Introduction
Meige syndrome is a focal dystonic movement disorder characterized by blepharospasm (double eyelid spasm) and oromandibular dystonia.[1] Dystonia involves abnormal involuntary posturing or body movements resulting from sustained muscle contractions, often arising from neurological or medical causes. In 1910, Dr Henry Meige, a French neurologist, observed abnormal contractions of midline facial muscles, including involuntary eyelid closure, in approximately 10 patients. He initially termed this condition "spasm facial median."[2][3] These patients also demonstrated a similar clinical manifestation in the muscles of the jaw and oropharynx.
In 1972, Dr George Paulson introduced the term "Meige syndrome" to describe patients experiencing facial muscle spasms, notably blepharospasm and dystonia of oromandibular muscles.[4] After several years, Gilbert introduced the term "Brueghel syndrome" to describe a case of jaw dystonia, distinguished from Meige syndrome by the absence of blepharospasm.[5][6]
The use of the term "Meige syndrome" for a type of dystonia based on historical perspectives can be problematic and confusing, especially considering that this physician did not suffer from the syndrome and was not the first doctor to describe this type of dystonia.[4] Medical associations, including the Council of Science Editors, have discouraged using possessive forms of eponyms.[7][8] Moreover, Meige syndrome is sometimes confused with Meigs' syndrome, which is unrelated to dystonia and defines the clinical presentation found in a specific type of benign ovarian tumor.[9]
Although the exact pathophysiology of Meige syndrome is unknown, it is believed to be related to abnormalities in the basal ganglia—a critical brain region essential for motor control.[10] Dystonia is thought to result from dysfunction in neurotransmitter systems, particularly affecting dopamine and gamma-aminobutyric acid (GABA).[11]
The course of Meige syndrome varies significantly from person to person. Symptoms typically begin gradually and may worsen over time, although they can also enter spontaneous remission or remain stable. The age of symptom onset may also differ. Some individuals experience symptoms in early adulthood, whereas others may not exhibit them until later in life. Managing and treating symptoms can be challenging due to their unpredictable nature and influence on day-to-day functioning.[12]
The lower face and jaw are typically the initial areas to exhibit signs of Meige syndrome. These signs manifest as involuntary spasms of the muscles involved in speech, swallowing, and chewing. Eventually, dystonic movements can extend to the lips, tongue, cheeks, and neck muscles, among other parts of the face.[13] In some cases, dystonia may extend to different parts of the body, resulting in generalized dystonia, while in others, it remains confined to the oromandibular region.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Primary Meige syndrome manifests without an identifiable underlying cause and is typically characterized by isolated dystonic movements in the face and jaw. In contrast, secondary Meige syndrome stems from identifiable triggers such as medications, neurodegenerative diseases, or structural brain lesions, often accompanied by additional neurological symptoms or signs indicative of the underlying cause.
Primary Meige Syndrome
Most Meige syndrome cases are classified as idiopathic, and the exact cause of the ailment is still unknown. However, several variables, including genetic, environmental, and neurochemical effects, have been linked to its pathophysiology.
Hereditary factors have been reported to be possibly involved in this syndrome, with studies indicating the significance of genetic components in generating the disease.[14] Meige syndrome can occasionally run in families, which suggests a hereditary susceptibility to the condition. Clinical manifestations of Meige syndrome have been observed in patients with p.Gly213Ser or p.Ala353thr mutations. Recent findings suggest that mutations in the GNAL gene (which encodes the guanine nucleotide-binding protein G, subunit alpha) have been reported to cause cranial and cervical dystonia. However, further evidence is required to confirm this association.[15][16]
Studies involving first-degree relatives of individuals with Meige syndrome have demonstrated a penetrance of approximately 20%, possibly indicating an autosomal dominant transmission.[17] The inheritance pattern is often complex, influenced by both environmental factors and multiple genes. Meige syndrome, along with other forms of dystonia, has been associated with mutations in TOR1A (DYT1), THAP1, and GNAL genes, which are involved in neurotransmitter control or neuronal excitability.[18][19]
Secondary Meige Syndrome
Although hereditary factors may predispose individuals to Meige syndrome, environmental triggers can also influence the development or severity of symptoms. These triggers may include psychological stress, exposure to certain drugs or toxins, or physical trauma such as surgery or a brain injury.[20] Although the exact mechanisms by which these environmental factors contribute to dystonia development remain unclear, interactions with genetic predisposition are believed to be involved.
Dysfunction in the basal ganglia and associated neuronal circuits is a crucial component of the pathogenesis of Meige syndrome. Changes in the levels or activities of neurotransmitters, including glutamate, GABA, and dopamine, have been implicated in dystonia development, as these neurotransmitters are vital for controlling motor function. Individuals with dystonia, including those with Meige syndrome, often exhibit structural abnormalities or alterations in brain regions involved in motor control, such as the thalamus, sensorimotor cortex, and basal ganglia. Dystonic symptoms may arise from these anomalies, disrupting the normal functioning of motor circuits.
Long-term use of neuroleptic medications, experienced by approximately one-fourth of patients, may lead to alterations in receptor function, resulting in facial or cervical dystonia. This denervation hypersensitivity is believed to be caused by increased central dopaminergic activity, supported by its improvement with dopamine-depleting agents. Medications that elevate central dopamine activity, such as antiemetics (eg, metoclopramide), antipsychotics, antidepressants, selective serotonin reuptake inhibitors, antihistamines, and dopaminergic agonists, can contribute to this condition. Various other factors, including head trauma, stroke, demyelination of the brain stem region, normal pressure hydrocephalus, cerebral hypoxia, postoperative bilateral thalamotomy, kernicterus, space-occupying lesions, and postencephalitis, have been implicated in the disorder's development.[20] Meige syndrome can be associated with other movement disorders, such as Parkinson disease, Wilson disease, olivopontocerebellar atrophy, or Lewy body disease.[21]
Comprehending the intricate interaction of genetic, environmental, and neurochemical elements involved in the genesis of Meige syndrome is essential for devising targeted treatment interventions to alleviate symptoms and improve the quality of life for affected individuals.
