Introduction
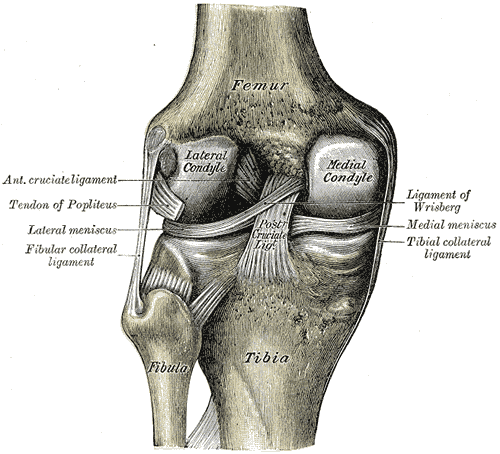
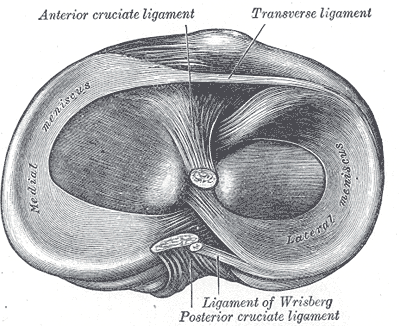
The anterior cruciate ligament (ACL) is one of 2 cruciate ligaments that aids in stabilizing the knee joint. It is a strong band made of connective tissue and collagenous fibers that originate from the anteromedial aspect of the intercondylar region of the tibial plateau and extends posterolaterally to attach to the medial aspect of the lateral femoral condyle, where there are two important landmarks; The lateral intercondylar ridge which defines the anterior boundary of the ACL, and the bifurcate ridge which separates the 2 ACL bundles. The ACL measures 32 mm long and is 7 to 12 mm wide. It has two bundles; an isometric anteromedial and a posterolateral bundle with more versatile length changes.[1]
The anteromedial bundle is the tightest in flexion and is mainly responsible for anterior tibial translation (85% of the stability), whereas the posterolateral bundle is the tightest in extension, with the main role in providing medial-lateral and rotational stability (secondary restraint).[2][3][4]
The ACL has 2200 N strength. The ACL and the posterior cruciate ligament (PCL) together form a cross (or an “x”) within the knee and prevent excessive forward or backward motion of the tibia relative to the femur during flexion and extension. Histologically, the ACL is composed of type I collagen (90%) and type III collagen (10%). It receives blood supply predominantly from the middle geniculate artery. Neurological innervation from the posterior articular nerve is a branch of the tibial nerve.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most ACL tears occur in athletes by non-contact mechanisms, non-contact pivoting injury where the tibia translates anteriorly while the knee is slightly flexed and in valgus.[6] A direct hit to the lateral knee has also been encountered as an injury mechanism. The most at-risk athletes for non-contact injury include skiers, soccer players, and basketball players.[7] The most at-risk athletes for contact injury are football players.[8]
Multiple intra-articular and extra-articular injuries can be associated with acute ACL ruptures.[9][10][11][12] Among those are meniscal tears; lateral meniscus injury in over half of acute ACL tears, whereas the medial meniscus is more involved in chronic cases. The PCL, LCL, and PLC could also be injured in association with an ACL injury. Chronic ACL deficiency seems to have deleterious effects on the knee, with the development of chondral injuries and complex unrepairable meniscal tears. Such as bucket handle medial meniscus tears.
Epidemiology
The ACL is the most commonly injured ligament in the knee, almost half of all knee injuries. The annual reported incidence in the United States alone is approximately 1 in 3500 people. There are approximately 400,000 ACL reconstructions every year in the united states.[13] However, data may not be accurate as there is no standard surveillance.
There is no age or gender bias; however, it has been suggested that women are at an increased risk of ACL injury secondary to a multitude of factors.[14] In athletes, the female-to-male ratio has been reported to be 4.5: 1. Female athletes tend to get ACL ruptures at a younger age and more in the supporting leg versus the kicking leg in males.
Among the factors increasing the female risk, as suggested by some studies that females may have weaker hamstrings (more quadriceps dominant) and preferential recruitment of the quadriceps muscle group while decelerating. Engaging the quadriceps musculature while slowing down places abnormally increased stresses on the ACL, as the quadriceps muscles are less effective at preventing anterior tibial translation versus the hamstring muscles. Additionally, females have weaker core stability than males.
Landing biomechanics in females may increase the risk of ACL injury with increased valgus angulation and extension of the knee.[15][16] One study utilizing video analysis demonstrated that female athletes are more likely to place their knees in increased valgus angulations when changing directions suddenly, which increases the stress on the ACL ligament, in addition to decreased hip and knee flexion and decreased fatigue resistance.
Other risk factors that might increase the risk of injury include anatomical risk factors such as increased body mass index, smaller femoral notch, impingement on the notch, smaller ACL, hypermobility, joint laxity, and previous ACL injury.
Certain risk factors related to specific sports participation were reported, such as soccer and basketball, with increased risk in females and males, respectively.
Moreover, hormones were reported to affect coordination, especially the preovulatory phase of the menstrual cycle. Females on OCP were noted to be less affected. Estrogen effects on the strength and flexibility of tissues such as ligaments may play a role and predispose females to injury; however, this remains controversial and has yet to be proven.
Collagen production (COL5A1 gene) was noted to be associated with lower injury risk in females.
History and Physical
History: One of the most common knee injuries is an ACL sprain or tear. Typically, injury occurs during activity/sports participation that involves sudden changes in the direction of movement, abrupt stopping or slowing down while running, or jumping and abnormal landing. Verifying the mechanism and time of the injury, ambulatory status, and joint stability can guide the diagnosis.
Most patients would complain of hearing and feeling a sudden "pop" with associated deep knee pain, and about 70% would experience immediate swelling due to haemarthrosis. Other reported symptoms are knee giving way, difficulty ambulating, and reduced knee range of movement.
Physical Examination
Inspection: patients may demonstrate quadriceps avoidance gait (no active knee extension). Varus knee malalignment should be noted as it increases the risk of ACL re-rupture and may warrant a concomitant procedure (knee realignment osteotomy) when performing ACL reconstruction.
Palpation: the involved knee will be swollen (haemarthrosis), and there may be joint line tenderness with an associated meniscal injury.
Movement: the knee may be locked due to associated meniscal injury. Other meniscal and ligamentous structures should be assessed.
Multiple provocative maneuvers can be employed to assess the ACL, including the anterior drawer, pivot shift, and Lachman tests.[17][18]
The Lachman test is the most sensitive in assessing ACL rupture, with 95% sensitivity and 94% specificity. The test is performed with the patient in the supine position and the knee in about 30 degrees of flexion. The clinician should stabilize the distal femur with one hand and, with the other hand, pull the tibia forward. The test is positive when there is increased anterior tibial translation relative to the femur.[19][20]
The test result is interpreted as either a firm endpoint (intact ACL) or no endpoint (ACL rupture). The grade of ACL rupture is classified based on the degree of anterior tibial translation in mm. Grade 1 (3-5 mm), grade 2 (5-10 mm), and grade 3 (> 10 mm translation). However, the injured side should always be compared with the good side. Additionally, the physician should be aware that a PCL tear may result in a "false" Lachman test interpretation due to translating the tibia from a posteriorly subluxated position.
