 2009 Sep 14 [PubMed PMID: 19752106]
Level 2 (mid-level) evidenceBurke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. American journal of preventive medicine. 2003 Feb:24(2):160-9 [PubMed PMID: 12568822]
Dharmage SC, Perret JL, Custovic A. Epidemiology of Asthma in Children and Adults. Frontiers in pediatrics. 2019:7():246. doi: 10.3389/fped.2019.00246. Epub 2019 Jun 18 [PubMed PMID: 31275909]
O'Toole J, Mikulic L, Kaminsky DA. Epidemiology and Pulmonary Physiology of Severe Asthma. Immunology and allergy clinics of North America. 2016 Aug:36(3):425-38. doi: 10.1016/j.iac.2016.03.001. Epub 2016 Jun 2 [PubMed PMID: 27401616]
Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, Bleecker ER, Lichtenstein LM. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. The American review of respiratory disease. 1991 Jul:144(1):51-8 [PubMed PMID: 2064141]
Riccio MM, Proud D. Evidence that enhanced nasal reactivity to bradykinin in patients with symptomatic allergy is mediated by neural reflexes. The Journal of allergy and clinical immunology. 1996 Jun:97(6):1252-63 [PubMed PMID: 8648021]
Brown RH, Croisille P, Mudge B, Diemer FB, Permutt S, Togias A. Airway narrowing in healthy humans inhaling methacholine without deep inspirations demonstrated by HRCT. American journal of respiratory and critical care medicine. 2000 Apr:161(4 Pt 1):1256-63 [PubMed PMID: 10764321]
Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, Zheng J, Schechtman KB, Ramkumar TP, Cochran R, Xueping E, Christie C, Newell J, Fain S, Altes TA, Castro M. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008 Dec:134(6):1183-1191. doi: 10.1378/chest.07-2779. Epub 2008 Jul 18 [PubMed PMID: 18641116]
Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, Zanin ME, Zuin R, Maestrelli P, Fabbri LM, Saetta M. Epithelial damage and angiogenesis in the airways of children with asthma. American journal of respiratory and critical care medicine. 2006 Nov 1:174(9):975-81 [PubMed PMID: 16917118]
Lommatzsch M, Virchow JC. Severe asthma: definition, diagnosis and treatment. Deutsches Arzteblatt international. 2014 Dec 12:111(50):847-55. doi: 10.3238/arztebl.2014.0847. Epub [PubMed PMID: 25585581]
Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ primary care respiratory medicine. 2018 Aug 14:28(1):31. doi: 10.1038/s41533-018-0098-2. Epub 2018 Aug 14 [PubMed PMID: 30108224]
Tse SM, Gold DR, Sordillo JE, Hoffman EB, Gillman MW, Rifas-Shiman SL, Fuhlbrigge AL, Tantisira KG, Weiss ST, Litonjua AA. Diagnostic accuracy of the bronchodilator response in children. The Journal of allergy and clinical immunology. 2013 Sep:132(3):554-559.e5. doi: 10.1016/j.jaci.2013.03.031. Epub 2013 May 14 [PubMed PMID: 23683464]
Galant SP, Morphew T, Amaro S, Liao O. Value of the bronchodilator response in assessing controller naïve asthmatic children. The Journal of pediatrics. 2007 Nov:151(5):457-62, 462.e1 [PubMed PMID: 17961685]
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, de Jongste JC, Kerstjens HA, Lazarus SC, Levy ML, O'Byrne PM, Partridge MR, Pavord ID, Sears MR, Sterk PJ, Stoloff SW, Sullivan SD, Szefler SJ, Thomas MD, Wenzel SE, American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. American journal of respiratory and critical care medicine. 2009 Jul 1:180(1):59-99. doi: 10.1164/rccm.200801-060ST. Epub [PubMed PMID: 19535666]
Arnold DH, Gebretsadik T, Abramo TJ, Moons KG, Sheller JR, Hartert TV. The RAD score: a simple acute asthma severity score compares favorably to more complex scores. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2011 Jul:107(1):22-8. doi: 10.1016/j.anai.2011.03.011. Epub 2011 Apr 22 [PubMed PMID: 21704881]
Tesse R, Borrelli G, Mongelli G, Mastrorilli V, Cardinale F. Treating Pediatric Asthma According Guidelines. Frontiers in pediatrics. 2018:6():234. doi: 10.3389/fped.2018.00234. Epub 2018 Aug 23 [PubMed PMID: 30191146]
National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. The Journal of allergy and clinical immunology. 2007 Nov:120(5 Suppl):S94-138 [PubMed PMID: 17983880]
Rodrigo GJ, Rodriquez Verde M, Peregalli V, Rodrigo C. Effects of short-term 28% and 100% oxygen on PaCO2 and peak expiratory flow rate in acute asthma: a randomized trial. Chest. 2003 Oct:124(4):1312-7 [PubMed PMID: 14555560]
Level 1 (high-level) evidenceCastro-Rodriguez JA, Rodrigo GJ. beta-agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta-analysis. The Journal of pediatrics. 2004 Aug:145(2):172-7 [PubMed PMID: 15289762]
Level 1 (high-level) evidenceKoh HP, Shamsudin NS, Tan MMY, Mohd Pauzi Z. The outcomes and acceptance of pressurized metered-dose inhaler bronchodilators with venturi mask modified spacer in the outpatient emergency department during the COVID-19 pandemic. Journal of clinical pharmacy and therapeutics. 2021 Aug:46(4):1129-1138. doi: 10.1111/jcpt.13410. Epub 2021 Mar 25 [PubMed PMID: 33768601]
Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, Douglas G, Muers M, Smith D, White J. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health technology assessment (Winchester, England). 2001:5(26):1-149 [PubMed PMID: 11701099]
Level 1 (high-level) evidenceGriffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. The Cochrane database of systematic reviews. 2013 Aug 21:(8):CD000060. doi: 10.1002/14651858.CD000060.pub2. Epub 2013 Aug 21 [PubMed PMID: 23966133]
Level 1 (high-level) evidenceRowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Annals of emergency medicine. 2000 Sep:36(3):181-90 [PubMed PMID: 10969218]
Level 1 (high-level) evidenceRowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA Jr. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. The Cochrane database of systematic reviews. 2000:2000(2):CD001490 [PubMed PMID: 10796650]
Level 1 (high-level) evidenceCheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Archives of disease in childhood. 2005 Jan:90(1):74-7 [PubMed PMID: 15613519]
Level 1 (high-level) evidenceGriffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. The Cochrane database of systematic reviews. 2016 Apr 29:4(4):CD011050. doi: 10.1002/14651858.CD011050.pub2. Epub 2016 Apr 29 [PubMed PMID: 27126744]
Level 1 (high-level) evidenceBleecker ER, Menzies-Gow AN, Price DB, Bourdin A, Sweet S, Martin AL, Alacqua M, Tran TN. Systematic Literature Review of Systemic Corticosteroid Use for Asthma Management. American journal of respiratory and critical care medicine. 2020 Feb 1:201(3):276-293. doi: 10.1164/rccm.201904-0903SO. Epub [PubMed PMID: 31525297]
Level 1 (high-level) evidenceNormansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. The Cochrane database of systematic reviews. 2016 May 13:2016(5):CD011801. doi: 10.1002/14651858.CD011801.pub2. Epub 2016 May 13 [PubMed PMID: 27176676]
Level 1 (high-level) evidenceKeeney GE, Gray MP, Morrison AK, Levas MN, Kessler EA, Hill GD, Gorelick MH, Jackson JL. Dexamethasone for acute asthma exacerbations in children: a meta-analysis. Pediatrics. 2014 Mar:133(3):493-9. doi: 10.1542/peds.2013-2273. Epub 2014 Feb 10 [PubMed PMID: 24515516]
Level 1 (high-level) evidencePiloni D, Tirelli C, Domenica RD, Conio V, Grosso A, Ronzoni V, Antonacci F, Totaro P, Corsico AG. Asthma-like symptoms: is it always a pulmonary issue? Multidisciplinary respiratory medicine. 2018:13():21. doi: 10.1186/s40248-018-0136-5. Epub 2018 Aug 3 [PubMed PMID: 30123502]
Bui DS, Lodge CJ, Perret JL, Lowe A, Hamilton GS, Thompson B, Giles G, Tan D, Erbas B, Pirkis J, Cicuttini F, Cassim R, Bowatte G, Thomas P, Garcia-Aymerich J, Hopper J, Abramson MJ, Walters EH, Dharmage SC. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: a prospective cohort study. The Lancet. Respiratory medicine. 2021 Apr:9(4):387-396. doi: 10.1016/S2213-2600(20)30413-6. Epub 2020 Nov 17 [PubMed PMID: 33217367]
Kapadia CR, Nebesio TD, Myers SE, Willi S, Miller BS, Allen DB, Jacobson-Dickman E, Drugs and Therapeutics Committee of the Pediatric Endocrine Society. Endocrine Effects of Inhaled Corticosteroids in Children. JAMA pediatrics. 2016 Feb:170(2):163-70. doi: 10.1001/jamapediatrics.2015.3526. Epub [PubMed PMID: 26720105]
Childhood Asthma Management Program Research Group, Szefler S, Weiss S, Tonascia J, Adkinson NF, Bender B, Cherniack R, Donithan M, Kelly HW, Reisman J, Shapiro GG, Sternberg AL, Strunk R, Taggart V, Van Natta M, Wise R, Wu M, Zeiger R. Long-term effects of budesonide or nedocromil in children with asthma. The New England journal of medicine. 2000 Oct 12:343(15):1054-63 [PubMed PMID: 11027739]
Guhan AR, Cooper S, Oborne J, Lewis S, Bennett J, Tattersfield AE. Systemic effects of formoterol and salmeterol: a dose-response comparison in healthy subjects. Thorax. 2000 Aug:55(8):650-6 [PubMed PMID: 10899240]
Osuorji I, Williams C, Hessney J, Patel T, Hsi D. Acute stress cardiomyopathy following treatment of status asthmaticus. Southern medical journal. 2009 Mar:102(3):301-3. doi: 10.1097/SMJ.0b013e31818f5bd8. Epub [PubMed PMID: 19204641]
Bernstein JA, Mansfield L. Step-up and step-down treatments for optimal asthma control in children and adolescents. The Journal of asthma : official journal of the Association for the Care of Asthma. 2019 Jul:56(7):758-770. doi: 10.1080/02770903.2018.1490752. Epub 2018 Sep 12 [PubMed PMID: 29972079]
Lou Y, Atherly A, Johnson T, Anderson M, Valdez C, Sabalot S. The impact of care management for high-risk pediatric asthmatics on healthcare utilization. The Journal of asthma : official journal of the Association for the Care of Asthma. 2021 Jan:58(1):133-140. doi: 10.1080/02770903.2019.1659311. Epub 2019 Sep 9 [PubMed PMID: 31496315]
Cook J, Beresford F, Fainardi V, Hall P, Housley G, Jamalzadeh A, Nightingale M, Winch D, Bush A, Fleming L, Saglani S. Managing the pediatric patient with refractory asthma: a multidisciplinary approach. Journal of asthma and allergy. 2017:10():123-130. doi: 10.2147/JAA.S129159. Epub 2017 Apr 20 [PubMed PMID: 28461761]