Epidemiology
Meige syndrome is relatively uncommon compared to other movement disorders. This condition presents with a wide range of clinical manifestations, typically characterized by segmental myodystonia leading to blepharospasm and abnormal muscular activity in the oromandibular and neck regions.[22] Patients with Meige syndrome are typically aged between 30 and 70, with a mean age of 55.7 years, although cases in teenagers have been reported.[23] Studies indicate an average onset age in the sixth decade of life.[24][25] Variable data regarding the prevalence of isolated blepharospasm and craniocervical dystonia are available, which are estimated to be around 2% to 20%.[26] Studies based on crude estimates of the prevalence of blepharospasm and segmental dystonia range from 16 to 133 cases per million.[27][28]
The precise sex ratio of Meige syndrome varies across studies, although there appears to be a tendency for the disorder to affect females more frequently. Some research indicates a relatively equal distribution between sexes, while others suggest a female-to-male ratio of approximately 2:1.[29] The higher prevalence in females may be attributed to unknown factors, possibly related to hormones, environmental influences, or genetics.[30] Hypotheses suggest that specific estrogen receptors in females make them prone to involuntary muscle spasms.[31]
Meige syndrome has been documented globally; however, the frequency of reporting and detection may differ based on factors such as geographical location, access to healthcare facilities, diagnostic capabilities, and awareness. Although extensive population-based research on the incidence of Meige syndrome is limited, case series and clinical reports from various countries across North America, Europe, Asia, and other continents have been reported. Studies conducted in the United States have reported prevalence estimates ranging from 13 to 130 individuals per million, while European studies have indicated estimates of approximately 36 individuals per million.[32]
Pathophysiology
Meige syndrome is characterized by anomalies in the basal ganglia-thalamocortical motor circuitry, leading to aberrant postures and involuntary muscular contractions, such as other kinds of dystonia. Current research suggests that aberrant sensory-motor integration, changes in cortical excitability, and malfunctioning neurotransmitter systems are among the possible pathophysiological causes underlying Meige syndrome, although these mechanisms are not fully elucidated.
Dopamine, glutamate, and GABA are the 3 important neurotransmitters involved in controlling motor function within the basal ganglia-thalamocortical circuitry. The pathophysiology of Meige syndrome has been associated with dysregulation of several neurotransmitter systems. The prevailing hypothesis for the development of Meige syndrome involves dopaminergic and cholinergic hyperactivity, although it may also stem from the reduced function of inhibitory neurons, such as GABAergic neurons, in the cortex. Notably, dystonia is believed to arise from diminished dopamine signaling in the basal ganglia, particularly within the striatum.
Dysfunction in the dopaminergic pathway can disrupt the balance between direct and indirect routes, leading to increased neuronal firing and abnormal muscle contractions.[33] GABA is the brain's main inhibitory neurotransmitter, crucial for regulating neuronal excitability.[34] Changes in GABAergic signaling, such as deficiencies in GABA production, release, or receptor function, may contribute to aberrant patterns of neuronal activity in the basal ganglia and cortical motor areas, exacerbating dystonic symptoms. Glutamate, the primary excitatory neurotransmitter in the central nervous system, mediates synaptic transmission within the basal ganglia-thalamocortical circuitry.[35]
Various environmental and genetic factors render a patient prone to craniocervical dystonia. Some researchers suggest that these patients exhibit abnormal sensorimotor processing, as evidenced by positron emission tomographic scans revealing decreased blood flow to the sensorimotor area in response to lower face vibrations. Silent functional magnetic resonance imaging (MRI) has shown reduced activation within the primary motor cortex (Brodmann area 4) and premotor cortex (Brodmann area 6) in the orofacial regions of Meige syndrome patients with isolated blepharospasm. This reduced activity may stem from disrupted regulation of cranial nerve nuclei in the brainstem by the basal ganglia. Brain imaging identifies a gray matter volume reduction in the cerebellum, superior frontal gyrus, insular cortex, and calcarine fissure in patients with craniocervical dystonia.[36][37]
Focal dystonias also exhibit a genetic component in their etiopathogenesis. At the cellular level, mutations in the TOR1A gene appear to cause a disturbed exchange of vesicles into and out of the nucleus, which in turn causes transcriptional dysregulation.[38][39] A similar mechanism is implicated in other primary focal dystonias, such as mutations in central nervous system transcriptional factors such as TAF1 and THAP1.[40] Studies involving animal and human models show evidence of specific genetic mutations that may lead to the disrupted development of the neuronal network.
History and Physical
The pattern of presentation of Meige syndrome varies among patients. Symptoms may initially manifest as unilateral blepharospasm before progressing to bilateral involvement (see Video. Typical Blepharospasm). One of the most challenging aspects of Meige syndrome is its diverse phenotypic forms, which can range from tonic spasms or prolonged closure of the eye to complete inability to open the eyes. Eyelid weakness or blepharoptosis is also prevalent.[25] This condition is characterized by progressive muscle dysfunction, typically beginning as focal neurological dysfunction such as essential blepharospasm or oromandibular dystonia before spreading to other muscle groups. Muscles of the neck (antecollis, retrocollis, and torticollis), respiratory muscles, or upper limb muscles (resulting in dystonic tremors) may become affected.[41] Commonly involved oromandibular muscles include the temporalis, masseter, and platysma. Involuntary lower facial and masticatory movements may encompass lip pursing, chewing, grimacing, jaw thrusting, and opening or closing/clenching actions.[42]
Patients frequently report a slow onset of symptoms, typically starting with occasional clenching of the jaw or facial twitching of the face. Over time, the frequency and intensity of involuntary movements and atypical postures may increase, indicating a progression of symptoms.[25] Studies suggest that the likelihood of dystonic contractions spreading to adjacent muscle groups is highest within the first year of symptom onset, with this risk persisting for the subsequent 3 to 5 years. In individuals with blepharospasm, factors such as older age at onset, female gender, and a history of head trauma may elevate the risk of symptom spread.[43]
Patients with Meige syndrome often use sensory tricks, which are sensory stimuli they learn to use to alleviate their dystonia symptoms. Common examples include sleeping, relaxing, talking, pulling the upper eyelid, blowing cheeks, walking, exposure to cold water, yawning, or drinking beverages.[44] More than half of patients with blepharospasm utilize 1 or multiple sensory tricks.[42] Additionally, patients may identify specific behaviors (eg, speaking and eating) or aggravating circumstances (eg, stress, exhaustion, and coffee) that exacerbate their symptoms. Recognizing these triggers can assist in treatment decisions and provide valuable insights into the pathophysiology of Meige syndrome.
A thorough evaluation of a patient's medical history is essential for assessing their risk of Meige syndrome. This includes inquiring about any history of drug use, neurological issues, or prior head trauma. Additionally, a family history of dystonia or other movement disorders may suggest a genetic predisposition to the condition.
The physical examination of individuals with Meige syndrome typically highlights characteristic clinical symptoms, including involuntary muscular spasms in the face, jaw, and neck. A comprehensive history and physical examination are essential to guide further evaluation and treatment. In addition, it is crucial to evaluate for associated symptoms, functional limitations, and potential triggers or exacerbating factors. A thorough neurological examination is indispensable for assessing reflexes, coordination, gait, motor function, and sensory trick function. Any presence of bradykinesia, tremors, dystonic movements, or other neurological symptoms warrants careful consideration for possible underlying neurological conditions.