The anterior drawer test is performed with the patient lying supine with their affected knee flexed to 90 degrees and the foot planted (Sometimes, it is easier for the clinician to stabilize the patient's foot by sitting on it). The clinician will grip the proximal tibia with both hands and pull it forward. The test is positive if there is an excessive anterior translation of the tibia relative to the femur. It may also be helpful to compare to the unaffected knee as patients may have increased laxity of the ACL that is not pathologic. This test has a sensitivity of 92% and specificity of 91% in chronic injuries but not acute injuries.[21]
The pivot shift test mimics the actual giving way event experienced in ACL-deficient knees. The test is performed by internally rotating the tibia while applying valgus stress on the knee and bringing the knee from extension to flexion. The anteriorly subluxated tibia in knee extension will reduce with a clunk in 20-30 degrees of flexion due to iliotibial band (ITB) tension.
The test necessitates intact ITB, MCL, and the absence of knee flexion contracture. This test can be difficult to perform in patients who are guarding, and some may not allow the clinician to perform the test. This is a highly specific test (98%) when positive but is insensitive (24%) due to the difficulty in evaluation secondary to patient pain and cooperation.[21][22]
The lever sign test: is performed by positioning a fulcrum (e.g., examiner's fist) under the proximal part of the supine patient's calf and applying a downward force to the quadriceps (distal thigh). The test is interpreted based on whether the ACL is intact or not; the patient's heel will either rise off of the examination couch or remain down.[23][24]
The KT-1000: This is performed with the knee in slight flexion and 10-30 degrees of external rotation. It helps assess and quantify anterior laxity.
It is important to evaluate for associated injuries such as medial or lateral collateral ligamentous injury, injury to the posterior collateral ligament, or meniscal injuries.[25]
Evaluation
Although ACL injury can be diagnosed clinically, imaging with magnetic resonance (MRI) is often utilized to confirm the diagnosis. MRI is the primary modality to diagnose ACL pathology, with a sensitivity of 86% and a specificity of 95%. Diagnosis may also be made with knee arthroscopy to differentiate complete from partial tears and chronic tears. Arthrography is considered the gold standard as it is 92% to 100% sensitive and 95% to 100% specific; however, it is rarely used as the initial step in diagnosis as it is invasive and requires anesthesia.
Magnetic Resonance Imaging (MRI) is 97% sensitive and 100 % specific: it confirms the diagnosis and assesses the presence of associated injuries. Normal ACL fibers are continuous and steeper than the intercondylar roof. ACL tears have primary and secondary signs.[26][27][28]
Primary Signs
- Sagittal view: will indicate changes associated directly with the ligamentous injury, while secondary signs are those changes closely related to the ACL injury. Primary signs include edema, an increased signal of the anterior cruciate ligament on T2 weighted or proton density images, discontinuity or complete absence of the fibers, and a change in the expected course of the ACL; too flat fibers in comparison with the intercondylar roof or Blumensaat's line. A flat appearance of the ACL fibers is more common in chronic cases where the ACL scars into the PCL. Tears usually occur within the midportion of the ligament, and signal changes are most often seen here and appear hyperintense.
- Coronal View: Empty notch sign where there is fluid against the lateral notch wall. Or discontinuity of the fibers.
Secondary signs include bone marrow edema (secondary to bone contusion) in more than half of ACL tears. The characteristic ACL bone bruising involves the middle third of the Lateral femoral condyle and the posterior third of the lateral tibial plateau. Other secondary signs include Segond fracture (as discussed below), associated medial collateral ligament injury, or anterior tibial translation greater than 7 mm of the tibia relative to the femur (best seen on lateral view), and tibial spine avulsion fracture.
Radiographs: (AP, lateral, skyline or merchant, or sunrise views) are generally non-contributory to diagnosing ACL injuries but help rule out fractures or other associated osseous injuries or may show evidence of effusion. In younger patients, avulsion of the tibial attachment may be seen. Other non-specific features that can be seen on radiographs include:
-
Segond fracture: An avulsion fracture of the anterolateral proximal tibia; attachment site of the lateral capsular ligaments (anterolateral ligament ALL).[29]
-
Arcuate fracture. An avulsion fracture of the proximal fibula; attachment site of the lateral collateral ligament and/or biceps femoris tendon. [30]
- Deep sulcus terminalis sign: This is a depression on the lateral femoral condyle at the junction between the weight-bearing tibial articular surface and patellar articular surface.
-
Joint effusion
-
Deep lateral sulcus sign: A notch on the lateral femoral condyle with a depth of 1.5 mm or more, best seen on the lateral view
Computed tomography (CT) is not generally utilized in evaluating the ACL and is only accurate in detecting an intact ACL. However, it plays a role in evaluating the available bone stock when planning for ACL revision. And it is the most sensitive and specific test for assessing bone loss in cases of tunnel widening and osteolysis.
Treatment / Management
ACL management should have an individualized approach. Both operative and non-operative treatments are acceptable.[31][32][31] Multiple factors should be considered when deciding on ACL management, including the patient's age and demands, activity level, sports participation, and the injury status of other supporting and stabilizing structures.[31](A1)
Acute treatment consists of the "RICE" therapy, which includes rest, ice, compression of the affected knee, and elevation of the affected lower extremity. Patients should be non-weight bearing and may utilize crutches or a wheelchair if necessary. Pain relief can be achieved with over-the-counter medications such as NSAIDs but is typically at the treating physician's discretion.
Non-operative management: this is indicated where there is reduced ACL laxity in low-demand patients or athletes involved in sports with no cutting or pivoting activities. These patients are usually managed with physiotherapy and lifestyle modifications. Non-operative treatment is indicated in partial ACL tears as well.[33] This involves an acute symptomatic treatment followed by 12 weeks of supervised physiotherapy, starting with regaining a full range of motion and progressing to quadriceps, hamstrings, hip abductors, and core muscle strengthening. A serial assessment to evaluate progression should be performed. Functional braces showed no superior functional stability.(A1)
However, non-operative management was reported to be associated with an increased risk of meniscal and cartilage damage due to repeated "giving way" episodes, especially if there are level I or II activities such as heavy manual labor and side-to-side sports, jumping, or cutting.
Operative Management
Two main operative management options are repair or reconstruction of the ruptured ACL.[34][35](A1)
ACL reconstruction: this is indicated in a complete ACL rupture in younger active or older active (> 40 years) high-demand patients. The primary objective is an anatomical ACL reconstruction to reinstitute the anterior and rotational stability, consequently lessening the chances of secondary meniscal or chondral injuries. Patients must be rehabilitated to achieve a full range of motion preoperative to reduce the risk of postoperative arthrofibrosis. Also, indicated in the pediatric population, however, activity restriction is unrealistic. A partial ACL rupture with functional instability would indicate reconstruction as well. Returning to sports participation following reconstruction hugely depends upon multiple demographic, functional, and psychological factors.
Surgical technique: arthroscopically assisted; preparation of the graft bed is either by clearance of the native ACL remnants completely or by leaving the stump as guidance for tunnel positioning and augmenting healing; there are no reported differences between these approaches. Also, there are no reported differences in outcomes between single and double-bundle reconstructions. Single bundle reconstruction still is the most common technique. A double-bundle reconstruction might improve native knee kinematics with better stability.
