Introduction
Hookworms are nematode parasites that usually get transmitted through infested soil. They usually affect the poorest individuals in tropical and subtropical areas. Two species are mainly responsible for human infections, Ancylostoma duodenale and Necator americanus. They can cause chronic infection of the intestinal tract, suck their host blood, leading to iron deficiency anemia in most cases. Moreover, pulmonary manifestations might occur by the effect of larval migration. While multiple medications are available to treat hookworm infections, prevention is still vital to fight complications.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Ancylostoma duodenale and Necator americanus are the principal species that infect humans. Ancylostoma ceylanicum is currently considered a significant cause of zoonotic infections in some regions of Asia. However, it doesn’t cause blood loss. Ancylostoma caninum, a dog hookworm, might cause enteritis and ileitis. Ancylostoma braziliense is the primary cause of cutaneous larva migrans.[4][5]
Epidemiology
Worldwide, about 470 million people have hookworm infections. Infection predominates within developing countries and leads to huge losses of economic productivity due to anemia worsening the already existing poverty and disease.
Necator americanus is the major cause of hookworm infections worldwide, while Ancylostoma duodenale tends to be endemic to the Mediterranean region and northern India and China.
Risk factors for developing hookworm infections include low socioeconomic background, exposure to infected soil, barefoot walking, poor sanitation, and personal hygiene. Children and pregnant women are at the highest risk. Transmission is affected by multiple factors such as warm and moist climate, contaminated water supply, and poor sanitation.[1][4][6][7][8]
Pathophysiology
In soil, the hookworm eggs hatch, and first-stage larvae called rhabditiform L1 larvae develop in few days. They molt twice to become the infective filariform L3, which is about 0.5 to 0.6 mm in length and can live for 3 to 4 weeks if suitable conditions were available. It waits in soil or on the grass until it comes into contact with human skin and initiates an infection.
The infection starts when larvae penetrate the victim's skin in a process that requires about 30 mins to 6 hours to complete, according to species. Occasionally, larvae might use buccal mucosa to invade the host and make their way down to the circulation. Cutaneous penetration usually goes unnoticed but sometimes might cause what is called 'ground' itch.
Skin penetration occurs under a chemical process that starts with the production of proteolytic enzymes from certain glands in the larvae. Necator americanus produce proteases that could break connective tissue components such as collagen and elastin. On the other hand, Ancylostoma larvae produce hyaluronidase enzyme, which cracks the dermal integrity and allows the larvae to migrate through the skin. One of the significant larval secretions is called Ancylostoma secreted proteins (ASPs), which are pivotal in developing the parasite, representing about one-third of its secreted proteins.
After skin penetration, the larvae migrate passively through the bloodstream to the right side of the heart and hence to the pulmonary vasculature. During pulmonary migration, it might cause a type-1 hypersensitivity reaction within the alveoli (Loeffler syndrome). They penetrate the alveoli and migrate through the bronchial tree to the pharynx and then to the intestinal tract.
Once the larvae reach the duodenum, and after molting twice, they become L5 immature worms that can use their teeth or cutting plates that line their buccal capsule to get fixed into the host's intestinal mucosa. Digestion of blood is helped by metalloproteases and anticoagulant peptides, which maintain the flow of liquid blood through mucosal injury. However, the process by which hemoglobin gets consumed in the parasite gut is poorly understood.
Worms mature in 4 to 6 weeks into adult sexually differentiated worms. After mating, the female produces up to 30000 eggs per day, which exit the host with feces to continue the life cycle.
Blood loss in heavily infected persons could reach up to 9.0 mL/day and occurs by two mechanisms. The first is through consumption of the parasite, which accounts for a small portion of blood loss. The second and the main loss occurs through the attachment site by leakage around it. Iron deficiency anemia occurs when the host becomes unable to compensate for blood loss, especially in heavy infections and nutritionally deprived individuals. The major risk factor for anemia is the worm burden, though, in children, anemia could occur with a lower worm burden.
Simultaneous protein loss might occur and result in symptomatic hypoalbuminemia and hypoproteinemia, leading to anasarca and worsen malnutrition.
Parasites can last for years in the host, and accordingly, it had to develop multiple strategies to ensure survival. The parasite uses broad-spectrum protease inhibitors to neutralize the effect of the host's immune defenses. While it helps the parasite protect itself from proteolytic enzymes, it worsens the host's malnutrition by interfering with absorption. Moreover, it induces apoptosis of T lymphocytes and, consequently, inhibition of local immune response. The down-regulated immune response to the parasite is mainly due to parasite-specific T cell hyporesponsiveness secondary to altered function of antigen-presenting cells, T cell apoptosis, and cytokines modulation.