Evaluation
Assessing Meige syndrome is crucial for establishing a diagnosis, identifying potential underlying causes, and guiding treatment decisions. The evaluation of Meige syndrome involves a comprehensive approach, including clinical assessment, neuroimaging scans, and laboratory investigations. As Meige syndrome cannot be diagnosed with a single specific test, a thorough evaluation is essential to rule out other potential causes and determine the severity of the condition.
Brain MRI plays a crucial role in ruling out structural abnormalities that may resemble or exacerbate dystonic symptoms, including tumors, vascular malformations, or other lesions.[45] Although brain MRI findings in Meige syndrome often appear normal, imaging can be valuable in certain cases to distinguish secondary causes of dystonia. Additionally, MRI and/or computed tomography (CT) of the brain can aid in ruling out stroke as a potential underlying cause.
Electrophysiological investigations are valuable in the differential diagnosis of Meige syndrome. Nerve conduction studies and electromyography (EMG) can provide essential information regarding muscle activation patterns and help exclude other neuromuscular conditions that can be mistaken for dystonia.[46] Surface EMG recordings are particularly useful for assessing the type and intensity of myofascial contractions in individuals with Meige syndrome.
Genetic testing is a valuable consideration in cases where there is a family history of dystonia or suspected genetic predisposition.[47] Genes linked to dystonia, such as TOR1A (DYT1), THAP1, and GNAL, may be the subject of targeted sequencing or panel testing to help identify pathogenic mutations that may be responsible for Meige syndrome.[32][48]
Metabolic and toxicological screening is valuable in cases with unusual symptoms or suspected secondary dystonia. Laboratory testing for metabolic diseases, such as Wilson disease or mitochondrial abnormalities, may be necessary. Additionally, investigating exposure to chemicals or drugs known to induce dystonia is essential. The workup should encompass a serum drug screen, SSA/SSB levels, Cu and ceruloplasmin levels, uric acid levels, and Beck's Depression Inventory.[49]
The European Federation of Neurological Societies (EFNS), the American Academy of Neurology, and other international societies have published clinical recommendations in the literature for diagnosing and treating movement disorders. These guidelines provide valuable insights into diagnosing and managing Meige syndrome, drawing upon the latest research findings and best practices.[50]
Treatment / Management
Meige syndrome care aims to alleviate symptoms, enhance functional outcomes, and improve the quality of life for affected individuals. Treatment approaches typically involve a combination of medications, botulinum toxin injections, surgical interventions, and supportive therapies.
Various oral medications, including muscle relaxants, dopamine receptor antagonists, and anticholinergic drugs, are utilized to address dystonic symptoms in Meige syndrome.[51] These medications aim to reduce muscle spasms and improve motor function, while individual differences in their effectiveness may exist.[52] Understanding the pathophysiology of Meige syndrome elucidates the efficacy of medications such as anticholinergics (eg, trihexyphenidyl), dopamine antagonists (eg, tiapride and tetrabenazine), and GABA receptor agonists (eg, benzodiazepines) in managing these symptoms.[4]
Additional medications for Meige syndrome management include antiepileptics (eg, valproic acid) and various psychoactive drugs. Eszopiclone and nitrazepam target specific subunits (omega-1 and omega-2) of the GABA receptor complex, thereby offering relief from eyelid spasms. Case reports suggest zolpidem's efficacy due to its high specificity for the GABA omega-1 receptor. Prolonged use of psychoactive drugs may induce eyelid spasming, which is more often associated with the use of typical antipsychotics, although reports also indicate blepharospasm worsening with olanzapine use.[53]
Botulinum toxin injections represent an effective treatment option for blepharospasm and facial dystonia (see Image. Botulinum Toxin Injections for Blepharospasm).[54] Botulinum A (also known as onabotulinumtoxinA or abobotulinumtoxinA) injections have demonstrated promising outcomes, particularly for patients with inadequate responses to oral medications or experiencing adverse effects from these medications.[55] Afflicted muscles receive botulinum toxin type A injections to induce temporary chemical denervation and reduce muscular hyperactivity.[56] Treatment typically occurs every 2 to 6 months and depends on an individual's response and the degree of symptoms.[57] However, prolonged and recurrent use of botulinum A injections may lead to therapeutic resistance due to antibody production.[58] Moreover, botulinum toxin injections can result in nearby muscle weakness or exacerbate existing dysphagia or dysarthria.[59](A1)
Deep brain stimulation (DBS) is a consideration for patients unresponsive to noninvasive treatments.[60] This surgical technique involves implanting electrodes into specific brain regions, such as the subthalamic nucleus or globus pallidus internus, to modulate neural activity and reduce dystonic symptoms.[61] Patients with severe, refractory Meige syndrome, inadequately responsive to medical treatments or botulinum toxin injections, may be suitable candidates for this intervention. When botulinum toxin and other conservative treatments fail to produce the desired results, DBS of the globus pallidus interna emerges as an effective option.[62] Electrode placement is meticulously planned, targeting the ventral and posterior segments of the globus pallidus interna, as these areas correspond to the facial region, while the cervicofacial area is situated more anteriorly.[63](A1)
Surgery can be an option for certain cases unresponsive to medical or local therapy, aiming to enhance both functional and aesthetic outcomes (see Image. Limited Myectomy for Blepharospasm).[64] Research indicates that blepharoplasty correction, coupled with selective myectomy and myotomy, can yield lasting improvements for patients with refractory Meige syndrome.[65][66][67](B3)
Differential Diagnosis
When evaluating patients with symptoms indicative of Meige syndrome, clinicians should consider a broad range of potential differential diagnoses, including:
- Xeromas
- Spinocerebellar ataxia
- Progressive supranuclear palsy
- Tardive dyskinesia
- Wilson disease
- Ischemic stroke
- Autoimmune or inflammatory conditions (such as multiple sclerosis, lupus erythematosus, and Behçet disease)
- Metabolic disorders (such as hypoxia and pontine myelinolysis)
- Neoplasms (such as meningioma and metastatic tumors)
- Myoclonus-dystonia syndrome
- Facial tic disorders
- Psychogenic craniocervical dystonia
- Parkinson disease
- Hemifacial spasm
- Generalized anxiety disorder
- Secondary dystonias
Prognosis
The prognosis for patients with Meige syndrome varies depending on the severity of symptoms, treatment response, and underlying etiological factors. Although Meige syndrome is a chronic illness that can significantly and negatively influence an individual's quality of life, many patients experience symptom improvement with appropriate management.