Tunnel Placement
Femur side; transtibial or tibia-independent, either inside-out or outside-in technique. Ideally, tunnel placement in the sagittal plane should be within 1-2 mm of the posterior femoral cortex. In the coronal plane, the aim is to position a more horizontal graft to reduce rotational laxity. So, using the clock face of the lateral wall, the graft should be positioned at 2 o'clock for the left knee or 10 o'clock for the right knee. Anteromedial and far medial drilling portals should be used to facilitate the proper femoral tunnel positioning. Drilling in over 70 degrees of flexion will avoid posterior wall blowout.[36][37]
Tibia side: Sagitally, more than one landmark would guide a final proper tunnel placement. The tunnel's center should be 10 to 11 mm anterior to the anterior border of the PCL, 6 mm anterior to the median eminence, and 9 mm posterior to the anterior inter-meniscal ligament. The tunnel trajectory should be less than 75 degrees from the horizontal in the coronal plane. This can be facilitated by adjusting the tibia starting point halfway between the tibial tubercle and the posterior medial edge of the tibia.[38][39]
Graft placement and fixation: preconditioning of the graft can reduce up to 50% of the stress relaxation. Tensioning at 20 or 40 N has no clinical effects on outcomes. Ideally, the graft should be fixed in 20 to 30 degrees of flexion. Multiple options are available for fixation, which can be used alone or in combination. Aperture or compression fixation can be achieved with an interference screw, whereas suspensory fixation can be achieved by cortical buttons, screws, and washer post or staple fixation.
ACL repair: There has been a resurgence in ACL repair techniques, especially in the pediatric population. Multiple techniques are used, including dynamic intra-ligamentary stabilization (DIS), internal brace ligament augmentation (IBLA), and biological enhancement, such as bridge-enhanced ACL repair (BEAR). The 2-year outcomes show comparable results.
Revision of ACL reconstruction is indicated in failed cases with instability affecting the desired activities. When considering a revision, the underlying etiology of the re-rupture must be identified, along with an assessment of any missed concomitant injuries. Other considerations include previous graft selection and considering stronger grafts, e.g., quadriceps tendon, hamstrings, or allografts. Allografts used for primary reconstruction have two folds higher risk of re-rupture compared to when used in revision cases. Reharversitng bone patellar tendon bone graft is contraindicated.
Combined fixation, aperture, and suspensory fixation should be considered. Also, if there is a previous tunnel dilation of 15 mm or the previous tunnel trajectory would compromise anatomical tunnel creation, bone grafting and staged procedure should be considered. Providing extra stability by anterolateral ligament reconstruction or lateral extra-articular tenodesis is still controversial. Finally, a conservative rehabilitation approach should be followed.
Graft selection for ACL reconstruction is a broad topic with pros and cons for each type of graft.[40][41](A1)
Quadrupled hamstring autograft: This is one of the most common types of grafts used in primary reconstruction. In revision cases, it can be harvested from the contralateral side whenever allograft is not an available or preferred option. Being autologous, so no immune reaction or risk of infection transmission. It can be harvested through a small incision, so less perioperative pain and no anterior knee pain associated with BPTB grafts. It is a strong graft with a maximum load to failure is 4000 N. However, a decreased peak flexion strength at three years was reported compared to BPTB grafts, in addition to reported hamstring weakness in female athletes with consequent increased re-rupture rate.
Complications reported following hamstring harvest include residual hamstring weakness and paresthesia due to saphenous nerve branch injury, where an oblique or horizontal incision can reduce this risk. A "windshield wiper" effect has been described when suspensory fixation away from the joint line would result in tunnel abrasion and dilation with knee range of movement.
Bone-patellar tendon-bone (BPTB) autograft is another commonly used graft type. It has the same advantages of being autologous as in the hamstring grafts, in addition to the faster incorporation due to bone-to-bone healing. It is considered the gold standard with a maximum load to failure is 2600 N ( Intact ACL is 1725 N). However, it has the highest incidence of postoperative anterior knee pain, especially with kneeling (10-30%). In addition to the risks of patella fracture and patellar tendon rupture. Higher risk of re-rupture in patients younger than 20 years old and when graft size is less than 8 mm.
Quadriceps tendon autograft: the incision for this graft is advantageous, being away from kneeling pressure areas. In pediatrics, the graft does not involve the physis compared to the BPTB graft. The maximum load to failure is 2185 N. It is either pure soft tissue or involves a patella bone block. Disadvantages are similar to the hamstring tendon autograft with suspensory fixation.
Allografts: are useful in revision settings, in addition to no donor site morbidity. However, they are more expensive, take longer to incorporate, and have a risk of disease transmission ( HIV < 1:1.6 million, hepatitis is greater)—it carries a high risk of graft re-rupture in young athletes, fourfold higher than autografts.
Graft processing: Fresh-frozen grafts have lower re-rupture rates than chemically treated or irradiated grafts. The reason is that the supercritical CO2 decreases the structural and mechanical properties of the grafts. Radiation has effects depending on its dose. More than 3 Mrads kill HIV but at the expense of reduced structural and mechanical properties of the graft. Between 2-2.8, Mrad, and 1 to 1.2, Mrad decreases stiffness by 30% and 20%, respectively—deep freezing and chlorhexidine gluconate (4%) damage cells without affecting the graft's strength.
Timing of ACL reconstruction:
Multiple factors dictate the ideal timing of ACL reconstruction. Additionally, the timing of addressing the associated injuries varies depending on the injured structure.[42][43](A1)
Meniscal tears: this should be addressed concurrently during the ACL reconstruction. Meniscal repair performed in the same setting as ACL reconstruction is reported to have a high healing rate.
Chondral injury: this can also be managed during ACL reconstruction. Staged procedures may be required based on the injury and the management modality. However, the presence of chondral injuries would compromise the long-term patient-reported outcomes following ACL reconstruction.
MCL injury: Grade I and II injuries, which are stable to valgus stress, can be managed non-operatively so that the MCL heals prior to ACL reconstruction. However, in Grade II or III injuries with instability to valgus stress requiring repair or reconstruction, this can be performed in the same stage as the ACL reconstruction. Failure to recognize and manage an associated high-grade MCL injury with valgus instability can compromise ACL reconstruction with high failure rates.[44]
Posterior cruciate ligament injury or posterolateral complex injury: This can be reconstructed concomitantly with ACL reconstruction or as a staged procedure. But, failure to diagnose and manage PCL or PLC injuries will result in varus instability and ACL graft overload.
Realignment osteotomy of the lower limb (high tibial or distal femoral osteotomies): malalignment in either coronal or sagittal planes should be managed before or at the same time as ACL reconstruction. Unmanaged limb malalignment is associated with a high ACL failure rate.
Pediatric consideration for ACL injury and reconstruction:
Non-operative management is indicated in low-demand, compliant children with isolated injuries. Partial ACL tears with no instability on objective testing (Lachman and Pivot shift) are indications for non-operative management. In contrast, a complete ACL tear with objective instability testing indicates operative management. The onset of menarche is the best determinant of skeletal maturity in females. The physis is open to children younger than 14 years old.[45][46][47]
Reconstruction
Intra-articular could be physeal sparing (all intra-epiphyseal), trans-physeal (males ≤13-16, females ≤ 12-14), or partial transphyseal; leaving one physis undisturbed either the distal femur or proximal tibia. There is no reported significant difference in growth disturbances between different techniques.
Combined intra- and extra-articular can be performed in males up to 12 years old and females up to 11 years old. The technique involves harvesting an ITB graft, freeing it up proximally while the distal attachment remains undisturbed. The graft is then looped through the knee in an over-the-top position and passed through the intercondylar notch and inferior to the inter-meniscal ligament anteriorly. Then the graft is sutured to the lateral femoral condyle and proximal tibia.
Adult-type reconstruction should be considered in males from 16 years old and females from 14 years old.
Graft Selection: Soft tissue grafts used in a trans-physeal approach rarely causes growth disturbances.
A high risk of physeal injury has been reported due to multiple factors which can be modified. A large tunnel diameter of more than 12mm is the most significant as it violates more than 7 to 9% of the physeal cross-sectional area, whereas an 8mm tunnel would compromise less than 3% of the physeal cross-sectional area.