The spectrum of dystonic symptoms in patients with Meige syndrome ranges from mild to severe, with some experiencing sporadic or intermittent contractions, whereas others endure incapacitating symptoms persistently. The degree of functional impairment and its effect on daily activities can impact treatment success and overall prognosis.
The prognosis of Meige syndrome is influenced by the effectiveness of treatments such as medications, botulinum toxin injections, and surgery. While botulinum toxin injections can alleviate muscular hyperactivity and improve motor function for many patients, individual responses to treatment may vary. Some individuals may require combination therapy or adjustments to their treatment regimen to achieve optimal results.
Although the progression of Meige syndrome can vary among individuals, it is generally considered a stable or slowly progressing condition. Some individuals may experience a gradual worsening of symptoms over time, but others may maintain stable symptoms. Close monitoring of patients' disease trajectories and their responses to treatment is essential for optimizing long-term outcomes and implementing any necessary adjustments to their management plans.
The prognosis of patients with Meige syndrome may also be affected by underlying genetic, metabolic, or anatomical issues. Unlike idiopathic forms of dystonia, secondary dystonias associated with neurodegenerative diseases, metabolic abnormalities, or structural lesions may have a less favorable outlook. Therefore, it is crucial to identify and address any underlying etiological causes to guide treatment decisions and manage prognostic expectations effectively.
With the proper care and support, many patients with Meige syndrome can achieve an acceptable quality of life despite the chronic nature of their condition. Multidisciplinary treatments, such as medical therapy, botulinum toxin injections, physical therapy, and supportive interventions, might help reduce symptoms, improve functional results, and enhance overall well-being.
Complications
Similar to other types of dystonia, Meige syndrome can result in multiple issues that affect an individual's social, psychological, and physical health. These adverse effects may arise from the chronic and debilitating nature of the condition and its impact on daily functioning and overall quality of life.
Common complications of Meige syndrome include functional impairment, pain or discomfort, challenges with speech and swallowing, social isolation and stigma, psychological morbidity, adverse reactions to medications, and treatment-related outcomes. The social, psychological, and physical ramifications of Meige syndrome can significantly diminish the quality of life for both patients and their caregivers. Implementing a comprehensive, multidisciplinary approach that integrates medical treatment, rehabilitative interventions, psychological support, and social assistance is essential for effectively managing this condition. Strategies aiming to minimize complications, optimize functional outcomes, and enhance overall well-being can substantially improve the lives of those affected by Meige syndrome.
Deterrence and Patient Education
Empowering patients with comprehensive information about their condition is vital for addressing Meige syndrome care, promoting treatment adherence, and minimizing complications. Effective patient education involves thorough discussions about symptoms, causes, potential impacts on daily life, available treatments, and coping strategies. This fosters informed decision-making and active engagement in their care journey.
Patients should be informed about the pathophysiology underlying Meige syndrome, including how abnormalities in neurotransmitters contribute to dystonic symptoms and the operation of dysfunctional basal ganglia-thalamocortical circuits. Clear explanations of the biological causes of a patient's ailment might help dispel myths and give them a sense of control over their health. Affected individuals should understand the distinctive signs and symptoms of Meige syndrome, such as discomfort, trouble speaking, functional impairment, and involuntary muscle spasms in the face, jaw, and neck. Encouraging awareness of symptoms and prompt reporting to healthcare professionals can facilitate early identification and intervention.
Patients should actively participate in discussions about the various treatment options for Meige syndrome, including botulinum toxin injections, surgery, supportive therapy, and oral medications. Each treatment approach has its own benefits and drawbacks, so involving patients in shared decision-making regarding their care is essential. This process should consider their individual treatment goals and preferences, ensuring that the chosen approach aligns with their unique treatment objectives and preferences.
Patients receiving botulinum toxin injections should receive thorough education about the treatment process, including how the toxin works, expected benefits, potential adverse effects, and the injection procedure itself. Additionally, it is important to provide guidance to patients on post-injection care, such as scheduling follow-up appointments for additional injections if necessary and monitoring for any signs of muscle weakness or adverse reactions.
Patients undergoing treatment for Meige syndrome should understand the significance of adhering to their treatment plans, which should include follow-up appointments, performing physical therapy exercises, and adhering to medication schedules. Medication adverse effects, budgetary limitations, or practical challenges can hinder treatment adherence, potentially compromising treatment effectiveness and exacerbating the condition. Addressing these obstacles is essential to optimize treatment outcomes and prevent worsening of the condition.
Motivating patients to adopt a healthy lifestyle is crucial as it can aid in symptom management and overall well-being for individuals with Meige syndrome. This may involve strategies to reduce stress, maintain regular exercise, prioritize adequate rest, and receive dietary guidance. Additionally, for some patients, avoiding known triggers such as alcohol or caffeine might prove beneficial. Equipping patients with the resources to actively participate in managing their condition involves tracking symptoms, noting medication effects, and developing coping strategies for dystonic movements and associated challenges.
Patients can empower themselves by mastering symptom management techniques such as adjusting posture, utilizing sensory tricks, and practicing relaxation exercises. Addressing the emotional and social aspects of Meige syndrome involves encouraging patients to seek support from healthcare professionals, support groups, or counseling services. Engaging in networking and peer support opportunities can provide encouragement, solidarity, and practical advice for navigating the condition's psychosocial challenges.
Pearls and Other Issues
Managing the complexities of Meige syndrome effectively demands a nuanced approach. Clinical pearls provide invaluable insights for healthcare professionals navigating the complexities of managing this challenging neurological disorder, from early recognition to tailored treatment plans and comprehensive patient education.
- Identifying hallmark symptoms, such as blepharospasm and oromandibular dystonia, promptly can facilitate timely diagnosis and intervention.
- Tailoring treatment plans, which may include botulinum toxin injections and DBS, to each patient's specific requirements and responses is paramount.
- Monitoring symptoms, treatment responses, and potential adverse effects regularly is crucial for the long-term management of Meige syndrome, as it allows for adjustments to treatment strategies as needed.
- Adopting additional therapies, such as physical and speech therapies and psychological support, can enhance patients' quality of life and functional outcomes.
- Involving multidisciplinary healthcare providers such as neurologists, ophthalmologists, rehabilitation therapists, and speech therapists can help coordinate care and maximize patient outcomes.
- Counseling patients about potential treatment-related adverse effects, such as weakness or worsening of dysphagia, helps manage expectations and minimize complications.
- Providing comprehensive education on the condition, treatment options, and potential complications empowers patients to actively participate in their care.
Enhancing Healthcare Team Outcomes
Patients with Meige syndrome benefit most from an interprofessional approach involving primary care clinicians, neurologists, neurosurgeons, pharmacists, nurses, social workers, and other healthcare professionals. Early intervention in patients with Meige syndrome is imperative for reducing morbidity and optimizing patient outcomes. Current treatment options for Meige syndrome include oral medication, botulinum toxin, DBS, and surgery in nonrespondent cases. Nurses are critical in patient education and facilitating communication within the team. Pharmacists review causative and therapeutic medications, check for drug interactions, and provide guidance on administration.
Evidence-based strategies maximize treatment plans and reduce adverse effects. Ethical principles guide treatment decisions and management, ensuring patient autonomy and informed consent. All healthcare professionals must understand their roles in promoting a multidisciplinary approach to patient care. Interprofessional communication facilitates smooth information sharing and cooperative decision-making within the team. Comprehensive care coordination is vital for seamlessly managing a patient's journey from diagnosis through treatment and follow-up, enhancing patient safety, and minimizing errors. By prioritizing principles of skill, strategy, ethics, interprofessional communication, and care coordination, healthcare professionals can deliver patient-centered care, leading to improved patient outcomes and heightened team performance.
Media
<p>Contributed by BCK Patel, MD, FRCS</p>
(Click Image to Enlarge)
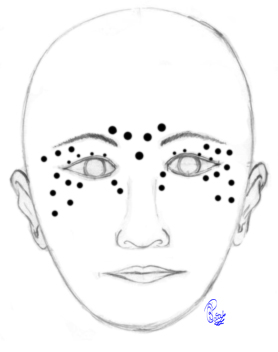
Botulinum Toxin Injections for Blepharospasm. Botulinum toxin injections may be administered at various sites, as depicted in the image. The dosage varies depending on the severity of the blepharospasm and apraxia of eyelid opening at each specific point. Pretarsal injections are administered to specifically counteract apraxia of eyelid opening. Injections just lateral to the lateral nasal wall aim to alleviate the squeezing of the nasalis muscle, which is observed in some patients. Injections into the corrugator and procerus muscles reduce the downward movement of the brow, consequently aiding eyelid control. Injections just below the brows provide a chemical lift to the brows, thereby improving the ability to open the eyelids. Caution is warranted to inject a minimal amount over the zygomaticus major and minor muscles to prevent the appearance of lower facial weakness following injections.
Contributed by BCK Patel, MD, FRCS
(Click Image to Enlarge)
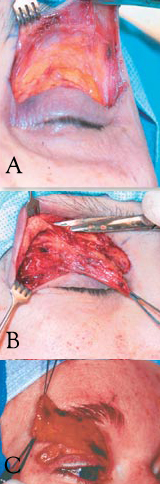
Limited Myectomy for Blepharospasm. The upper eyelid orbital and preseptal orbicularis muscle are dissected from the skin through an eyelid crease incision. The degree to which the pretarsal muscle is removed is determined by the extent of apraxia of eyelid opening (A). Care is exercised to safeguard the skin through meticulous dissection (B). The image illustrates en bloc removal of the orbicularis muscle (C).
Contributed by BCK Patel, MD, FRCS
References
Wang X, Mao Z, Cui Z, Xu X, Pan L, Liang S, Ling Z, Yu X. Predictive factors for long-term clinical outcomes of deep brain stimulation in the treatment of primary Meige syndrome. Journal of neurosurgery. 2019 Apr 5:132(5):1367-1375. doi: 10.3171/2019.1.JNS182555. Epub 2019 Apr 5 [PubMed PMID: 30952124]
Level 2 (mid-level) evidenceMarion MH. Henry Meige: The man and his understanding of dystonia, at the turn of the 19th to 20th century. Revue neurologique. 2022 Jun:178(6):532-538. doi: 10.1016/j.neurol.2021.07.024. Epub 2021 Nov 17 [PubMed PMID: 34799079]
Level 3 (low-level) evidenceTolosa E, Kulisevsky J, Fahn S. Meige syndrome: primary and secondary forms. Advances in neurology. 1988:50():509-15 [PubMed PMID: 2899953]
Level 3 (low-level) evidenceLeDoux MS. Meige syndrome: what's in a name? Parkinsonism & related disorders. 2009 Aug:15(7):483-9. doi: 10.1016/j.parkreldis.2009.04.006. Epub 2009 May 19 [PubMed PMID: 19457699]
Sellal F, Frismand S. Cervico-facial dystonia as depicted in sculpture before its scientific description. Revue neurologique. 2019 Mar:175(3):198-200. doi: 10.1016/j.neurol.2018.05.006. Epub 2019 Jan 15 [PubMed PMID: 30658849]
Aires A, Gomes T, Linhares P, Cunha F, Rosas MJ, Vaz R. The impact of deep brain stimulation on health related quality of life and disease-specific disability in Meige Syndrome (MS). Clinical neurology and neurosurgery. 2018 Aug:171():53-57. doi: 10.1016/j.clineuro.2018.05.012. Epub 2018 May 25 [PubMed PMID: 29807200]
Level 2 (mid-level) evidenceJana N, Barik S, Arora N. Current use of medical eponyms--a need for global uniformity in scientific publications. BMC medical research methodology. 2009 Mar 9:9():18. doi: 10.1186/1471-2288-9-18. Epub 2009 Mar 9 [PubMed PMID: 19272131]
Ayesu K, Nguyen B, Harris S, Carlan S. The case for consistent use of medical eponyms by eliminating possessive forms. Journal of the Medical Library Association : JMLA. 2018 Jan:106(1):127-129. doi: 10.5195/jmla.2018.284. Epub 2018 Jan 2 [PubMed PMID: 29339943]