Other factors include oblique tunnel trajectory, high-speed tunnel reaming, aperture fixation with an interference screw, dissection close to the perichondral ring of LaCroix, suturing near the tibial tubercle, and lateral extra-articular tenodesis. Overall physeal disruption with no growth disturbance has been reported in 10% of cases.
Differential Diagnosis
- ACL tear
- Epiphyseal fracture of femur or tibia
- Medial collateral knee ligament injury
- Meniscal tear
- Osteochondral fracture
- Patellar dislocation
- Posterior cruciate ligament injury
- Tibial spine fracture
Prognosis
ACL-deficient knees are more prone to the progression of arthritis with further injuries to the chondral and meniscal structures. ACL reconstruction restores native knee kinematics, and a high level of return to sports participation has been reported.
Complications
There are a variety of complications that can happen intraoperative or postoperative.[48][49] Intraoperative such as graft tunnel mismatch, e.g., in BPTB graft, if longer than the combined femoral tunnel, tibial tunnel, and intra-articular portion, will result in prominence of the tibial bone plug and may compromise distal fixation. This can be encountered in BPTB allograft, patella Alta and non-transtibial drilling techniques. However, it is an avoidable situation that is tackled by accurately measuring the tunnel and tailoring the graft accordingly, which can be accomplished by twisting the graft to shorten it.
Tunnel malpositioning: on the femoral side, a vertical tunnel in the coronal plane, instead of a more horizontal one, would result in persistent rotational instability (positive pivot shift test). In the sagittal plane: anterior misplacement yields a tight knee in flexion and loose in extension, and vice versa with posterior misplacement. Clearing the resident ridge would allow a clear view and avoid anterior-posterior misplacement. On the tibial side: too anterior misplacement yields a tight knee in flexion and roof impingement in extension. A posterior misplacement will result in an ACL graft impingement on the PCL.[50]
Another scenario is a posterior wall blowout. This can be overcome by adequate posterior wall exposure and evaluation of the wall after drilling. Also, drilling the tunnel with 70 to 90 degrees of flexion helps avoid this complication. Posterior wall blowout can be managed with redrilling with anteriorly directed trajectory if the defect is minimal and proceed with the same planned fixation. Otherwise, if there is a significant defect, the same tunnel can be used with suspensory fixation and supplementary interference screw.
Graft failure due to various other issues, such as hardware failure, could be due to inadequate fixation, e.g., graft screw divergence > 30 degrees—attritional graft failure due to small graft diameter (< 8 mm). Intra-articular femoral bone plug dislodgement in the BPTB graft would require revision surgery. Missed diagnosis of an associated ligamentous injury or bony malalignment. Improper rehabilitation with too aggressive exercises such as open-chain exercises.
Infection and septic arthritis: This is < 1% of all cases when it occurs and is mostly superficial, with S. epidermidis (coagulase-negative staph) being the most commonly involved pathogen. Routine graft soaking in vancomycin may lower infection risk. Risk factors for infection include graft contamination during intraoperative handling or falling on the floor. Grafts dropped on the floor: this can be managed with multiple soakings in a variety of antibiotic solutions with no increased risk of infection reported.
Patients usually present with the usual infection symptoms and signs such as pain, swelling, erythema, and raised WBC at 2 to 14 days postoperatively. Diagnosis is confirmed with aspiration and gm stain and cultures. Management for intraoperative events, the graft should be soaked in antibiotic solutions before insertion and fixation. Postoperative management involves immediate incision and drainage. The graft can be retained with multiple I &Ds and antibiotics for a minimum of 6 weeks. This philosophy is more likely to work with staph epidermidis but less likely to work with staph aureus.
Stiffness and arthrofibrosis: This is the most common complication following ACL reconstruction, mainly due to a preoperative reduced range of motion. Patients present with reduced patellar movement. Management starts preoperatively with prevention and emphasizes the importance of pre-hab to regain full ROM before surgery—planning surgery after swelling resolution reduces the incidence of arthrofibrosis. Intraoperatively, precision in tunnel positioning is crucial to achieving full ROM, and cryotherapy and aggressive physiotherapy if encountered up to 12 weeks postoperatively. However, if more than 12 weeks postoperatively, lysis of adhesions and manipulation under anesthesia might be indicated.
Infrapatellar contracture syndrome: an uncommon cause of postoperative stiffness with reduced patellar translation.
Patella Tendon Rupture: with evidence of patella Alta on radiographs.
Complex Regional Pain Syndrome
Patella fracture: in BPTB graft and quadriceps tendon graft with bone plug. The majority of the fracture will occur 8 to 12 weeks postoperatively.
Tunnel osteolysis: Unless graft laxity or knee instability exists, tunnel osteolysis should be managed by observation.
Osteoarthritis in the long term: Could be related to associated meniscal injuries. Increased incidence has been reported in patients above 50 years old at the time of ACL reconstruction.[51]
Saphenous nerve irritation has been reported with harvesting hamstring autograft.
Cyclops lesion: This is a fibroproliferative tissue block extension. A click may be heard at the terminal extension.[52]
Postoperative and Rehabilitation Care
Immediate postoperative care involves extensive cryotherapy. Patients should be encouraged to fully weight bear as tolerated to reduce patellofemoral pain. The importance of early full passive extension, especially with associated MCL injury or patella dislocation, should be emphasized.
Early rehabilitation should be commenced with exercises that do not exert excessive stress on the graft. At three weeks postoperatively, eccentric strengthening increases quadriceps volume and strength. Other exercises include isometric hamstring contractions at any angle. Isometric quadriceps or simultaneous quadriceps and hamstring contractions. Active knee ROM (35-90 degrees), in addition to core muscles and gluteal strengthening. Closed chain exercises should be emphasized, such as squats or leg-press.
During early rehabilitation: certain exercises should be avoided, such as isokinetic quadriceps strengthening (15-30°), open chain quadriceps strengthening, leg extensions similar to the anterior drawer, and Lachman maneuvers.
Return to play: this is a controversial area with no definite criteria on return to sports, either timing or type of sports involved. Previously, there was an agreement that return should not be earlier than nine months postoperatively. However, patients should be able to demonstrate the ability to perform certain sport-specific activities and complete a series of functional tests such as various single- and double-leg hopping and jumping. Dynamic valgus has been shown to increase the risk of ipsilateral and contralateral rupture.
Re-rupture: Higher rates have been reported with early return to sport before clearance, which is ideally a joint decision between surgeon and patient.
For injury prevention, certain factors can be employed with particular reference to female athletes who might benefit from neuromuscular training, plyometrics (jump training), training to land from jumping in less valgus and more knee flexion, in addition to increasing hamstring strength to decrease quadriceps dominance ratio.