Level 3 (low-level) evidenceLurie S. Meigs' syndrome: the history of the eponym. European journal of obstetrics, gynecology, and reproductive biology. 2000 Oct:92(2):199-204 [PubMed PMID: 10996681]
Zheng W, Lv G, Lu Y, Liu J, Hao Q, Ding H, Liu Y, Liu R. Bilateral Pallidal Deep Brain Stimulation in Meige Syndrome: Effects on Motor Function, Neuropsychological Status, and Mood. Neurosurgery. 2023 May 1:92(5):1073-1079. doi: 10.1227/neu.0000000000002335. Epub 2023 Jan 9 [PubMed PMID: 36728352]
Eftekhari K, Choe CH, Vagefi MR, Gausas RE, Eckstein LA. Oral methylphenidate for the treatment of refractory facial dystonias. Ophthalmic plastic and reconstructive surgery. 2015 May-Jun:31(3):e65-6. doi: 10.1097/IOP.0000000000000079. Epub [PubMed PMID: 25951177]
Younce JR, Cascella RH, Berman BD, Jinnah HA, Bellows S, Feuerstein J, Wagle Shukla A, Mahajan A, Chang FCF, Duque KR, Reich S, Richardson SP, Deik A, Stover N, Luna JM, Norris SA. Anatomical categorization of isolated non-focal dystonia: novel and existing patterns using a data-driven approach. Dystonia (Lausanne, Switzerland). 2023:2():. pii: 11305. doi: 10.3389/dyst.2023.11305. Epub 2023 Jun 8 [PubMed PMID: 37920445]
Balint B, Bhatia KP. Dystonia: an update on phenomenology, classification, pathogenesis and treatment. Current opinion in neurology. 2014 Aug:27(4):468-76. doi: 10.1097/WCO.0000000000000114. Epub [PubMed PMID: 24978640]
Level 3 (low-level) evidencePedrero-Escalas MF, García-López I, Santiago-Pérez S, Vivancos F, Gavilán J. Clinical experience with patients with spasmodic dysphonia and primary Meige syndrome. Acta otorrinolaringologica espanola. 2019 Jan-Feb:70(1):1-5. doi: 10.1016/j.otorri.2017.11.007. Epub 2018 Apr 30 [PubMed PMID: 29716720]
Jochim A, Li Y, Gora-Stahlberg G, Mantel T, Berndt M, Castrop F, Dresel C, Haslinger B. Altered functional connectivity in blepharospasm/orofacial dystonia. Brain and behavior. 2018 Jan:8(1):e00894. doi: 10.1002/brb3.894. Epub 2017 Dec 18 [PubMed PMID: 29568690]
Horisawa S, Ochiai T, Goto S, Nakajima T, Takeda N, Kawamata T, Taira T. Long-term outcome of pallidal stimulation for Meige syndrome. Journal of neurosurgery. 2018 Jan 19:130(1):84-89. doi: 10.3171/2017.7.JNS17323. Epub [PubMed PMID: 29350600]
Kumar KR, Lohmann K, Masuho I, Miyamoto R, Ferbert A, Lohnau T, Kasten M, Hagenah J, Brüggemann N, Graf J, Münchau A, Kostic VS, Sue CM, Domingo AR, Rosales RL, Lee LV, Freimann K, Westenberger A, Mukai Y, Kawarai T, Kaji R, Klein C, Martemyanov KA, Schmidt A. Mutations in GNAL: a novel cause of craniocervical dystonia. JAMA neurology. 2014 Apr:71(4):490-4. doi: 10.1001/jamaneurol.2013.4677. Epub [PubMed PMID: 24535567]
Koptielow J, Szyłak E, Szewczyk-Roszczenko O, Roszczenko P, Kochanowicz J, Kułakowska A, Chorąży M. Genetic Update and Treatment for Dystonia. International journal of molecular sciences. 2024 Mar 22:25(7):. doi: 10.3390/ijms25073571. Epub 2024 Mar 22 [PubMed PMID: 38612382]
Sarva H, Rodriguez-Porcel F, Rivera F, Gonzalez CD, Barkan S, Tripathi S, Gatto E, Ruiz PG, Rare Movement Disorders Study Group of the International Parkinsons and Movement Disorders Society. The role of genetics in the treatment of dystonia with deep brain stimulation: Systematic review and Meta-analysis. Journal of the neurological sciences. 2024 Apr 15:459():122970. doi: 10.1016/j.jns.2024.122970. Epub 2024 Mar 20 [PubMed PMID: 38520940]
Level 1 (high-level) evidenceArzul L, Henoux M, Marion F, Corre P. [Bilateral chronic dislocation of the temporomandibular joints and Meige syndrome]. Revue de stomatologie, de chirurgie maxillo-faciale et de chirurgie orale. 2015 Apr:116(2):106-10. doi: 10.1016/j.revsto.2015.01.004. Epub 2015 Mar 3 [PubMed PMID: 25742702]
Gautam P, Bhatia MS, Kaur J, Rathi A. Meige's syndrome. Industrial psychiatry journal. 2016 Jul-Dec:25(2):232-233. doi: 10.4103/0972-6748.207853. Epub [PubMed PMID: 28659707]
Gn S, Nag A. Management of Oromandibular Dystonia: A Case Report and Literature Update. Case reports in dentistry. 2017:2017():3514393. doi: 10.1155/2017/3514393. Epub 2017 Jun 19 [PubMed PMID: 28706744]
Level 3 (low-level) evidenceO'Riordan S, Raymond D, Lynch T, Saunders-Pullman R, Bressman SB, Daly L, Hutchinson M. Age at onset as a factor in determining the phenotype of primary torsion dystonia. Neurology. 2004 Oct 26:63(8):1423-6 [PubMed PMID: 15505159]
Scorr LM, Cho HJ, Kilic-Berkmen G, McKay JL, Hallett M, Klein C, Baumer T, Berman BD, Feuerstein JS, Perlmutter JS, Berardelli A, Ferrazzano G, Wagle-Shukla A, Malaty IA, Jankovic J, Bellows ST, Barbano RL, Vidailhet M, Roze E, Bonnet C, Mahajan A, LeDoux MS, Fung VSC, Chang FCF, Defazio G, Ercoli T, Factor S, Wojno T, Jinnah HA. Clinical Features and Evolution of Blepharospasm: A Multicenter International Cohort and Systematic Literature Review. Dystonia (Lausanne, Switzerland). 2022:1():. pii: 10359. doi: 10.3389/dyst.2022.10359. Epub 2022 May 16 [PubMed PMID: 36248010]
Level 1 (high-level) evidencePandey S, Sharma S. Meige's syndrome: History, epidemiology, clinical features, pathogenesis and treatment. Journal of the neurological sciences. 2017 Jan 15:372():162-170. doi: 10.1016/j.jns.2016.11.053. Epub 2016 Nov 23 [PubMed PMID: 28017205]
Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. The Lancet. Neurology. 2004 Nov:3(11):673-8 [PubMed PMID: 15488460]
Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. Journal of neurology. 2000 Oct:247(10):787-92 [PubMed PMID: 11127535]
Level 2 (mid-level) evidenceDefazio G, Livrea P. Epidemiology of primary blepharospasm. Movement disorders : official journal of the Movement Disorder Society. 2002 Jan:17(1):7-12 [PubMed PMID: 11835433]
Jankovic J, Orman J. Blepharospasm: demographic and clinical survey of 250 patients. Annals of ophthalmology. 1984 Apr:16(4):371-6 [PubMed PMID: 6144283]
Level 3 (low-level) evidenceZhan S, Sun F, Pan Y, Liu W, Huang P, Cao C, Zhang J, Li D, Sun B. Bilateral deep brain stimulation of the subthalamic nucleus in primary Meige syndrome. Journal of neurosurgery. 2018 Mar:128(3):897-902. doi: 10.3171/2016.12.JNS16383. Epub 2017 May 26 [PubMed PMID: 28548593]
Cabuk KS, Coban G, Yalcinkaya Cakir G, Sezal Serefoglu Z, Asik Nacaroglu S, Ozturk Karabulut G, Fazil K. Histopathological View of Benign Essential Blepharospasm: Orbicularis Oculi Hormone Receptor Levels. Beyoglu eye journal. 2023:8(2):110-114. doi: 10.14744/bej.2023.16779. Epub 2023 May 1 [PubMed PMID: 37521878]
Ma H, Qu J, Ye L, Shu Y, Qu Q. Blepharospasm, Oromandibular Dystonia, and Meige Syndrome: Clinical and Genetic Update. Frontiers in neurology. 2021:12():630221. doi: 10.3389/fneur.2021.630221. Epub 2021 Mar 29 [PubMed PMID: 33854473]
Jankovic J, Orman J. Tetrabenazine therapy of dystonia, chorea, tics, and other dyskinesias. Neurology. 1988 Mar:38(3):391-4 [PubMed PMID: 3279337]
Yoshimura R, Kakihara S, Soya A, Ueda N, Shinkai K, Nakamura J. Effect of clonazepam treatment on antipsychotic drug-induced Meige syndrome and changes in plasma levels of GABA, HVA, and MHPG during treatment. Psychiatry and clinical neurosciences. 2001 Oct:55(5):543-6 [PubMed PMID: 11555353]
Lee SA, Kim JS, Ahn JH, Choi KG. Sulpiride in Meige's syndrome: possible role of glutamate. Yonsei medical journal. 1988:29(1):62-5 [PubMed PMID: 2898184]
Piccinin CC, Piovesana LG, Santos MC, Guimarães RP, De Campos BM, Rezende TJ, Campos LS, Torres FR, Amato-Filho AC, França MC Jr, Lopes-Cendes I, Cendes F, D'Abreu A. Diffuse decreased gray matter in patients with idiopathic craniocervical dystonia: a voxel-based morphometry study. Frontiers in neurology. 2014:5():283. doi: 10.3389/fneur.2014.00283. Epub 2015 Jan 8 [PubMed PMID: 25620953]
Valls-Sole J, Defazio G. Blepharospasm: Update on Epidemiology, Clinical Aspects, and Pathophysiology. Frontiers in neurology. 2016:7():45. doi: 10.3389/fneur.2016.00045. Epub 2016 Mar 31 [PubMed PMID: 27064462]
Fan Y, Si Z, Wang L, Zhang L. DYT-TOR1A dystonia: an update on pathogenesis and treatment. Frontiers in neuroscience. 2023:17():1216929. doi: 10.3389/fnins.2023.1216929. Epub 2023 Aug 10 [PubMed PMID: 37638318]
Kim S, Phan S, Shaw TR, Ellisman MH, Veatch SL, Barmada SJ, Pappas SS, Dauer WT. TorsinA is essential for the timing and localization of neuronal nuclear pore complex biogenesis. bioRxiv : the preprint server for biology. 2023 Apr 27:():. pii: 2023.04.26.538491. doi: 10.1101/2023.04.26.538491. Epub 2023 Apr 27 [PubMed PMID: 37162852]
Pandey S, Bhattad S, Dinesh S. Tremor in Primary Monogenic Dystonia. Current neurology and neuroscience reports. 2021 Jul 15:21(9):48. doi: 10.1007/s11910-021-01135-w. Epub 2021 Jul 15 [PubMed PMID: 34264428]
Wu X, Xue T, Pan S, Xing W, Huang C, Zhang J, Zhao G. Pallidal versus subthalamic deep brain stimulation for Meige syndrome: A systematic review and meta-analysis. Heliyon. 2024 Mar 30:10(6):e27945. doi: 10.1016/j.heliyon.2024.e27945. Epub 2024 Mar 10 [PubMed PMID: 38510025]
Level 1 (high-level) evidencePeckham EL,Lopez G,Shamim EA,Richardson SP,Sanku S,Malkani R,Stacy M,Mahant P,Crawley A,Singleton A,Hallett M, Clinical features of patients with blepharospasm: a report of 240 patients. European journal of neurology. 2011 Mar; [PubMed PMID: 20649903]
Broussolle E, Laurencin C, Bernard E, Thobois S, Danaila T, Krack P. Early Illustrations of Geste Antagoniste in Cervical and Generalized Dystonia. Tremor and other hyperkinetic movements (New York, N.Y.). 2015:5():332. doi: 10.7916/D8KD1X74. Epub 2015 Sep 21 [PubMed PMID: 26417535]
Marsden CD. Blepharospasm-oromandibular dystonia syndrome (Brueghel's syndrome). A variant of adult-onset torsion dystonia? Journal of neurology, neurosurgery, and psychiatry. 1976 Dec:39(12):1204-9 [PubMed PMID: 1011031]
Liu B, Mao Z, Cui Z, Ling Z, Xu X, He K, Cui M, Feng Z, Yu X, Zhang Y. Cerebellar gray matter alterations predict deep brain stimulation outcomes in Meige syndrome. NeuroImage. Clinical. 2023:37():103316. doi: 10.1016/j.nicl.2023.103316. Epub 2023 Jan 4 [PubMed PMID: 36610311]
Huang C, Miao S, Chu H, Muheremu A, Wu J, Zhou R, Zuo H, Ma Y. Application of electrophysiological methods and magnetic resonance tomographic angiography in the differentiation between hemifacial spasm and Meige syndrome. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2016 May:37(5):769-75. doi: 10.1007/s10072-016-2492-2. Epub 2016 Feb 2 [PubMed PMID: 26838523]
Teng X, Qu Q, Shu Y, Gong J, Xu B, Qu J. Genetic screening in patients of Meige syndrome and blepharospasm. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2022 Jun:43(6):3683-3694. doi: 10.1007/s10072-022-05900-8. Epub 2022 Jan 19 [PubMed PMID: 35044558]