Advanced Updates on Postoperative Rehabilitation
Neuromuscular electrical stimulation (NMES): multiple studies assessed the outcomes of Neuromuscular electrical stimulation (NMES) following ACLR.[53][54][55]
A significant clinical effect was demonstrated by adding 2 to 6 sessions of NMES per week to standard rehabilitation protocol, with improved quadriceps strength in the first 4 to 12 weeks postoperatively.[53]
Open Kinetic Chain Exercises: No significant differences were reported with regard to knee laxity, the strength of the muscle, and self-reported function and physical function between the two measures at any follow-up time (4 weeks to 19 months), regardless of the graft type. No difference between the early (< 4 weeks) versus delayed (12 weeks) start of the open kinetic chain exercises with knee flexion limited to a range of 45 to 90 degrees of flexion.[56][57]
No superiority was reported for structured in-person rehabilitation versus structured home-based rehabilitation for quadriceps and hamstring muscle strength, knee laxity, and functionality in both the short and long term following injury and surgery.[58]
Knee bracing following ACLR was found to be not advantageous for knee laxity and physical function.[59]
Preoperative rehabilitation of muscular strengthening and neuromuscular stability training for 3–6 weeks improves self-reported and physical function three months postoperatively, but it does not affect the return to sports outcomes.[60]
Cryotherapy with cold compression devices in the first 24 to 48 hours post-ACLR reduces pain and limits analgesic use compared to no cryotherapy.[61][55]
Following ACLR, Low to very low levels of evidence supports psychological interventions, whole body vibration, protein-based supplement use to improve quadriceps volume and strength, blood flow restriction training to improve quadriceps size and lean muscle mass, neuromuscular control exercises, and continuous passive motion.[62][63][64][65][66][65][64][62]
Pearls and Other Issues
Return to activity is variable and patient-dependent. The average return to full activity and/or sports participation is estimated to be between 6 to 12 months after surgical reconstruction, depending upon their progress with PT and the type of sport/activity to which they are returning. However, some studies have shown that the graft takes up to 18 months or more to become fully functional and incorporated. Early/premature return to activity can lead to re-injury and graft failure.
Enhancing Healthcare Team Outcomes
The diagnosis and management of ACL are best by an interprofessional team that includes an emergency department clinician, orthopedic surgeon, sports clinician, nursing staff, and physical therapist. The initial treatment of ACL is RICE therapy. Depending on their severity, ACL injuries can be managed nonoperatively or operatively.
The patient with an anterior cruciate ligament injury should be referred to an orthopedic surgeon to discuss treatment options and a physical therapist (PT) for rehabilitation. Care coordination between PT and the treating clinician is often the task of a specialty-trained orthopedic nurse, who can also counsel the patient on their condition and treatment. The outcomes for patients with ACL injury are good, but the recovery may take at least 3 to 9 months of intense physical therapy.[3][67]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
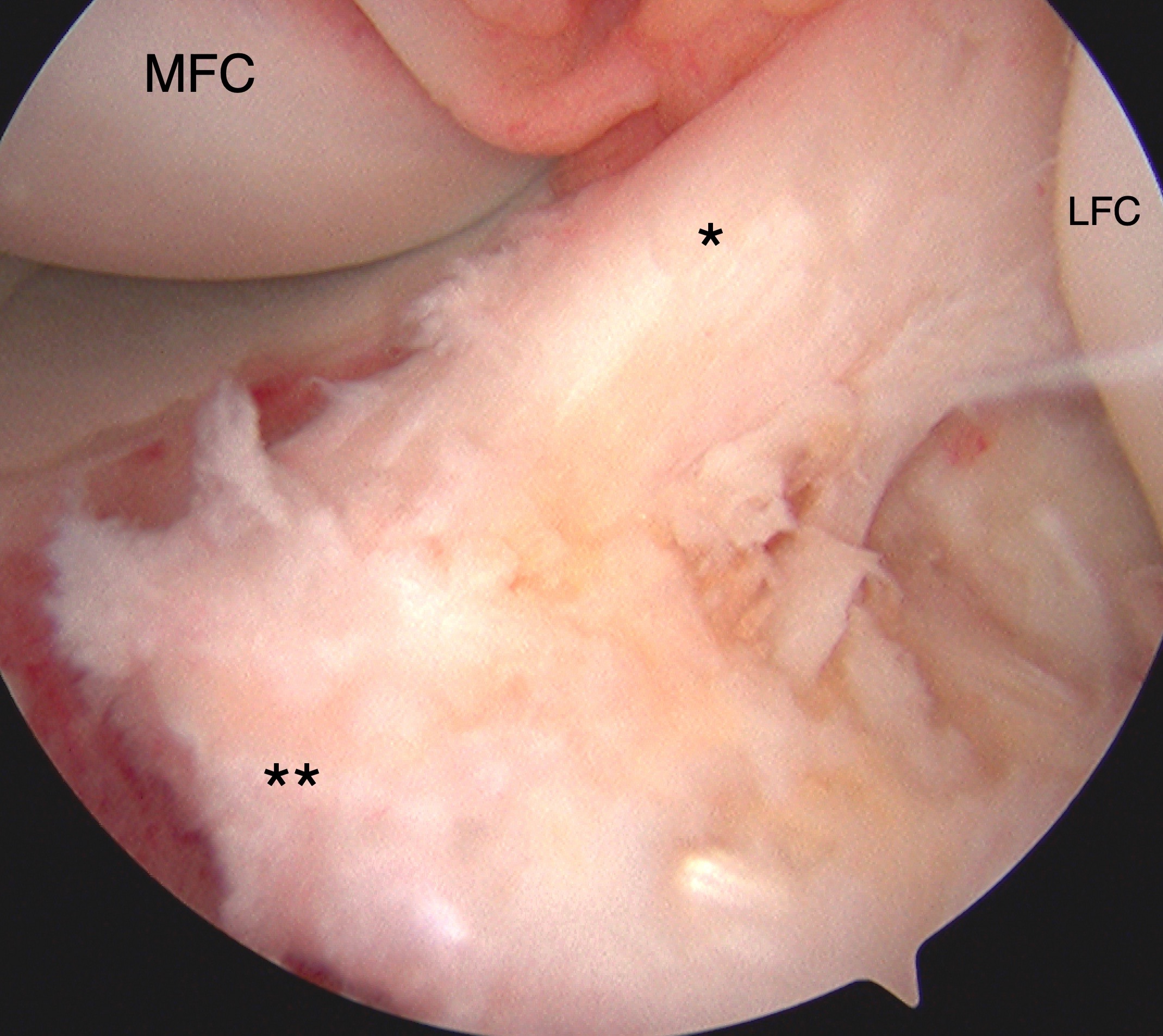
Tibial Eminence Arthroscopic Reduction and Internal Fixation. Tibial eminence avulsion injury confirmed by direct visualization during knee arthroscopy. Arthroscopy-assisted internal fixation was performed using sutures for anchoring. The anterior cruciate ligament (ACL, marked by *) and insertional footprint on the tibia (marked by **) are shown. The medial (MFC) and lateral (LFC) femoral condyles are also labeled for reference.
Contributed by Matthew A Varacallo MD
References
Giuliani JR, Kilcoyne KG, Rue JP. Anterior cruciate ligament anatomy: a review of the anteromedial and posterolateral bundles. The journal of knee surgery. 2009 Apr:22(2):148-54 [PubMed PMID: 19476182]
Gupta R, Malhotra A, Sood M, Masih GD. Is anterior cruciate ligament graft rupture (after successful anterior cruciate ligament reconstruction and return to sports) actually a graft failure or a re-injury? Journal of orthopaedic surgery (Hong Kong). 2019 Jan-Apr:27(1):2309499019829625. doi: 10.1177/2309499019829625. Epub [PubMed PMID: 30782075]
Hoogeslag RAG, Brouwer RW, Boer BC, de Vries AJ, Huis In 't Veld R. Acute Anterior Cruciate Ligament Rupture: Repair or Reconstruction? Two-Year Results of a Randomized Controlled Clinical Trial. The American journal of sports medicine. 2019 Mar:47(3):567-577. doi: 10.1177/0363546519825878. Epub [PubMed PMID: 30822124]
Level 1 (high-level) evidenceBarfod KW, Rasmussen R, Blaabjerg B, Hölmich P, Lind M. [Return to play after anterior cruciate ligament reconstruction]. Ugeskrift for laeger. 2019 Feb 18:181(8):. pii: V11170846. Epub [PubMed PMID: 30821236]
Siegel L, Vandenakker-Albanese C, Siegel D. Anterior cruciate ligament injuries: anatomy, physiology, biomechanics, and management. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2012 Jul:22(4):349-55. doi: 10.1097/JSM.0b013e3182580cd0. Epub [PubMed PMID: 22695402]
Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. British journal of sports medicine. 2007 Aug:41 Suppl 1(Suppl 1):i47-51 [PubMed PMID: 17646249]
Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. Journal of athletic training. 2008 Jul-Aug:43(4):396-408. doi: 10.4085/1062-6050-43.4.396. Epub [PubMed PMID: 18668173]
Boden BP, Dean GS, Feagin JA Jr, Garrett WE Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000 Jun:23(6):573-8 [PubMed PMID: 10875418]
Pike AN, Patzkowski JC, Bottoni CR. Meniscal and Chondral Pathology Associated With Anterior Cruciate Ligament Injuries. The Journal of the American Academy of Orthopaedic Surgeons. 2019 Feb 1:27(3):75-84. doi: 10.5435/JAAOS-D-17-00670. Epub [PubMed PMID: 30320730]
Bollier M, Smith PA. Anterior cruciate ligament and medial collateral ligament injuries. The journal of knee surgery. 2014 Oct:27(5):359-68. doi: 10.1055/s-0034-1381961. Epub 2014 Jun 20 [PubMed PMID: 24949985]
Fanelli GC, Beck JD, Edson CJ. Combined PCL-ACL lateral and medial side injuries: treatment and results. Sports medicine and arthroscopy review. 2011 Jun:19(2):120-30. doi: 10.1097/JSA.0b013e318219149c. Epub [PubMed PMID: 21540709]
Dean RS, LaPrade RF. ACL and Posterolateral Corner Injuries. Current reviews in musculoskeletal medicine. 2020 Feb:13(1):123-132. doi: 10.1007/s12178-019-09581-3. Epub [PubMed PMID: 31884674]
Kaeding CC, Léger-St-Jean B, Magnussen RA. Epidemiology and Diagnosis of Anterior Cruciate Ligament Injuries. Clinics in sports medicine. 2017 Jan:36(1):1-8. doi: 10.1016/j.csm.2016.08.001. Epub 2016 Oct 4 [PubMed PMID: 27871652]
Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. The Journal of the American Academy of Orthopaedic Surgeons. 2013 Jan:21(1):41-50. doi: 10.5435/JAAOS-21-01-41. Epub [PubMed PMID: 23281470]
Davey A, Endres NK, Johnson RJ, Shealy JE. Alpine Skiing Injuries. Sports health. 2019 Jan/Feb:11(1):18-26. doi: 10.1177/1941738118813051. Epub [PubMed PMID: 30782106]
Vaudreuil NJ, Rothrauff BB, de Sa D, Musahl V. The Pivot Shift: Current Experimental Methodology and Clinical Utility for Anterior Cruciate Ligament Rupture and Associated Injury. Current reviews in musculoskeletal medicine. 2019 Mar:12(1):41-49. doi: 10.1007/s12178-019-09529-7. Epub [PubMed PMID: 30706283]
Eberl R. [Anterior cruciate ligament rupture in children with open growth plate : Diagnostics and treatment]. Der Unfallchirurg. 2019 Jan:122(1):17-21. doi: 10.1007/s00113-018-0589-1. Epub [PubMed PMID: 30635672]
Palazzolo A, Rosso F, Bonasia DE, Saccia F, Rossi R, Knee Committee SIGASCOT. Uncommon Complications after Anterior Cruciate Ligament Reconstruction. Joints. 2018 Sep:6(3):188-203. doi: 10.1055/s-0038-1675799. Epub 2018 Nov 30 [PubMed PMID: 30582108]
Coffey R, Bordoni B. Lachman Test. StatPearls. 2023 Jan:(): [PubMed PMID: 32119302]
Sokal PA, Norris R, Maddox TW, Oldershaw RA. The diagnostic accuracy of clinical tests for anterior cruciate ligament tears are comparable but the Lachman test has been previously overestimated: a systematic review and meta-analysis. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2022 Oct:30(10):3287-3303. doi: 10.1007/s00167-022-06898-4. Epub 2022 Feb 12 [PubMed PMID: 35150292]
Level 1 (high-level) evidenceKim SJ, Kim HK. Reliability of the anterior drawer test, the pivot shift test, and the Lachman test. Clinical orthopaedics and related research. 1995 Aug:(317):237-42 [PubMed PMID: 7671485]
Huang W, Zhang Y, Yao Z, Ma L. Clinical examination of anterior cruciate ligament rupture: a systematic review and meta-analysis. Acta orthopaedica et traumatologica turcica. 2016:50(1):22-31. doi: 10.3944/AOTT.2016.14.0283. Epub [PubMed PMID: 26854045]
Level 1 (high-level) evidenceLelli A, Di Turi RP, Spenciner DB, Dòmini M. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2016 Sep:24(9):2794-2797. doi: 10.1007/s00167-014-3490-7. Epub 2014 Dec 25 [PubMed PMID: 25536951]
Sobrado MF, Bonadio MB, Ribeiro GF, Giglio PN, Helito CP, Demange MK. LEVER SIGN TEST FOR CHRONIC ACL INJURY: A COMPARISON WITH LACHMAN AND ANTERIOR DRAWER TESTS. Acta ortopedica brasileira. 2021 May-Jun:29(3):132-136. doi: 10.1590/1413-785220212903238345. Epub [PubMed PMID: 34290559]
Arneja S, Leith J. Review article: Validity of the KT-1000 knee ligament arthrometer. Journal of orthopaedic surgery (Hong Kong). 2009 Apr:17(1):77-9 [PubMed PMID: 19398799]
Atik OŞ, Çavuşoğlu AT, Ayanoğlu T. Is magnetic resonance imaging reliable for the evaluation of the ruptured or healed anterior cruciate ligament? Eklem hastaliklari ve cerrahisi = Joint diseases & related surgery. 2015:26(1):38-40. doi: 10.5606/ehc.2015.09. Epub [PubMed PMID: 25741919]
Wu F, Colak C, Subhas N. Preoperative and Postoperative Magnetic Resonance Imaging of the Cruciate Ligaments. Magnetic resonance imaging clinics of North America. 2022 May:30(2):261-275. doi: 10.1016/j.mric.2021.11.006. Epub 2022 Apr 13 [PubMed PMID: 35512889]
Xu B, Zhang H, Li B, Wang W. Comparison of magnetic resonance imaging for patients with acute and chronic anterior cruciate ligament tears. Medicine. 2018 Mar:97(10):e0001. doi: 10.1097/MD.0000000000010001. Epub [PubMed PMID: 29517656]