Liu J, Ding H, Liu R. Mutation of ADCY5 gene in patients with Meige syndrome. Asian journal of surgery. 2022 Jul:45(7):1487-1488. doi: 10.1016/j.asjsur.2022.02.061. Epub 2022 Mar 12 [PubMed PMID: 35288007]
Guan H, Geng Z, Yuan W, Chang B. Association of Serum Uric Acid Levels in Meige's Syndrome. Frontiers in neuroscience. 2021:15():755056. doi: 10.3389/fnins.2021.755056. Epub 2021 Oct 1 [PubMed PMID: 34658782]
Tamás G, Abrantes C, Valadas A, Radics P, Albanese A, Tijssen MAJ, Ferreira JJ. Quality and reporting of guidelines on the diagnosis and management of dystonia. European journal of neurology. 2018 Feb:25(2):275-283. doi: 10.1111/ene.13488. Epub 2017 Dec 22 [PubMed PMID: 29053896]
Level 2 (mid-level) evidenceJinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurologic clinics. 2015 Feb:33(1):77-100. doi: 10.1016/j.ncl.2014.09.002. Epub [PubMed PMID: 25432724]
Karp BI. Botulinum toxin physiology in focal hand and cranial dystonia. Toxins. 2012 Nov 20:4(11):1404-14. doi: 10.3390/toxins4111404. Epub 2012 Nov 20 [PubMed PMID: 23202323]
Sobstyl M, Ząbek M. [Deep brain stimulation in the treatment of torticollis and Meige syndrome]. Neurologia i neurochirurgia polska. 2011 Nov-Dec:45(6):590-9 [PubMed PMID: 22212990]
Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, Armstrong MJ, Gloss D, Potrebic S, Jankovic J, Karp BP, Naumann M, So YT, Yablon SA. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016 May 10:86(19):1818-26. doi: 10.1212/WNL.0000000000002560. Epub 2016 Apr 18 [PubMed PMID: 27164716]
Level 1 (high-level) evidenceHassell TJW, Charles D. Treatment of Blepharospasm and Oromandibular Dystonia with Botulinum Toxins. Toxins. 2020 Apr 22:12(4):. doi: 10.3390/toxins12040269. Epub 2020 Apr 22 [PubMed PMID: 32331272]
Karp BI, Alter K. Botulinum Toxin Treatment of Blepharospasm, Orofacial/Oromandibular Dystonia, and Hemifacial Spasm. Seminars in neurology. 2016 Feb:36(1):84-91. doi: 10.1055/s-0036-1571952. Epub 2016 Feb 11 [PubMed PMID: 26866500]
Duarte A, Coutinho L, Germiniani FMB, Teive HAG. Effects of onabotulinum toxin type A injections in patients with Meige's syndrome. Arquivos de neuro-psiquiatria. 2024 Apr:82(4):1-7. doi: 10.1055/s-0044-1785691. Epub 2024 Apr 19 [PubMed PMID: 38641339]
Wu WQ, Li K, Chu LL, Shen TT, Li Y, Xu YY, Zhang QL, Liu CF, Liu J, Zhou XP, Luo WF. Association analyses between the variants of SNAP25, SV2C and ST3GAL2 and the efficacy of botulinum toxin A in the treatment of the primary Meige syndrome. Heliyon. 2024 Apr 30:10(8):e28543. doi: 10.1016/j.heliyon.2024.e28543. Epub 2024 Mar 31 [PubMed PMID: 38628704]
Zheng H, Wu L, Tian S, Liu M, Zhan Q, Yu X, Xie Y, Zhong X, Wu W. Effect of botulinum toxin type A on non-motor symptoms and quality of life in Meige syndrome. Frontiers in neurology. 2023:14():1115482. doi: 10.3389/fneur.2023.1115482. Epub 2023 Feb 9 [PubMed PMID: 36846150]
Level 2 (mid-level) evidenceRodrigues FB, Duarte GS, Prescott D, Ferreira J, Costa J. Deep brain stimulation for dystonia. The Cochrane database of systematic reviews. 2019 Jan 10:1(1):CD012405. doi: 10.1002/14651858.CD012405.pub2. Epub 2019 Jan 10 [PubMed PMID: 30629283]
Level 1 (high-level) evidenceAragão VT, Barbosa Casagrande SC, Listik C, Teixeira MJ, Barbosa ER, Cury RG. Rescue Subthalamic Deep Brain Stimulation for Refractory Meige Syndrome. Stereotactic and functional neurosurgery. 2021:99(5):451-453. doi: 10.1159/000515722. Epub 2021 Apr 23 [PubMed PMID: 33895729]
Odorfer TM, Volkmann J. Deep Brain Stimulation for Focal or Segmental Craniocervical Dystonia in Patients Who Have Failed Botulinum Neurotoxin Therapy-A Narrative Review of the Literature. Toxins. 2023 Oct 9:15(10):. doi: 10.3390/toxins15100606. Epub 2023 Oct 9 [PubMed PMID: 37888637]
Level 3 (low-level) evidenceLimotai N, Go C, Oyama G, Hwynn N, Zesiewicz T, Foote K, Bhidayasiri R, Malaty I, Zeilman P, Rodriguez R, Okun MS. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. Journal of neurology. 2011 Nov:258(11):2069-74. doi: 10.1007/s00415-011-6075-0. Epub 2011 May 7 [PubMed PMID: 21553081]
Level 3 (low-level) evidenceLai CS, Ramachandran S, Lee CC, Lai YW, Chang YP, Huang SH. Evaluation of Blepharoptosis in Patients With Refractory Blepharospasm by VISA-Video Recordings, Idiosyncratic Expressions, Sensory Tricks, and Ancillary Procedures. Annals of plastic surgery. 2023 May 1:90(5S Suppl 2):S172-S176. doi: 10.1097/SAP.0000000000003371. Epub 2023 Feb 18 [PubMed PMID: 37192418]
Pariseau B, Worley MW, Anderson RL. Myectomy for blepharospasm 2013. Current opinion in ophthalmology. 2013 Sep:24(5):488-93. doi: 10.1097/ICU.0b013e3283645aee. Epub [PubMed PMID: 23925062]
Level 3 (low-level) evidenceLai HT, Chen AD, Lee SS, Lin YH, Lai CS. Myotomy In Situ for Essential Blepharospasm Refractory to Botulinum Toxin. Annals of plastic surgery. 2020 Jan:84(1S Suppl 1):S74-S79. doi: 10.1097/SAP.0000000000002182. Epub [PubMed PMID: 31833891]
Lai CS, Wang YC, Ramachandran S, Chang YP, Huang SH, Hsieh MW. Selective Myectomy and Myotomy In Situ for the Management of Refractory Blepharospasm in Meige Syndrome. Annals of plastic surgery. 2023 Apr 1:90(1 Suppl 1):S84-S88. doi: 10.1097/SAP.0000000000003367. Epub 2023 Jan 18 [PubMed PMID: 36752539]