Shaikh H, Herbst E, Rahnemai-Azar AA, Bottene Villa Albers M, Naendrup JH, Musahl V, Irrgang JJ, Fu FH. The Segond Fracture Is an Avulsion of the Anterolateral Complex. The American journal of sports medicine. 2017 Aug:45(10):2247-2252. doi: 10.1177/0363546517704845. Epub 2017 May 12 [PubMed PMID: 28499093]
Kushare I, Ghanta RB, Ditzler M, Jadhav SP. Arcuate sign-fibular head avulsion fracture and associated injuries in the pediatric and adolescent population. Emergency radiology. 2021 Aug:28(4):723-727. doi: 10.1007/s10140-021-01910-9. Epub 2021 Feb 10 [PubMed PMID: 33566239]
Diermeier T, Rothrauff BB, Engebretsen L, Lynch AD, Ayeni OR, Paterno MV, Xerogeanes JW, Fu FH, Karlsson J, Musahl V, Svantesson E, Hamrin Senorski E, Rauer T, Meredith SJ, Panther Symposium ACL Treatment Consensus Group. Treatment after anterior cruciate ligament injury: Panther Symposium ACL Treatment Consensus Group. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2020 Aug:28(8):2390-2402. doi: 10.1007/s00167-020-06012-6. Epub 2020 May 9 [PubMed PMID: 32388664]
Level 3 (low-level) evidenceBeard DJ, Davies L, Cook JA, Stokes J, Leal J, Fletcher H, Abram S, Chegwin K, Greshon A, Jackson W, Bottomley N, Dodd M, Bourke H, Shirkey BA, Paez A, Lamb SE, Barker K, Phillips M, Brown M, Lythe V, Mirza B, Carr A, Monk P, Morgado Areia C, O'Leary S, Haddad F, Wilson C, Price A, ACL SNNAP Study Group. Rehabilitation versus surgical reconstruction for non-acute anterior cruciate ligament injury (ACL SNNAP): a pragmatic randomised controlled trial. Lancet (London, England). 2022 Aug 20:400(10352):605-615. doi: 10.1016/S0140-6736(22)01424-6. Epub [PubMed PMID: 35988569]
Level 1 (high-level) evidenceGiummarra M, Vocale L, King M. Efficacy of non-surgical management and functional outcomes of partial ACL tears. A systematic review of randomised trials. BMC musculoskeletal disorders. 2022 Apr 8:23(1):332. doi: 10.1186/s12891-022-05278-w. Epub 2022 Apr 8 [PubMed PMID: 35395764]
Level 1 (high-level) evidenceMusahl V, Engler ID, Nazzal EM, Dalton JF, Lucidi GA, Hughes JD, Zaffagnini S, Della Villa F, Irrgang JJ, Fu FH, Karlsson J. Current trends in the anterior cruciate ligament part II: evaluation, surgical technique, prevention, and rehabilitation. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2022 Jan:30(1):34-51. doi: 10.1007/s00167-021-06825-z. Epub 2021 Dec 5 [PubMed PMID: 34865182]
Pang L, Li P, Li T, Li Y, Zhu J, Tang X. Arthroscopic Anterior Cruciate Ligament Repair Versus Autograft Anterior Cruciate Ligament Reconstruction: A Meta-Analysis of Comparative Studies. Frontiers in surgery. 2022:9():887522. doi: 10.3389/fsurg.2022.887522. Epub 2022 Apr 20 [PubMed PMID: 35521430]
Level 1 (high-level) evidenceLiu W, Wu Y, Wang X, Kuang S, Su C, Xiong Y, Tang H, Xiao Y, Gao S. ACL stump and ACL femoral landmarks are equally reliable in ACL reconstruction for assisting ACL femoral tunnel positioning. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2023 Jan:31(1):219-228. doi: 10.1007/s00167-022-07084-2. Epub 2022 Aug 10 [PubMed PMID: 35947159]
Ellapparadja P, Joseph I, Selvaratnam V. How to Achieve an Accurate Anatomical Femoral Tunnel Technique in ACL Reconstruction in the Early Years of Your Consultancy? Femoral Offset Aimer Technique: Consistent and Reproducible Technique. The journal of knee surgery. 2020 Dec:33(12):1201-1205. doi: 10.1055/s-0039-1692993. Epub 2019 Aug 2 [PubMed PMID: 31378859]
Arno S, Bell CP, Alaia MJ, Singh BC, Jazrawi LM, Walker PS, Bansal A, Garofolo G, Sherman OH. Does Anteromedial Portal Drilling Improve Footprint Placement in Anterior Cruciate Ligament Reconstruction? Clinical orthopaedics and related research. 2016 Jul:474(7):1679-89. doi: 10.1007/s11999-016-4847-7. Epub 2016 Apr 22 [PubMed PMID: 27106125]
Haro MS, Riff A, Bach BR Jr. Tips for successful transtibial anterior cruciate ligament reconstruction. The journal of knee surgery. 2014 Oct:27(5):331-42. doi: 10.1055/s-0034-1384216. Epub 2014 Jun 25 [PubMed PMID: 24964163]
Sim K, Rahardja R, Zhu M, Young SW. Optimal Graft Choice in Athletic Patients with Anterior Cruciate Ligament Injuries: Review and Clinical Insights. Open access journal of sports medicine. 2022:13():55-67. doi: 10.2147/OAJSM.S340702. Epub 2022 Jul 1 [PubMed PMID: 35800660]
Dhillon J, Kraeutler MJ, Belk JW, McCarty EC, McCulloch PC, Scillia AJ. Autograft and Nonirradiated Allograft for Anterior Cruciate Ligament Reconstruction Demonstrate Similar Clinical Outcomes and Graft Failure Rates: An Updated Systematic Review. Arthroscopy, sports medicine, and rehabilitation. 2022 Aug:4(4):e1513-e1521. doi: 10.1016/j.asmr.2022.04.008. Epub 2022 Jun 6 [PubMed PMID: 36033181]
Level 1 (high-level) evidenceShen X, Liu T, Xu S, Chen B, Tang X, Xiao J, Qin Y. Optimal Timing of Anterior Cruciate Ligament Reconstruction in Patients With Anterior Cruciate Ligament Tear: A Systematic Review and Meta-analysis. JAMA network open. 2022 Nov 1:5(11):e2242742. doi: 10.1001/jamanetworkopen.2022.42742. Epub 2022 Nov 1 [PubMed PMID: 36394870]
Level 1 (high-level) evidenceRodriguez AN, LaPrade RF, Geeslin AG. Combined Meniscus Repair and Anterior Cruciate Ligament Reconstruction. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2022 Mar:38(3):670-672. doi: 10.1016/j.arthro.2022.01.003. Epub [PubMed PMID: 35248223]
Razi M, Soufali AP, Ziabari EZ, Dadgostar H, Askari A, Arasteh P. Treatment of Concomitant ACL and MCL Injuries: Spontaneous Healing of Complete ACL and MCL Tears. The journal of knee surgery. 2021 Oct:34(12):1329-1336. doi: 10.1055/s-0040-1708858. Epub 2020 Apr 8 [PubMed PMID: 32268406]
Chipman DE, Pascual-Leone N, Cordasco FA, Green DW. Anterior Cruciate Ligament Reconstruction in Skeletally Immature Athletes Using All-Epiphyseal Techniques. Clinics in sports medicine. 2022 Oct:41(4):569-577. doi: 10.1016/j.csm.2022.05.002. Epub [PubMed PMID: 36210159]
Patel NM, DeFrancesco CJ, Talathi NS, Bram JT, Ganley TJ. All-epiphyseal Anterior Cruciate Ligament Reconstruction Does Not Increase the Risk of Complications Compared With Pediatric Transphyseal Reconstruction. The Journal of the American Academy of Orthopaedic Surgeons. 2019 Aug 15:27(16):e752-e757. doi: 10.5435/JAAOS-D-18-00276. Epub [PubMed PMID: 30531545]
Pascual-Leone N, Gross PW, Meza BC, Fabricant PD. Techniques in Pediatric Anterior Cruciate Ligament Reconstruction. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2022 Oct:38(10):2784-2786. doi: 10.1016/j.arthro.2022.08.003. Epub [PubMed PMID: 36192042]
Heard WM, Chahal J, Bach BR Jr. Recognizing and managing complications in ACL reconstruction. Sports medicine and arthroscopy review. 2013 Jun:21(2):106-12. doi: 10.1097/JSA.0b013e318290070c. Epub [PubMed PMID: 23649158]
Dayan E, Maderazo A, Fitzpatrick D. Magnetic Resonance Imaging of Complications of Anterior Cruciate Ligament Reconstruction. American journal of orthopedics (Belle Mead, N.J.). 2015 Dec:44(12):569-71 [PubMed PMID: 26665245]
de Padua VBC, Saithna A, Chagas EFB, Zutin TLM, Piazzalunga LF, Patriarcha LF, Gelas PJL, Helito CP. Rate of Tibial Tunnel Malposition Is Not Changed by Drilling Entirely Within the Stump of Preserved Remnants During ACL Reconstruction: A Prospective Comparative 3D-CT Study. Orthopaedic journal of sports medicine. 2021 Oct:9(10):23259671211037324. doi: 10.1177/23259671211037324. Epub 2021 Oct 6 [PubMed PMID: 34646899]
Level 2 (mid-level) evidenceWang LJ, Zeng N, Yan ZP, Li JT, Ni GX. Post-traumatic osteoarthritis following ACL injury. Arthritis research & therapy. 2020 Mar 24:22(1):57. doi: 10.1186/s13075-020-02156-5. Epub 2020 Mar 24 [PubMed PMID: 32209130]
Noailles T, Chalopin A, Boissard M, Lopes R, Bouguennec N, Hardy A. Incidence and risk factors for cyclops syndrome after anterior cruciate ligament reconstruction: A systematic literature review. Orthopaedics & traumatology, surgery & research : OTSR. 2019 Nov:105(7):1401-1405. doi: 10.1016/j.otsr.2019.07.007. Epub 2019 Aug 9 [PubMed PMID: 31405748]
Level 1 (high-level) evidenceHauger AV, Reiman MP, Bjordal JM, Sheets C, Ledbetter L, Goode AP. Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2018 Feb:26(2):399-410. doi: 10.1007/s00167-017-4669-5. Epub 2017 Aug 17 [PubMed PMID: 28819679]
Kim KM, Croy T, Hertel J, Saliba S. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. The Journal of orthopaedic and sports physical therapy. 2010 Jul:40(7):383-91. doi: 10.2519/jospt.2010.3184. Epub [PubMed PMID: 20592480]
Level 1 (high-level) evidenceGatewood CT, Tran AA, Dragoo JL. The efficacy of post-operative devices following knee arthroscopic surgery: a systematic review. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2017 Feb:25(2):501-516. doi: 10.1007/s00167-016-4326-4. Epub 2016 Oct 1 [PubMed PMID: 27695905]
Level 1 (high-level) evidenceJewiss D, Ostman C, Smart N. Open versus Closed Kinetic Chain Exercises following an Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Journal of sports medicine (Hindawi Publishing Corporation). 2017:2017():4721548. doi: 10.1155/2017/4721548. Epub 2017 Aug 17 [PubMed PMID: 28913413]
Level 1 (high-level) evidencePerriman A, Leahy E, Semciw AI. The Effect of Open- Versus Closed-Kinetic-Chain Exercises on Anterior Tibial Laxity, Strength, and Function Following Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. The Journal of orthopaedic and sports physical therapy. 2018 Jul:48(7):552-566. doi: 10.2519/jospt.2018.7656. Epub 2018 Apr 23 [PubMed PMID: 29685058]
Level 1 (high-level) evidenceGamble AR, Pappas E, O'Keeffe M, Ferreira G, Maher CG, Zadro JR. Intensive supervised rehabilitation versus less supervised rehabilitation following anterior cruciate ligament reconstruction? A systematic review and meta-analysis. Journal of science and medicine in sport. 2021 Sep:24(9):862-870. doi: 10.1016/j.jsams.2021.03.003. Epub 2021 Mar 10 [PubMed PMID: 33736965]
Level 1 (high-level) evidenceYang XG, Feng JT, He X, Wang F, Hu YC. The effect of knee bracing on the knee function and stability following anterior cruciate ligament reconstruction: A systematic review and meta-analysis of randomized controlled trials. Orthopaedics & traumatology, surgery & research : OTSR. 2019 Oct:105(6):1107-1114. doi: 10.1016/j.otsr.2019.04.015. Epub 2019 Jul 3 [PubMed PMID: 31279767]
Level 1 (high-level) evidenceCarter HM, Littlewood C, Webster KE, Smith BE. The effectiveness of preoperative rehabilitation programmes on postoperative outcomes following anterior cruciate ligament (ACL) reconstruction: a systematic review. BMC musculoskeletal disorders. 2020 Oct 3:21(1):647. doi: 10.1186/s12891-020-03676-6. Epub 2020 Oct 3 [PubMed PMID: 33010802]
Level 1 (high-level) evidenceMartimbianco AL, Gomes da Silva BN, de Carvalho AP, Silva V, Torloni MR, Peccin MS. Effectiveness and safety of cryotherapy after arthroscopic anterior cruciate ligament reconstruction. A systematic review of the literature. Physical therapy in sport : official journal of the Association of Chartered Physiotherapists in Sports Medicine. 2014 Nov:15(4):261-8. doi: 10.1016/j.ptsp.2014.02.008. Epub 2014 Mar 13 [PubMed PMID: 24713365]
Level 1 (high-level) evidenceCoronado RA, Bird ML, Van Hoy EE, Huston LJ, Spindler KP, Archer KR. Do psychosocial interventions improve rehabilitation outcomes after anterior cruciate ligament reconstruction? A systematic review. Clinical rehabilitation. 2018 Mar:32(3):287-298. doi: 10.1177/0269215517728562. Epub 2017 Aug 24 [PubMed PMID: 28836467]
Level 1 (high-level) evidenceSeixas A, Sañudo B, Sá-Caputo D, Taiar R, Bernardo-Filho M. Whole-Body Vibration for Individuals with Reconstructed Anterior Cruciate Ligament: A Systematic Review. BioMed research international. 2020:2020():7362069. doi: 10.1155/2020/7362069. Epub 2020 May 1 [PubMed PMID: 32462013]
Level 1 (high-level) evidenceQiu J, Ong MT, Leong HT, He X, Fu SC, Yung PS. Effects of Whole-Body Vibration Therapy on Quadriceps Function in Patients With Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sports health. 2022 Mar-Apr:14(2):216-226. doi: 10.1177/19417381211004937. Epub 2021 Apr 5 [PubMed PMID: 33813953]
Level 1 (high-level) evidenceGreif DN, Emerson CP, Allegra P, Arizpe A, Mansour KL, Cade WH 2nd, Baraga MG. Supplement Use in Patients Undergoing Anterior Cruciate Ligament Reconstruction: A Systematic Review. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2020 Sep:36(9):2537-2549. doi: 10.1016/j.arthro.2020.04.047. Epub 2020 May 8 [PubMed PMID: 32438028]
Level 1 (high-level) evidenceArumugam A, Björklund M, Mikko S, Häger CK. Effects of neuromuscular training on knee proprioception in individuals with anterior cruciate ligament injury: a systematic review and GRADE evidence synthesis. BMJ open. 2021 May 18:11(5):e049226. doi: 10.1136/bmjopen-2021-049226. Epub 2021 May 18 [PubMed PMID: 34006560]
Level 1 (high-level) evidenceThoma LM, Grindem H, Logerstedt D, Axe M, Engebretsen L, Risberg MA, Snyder-Mackler L. Coper Classification Early After Anterior Cruciate Ligament Rupture Changes With Progressive Neuromuscular and Strength Training and Is Associated With 2-Year Success: The Delaware-Oslo ACL Cohort Study. The American journal of sports medicine. 2019 Mar:47(4):807-814. doi: 10.1177/0363546519825500. Epub 2019 Feb 21 [PubMed PMID: 30790527]