Interestingly, in individuals with hookworm infections, same as other helminths, patients tend to have a wider variety of gut microbiota. This observation, along with immune regulatory mechanisms in hookworms, prompted research into the potential use of hookworms to treat immune-mediated gastrointestinal diseases like celiac disease and inflammatory bowel disease.[1][5][7][9][10]
History and Physical
Hookworm infections are usually asymptomatic. Symptoms usually relate to the stage of parasite development and the site of the affected host. It usually starts at the time of skin penetration in the form of a localized erythematous reaction (ground itch).[6][10]
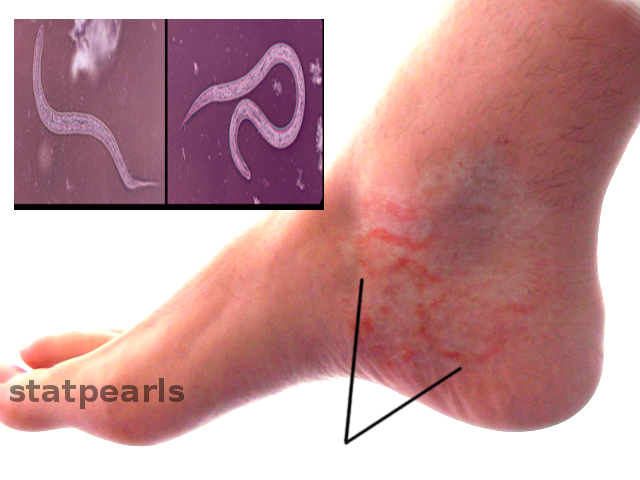
Hookworm related cutaneous larvae migrans (creeping eruptions) have links to zoonotic hookworms. It is endemic in many developing countries, while travelers and health professionals are the sectors that are usually affected in developed countries. It starts as an erythematous papule that develops afterward into the characteristic serpiginous, 1 to 5 cm tunnels beneath the skin, which occur as larvae become unable to penetrate the deep layers of the skin and stay in its superficial layers. Infection often affects hands and feet as the usual contact site with soil.[1][11]
During the pulmonary stage, the infection might express itself in the form of cough, sneezing, bronchitis, hemoptysis, and eosinophilic pneumonia (Loeffler syndrome), usually self-limiting and doesn’t need any intervention. With peroral infection, nausea, vomiting, pharyngeal irritation, cough, and dyspnoea might occur (Wakana syndrome).
Once worms reach the small intestine, nonspecific abdominal symptoms might occur, such as abdominal pain and distension, diarrhea, occult fecal blood, and occasionally melena. The small size of the parasite makes surgical complications unlikely.[1][10]
The major feature of hookworm infection is iron deficiency anemia secondary to blood loss, either through direct parasite consumption or blood leakage from the parasite attachment site to the gut.[12]
Additionally, hypoalbuminemia might lead to edema formation and generalized anasarca. Occasionally, some patients crave soil and ingest dirt (geophagia).[1][10][7]
Evaluation
Clinical features of hookworm infections are usually non-specific and could be misleading. Proper understanding of the epidemiology, clinical features, and laboratory findings is crucial in diagnosis.[1][10]
Stool microscopy is the mainstay tool for diagnosis but with some limitations. It is useful in identifying and quantifying hookworm eggs. In hospitals, labs tend to use egg concentration techniques, while for screening and public health control, simple tests as Kato-Katz techniques are options; those are usually used in epidemiological studies because they provide an indirect measure of worm burden. Precision is limited by variations in egg production, especially with less severe infections. IgG4 assay might identify recent infection but remains non-specific.[5][10]
Eosinophilia raises suspicion of hookworm infection but non-specific. Systemic and mucosal eosinophilia is widely present in hookworm infections. It is detectable in the blood even before reaching the intestine and peaks after adult worms reach intestinal mucosa.[1]
Capsule endoscopy might show parasites, but it is rarely used to diagnose infection. Computer-aided detection of hookworms on capsule endoscopy images is still challenging; the ultimate goal is to use automatic detection models to assist diagnosis more accurately than experienced endoscopists.[1][10][13]
Treatment / Management
The main drugs used for hookworm infections are mebendazole and albendazole. Data support 400 mg single-dose albendazole therapy over a 500 mg single dose of mebendazole. Three consecutive daily doses of either drug demonstrate superior cure and egg reduction rates, but it is less convenient for mass treatment campaigns. Alternatively, a 3-day regimen of 100 mg twice daily, mebendazole, is suitable for stable uncomplicated cases. Also, pyrantel pamoate 11 mg/kg (up to a maximum of 1 g) orally daily for three days could be an option.[1]
A systematic meta-analysis showed that the efficacy of single-dose oral albendazole, mebendazole, and pyrantel pamoate against hookworm infections was 72% (95% CI, 59%-81%; 742 patients), 15% (95% CI, 1%-27%; 853 patients), and 31% (95% CI, 19%-42%; 152 patients).[14](B3)
Treatment efficacy varies according to the severity of infection, geographical distribution, and age groups. Both mebendazole and albendazole are usually safe with few transient side effects such as dizziness, headache, and abdominal upset.[1][15]
Pregnant and lactating women have an increased risk of anemia from hookworm infections. Albendazole and mebendazole were both pregnancy category C under the prior FDA system; data on their use in pregnant women are limited. It is not known whether albendazole or mebendazole gets excreted in human milk. While albendazole requires caution in breastfeeding, the WHO allows the use of mebendazole in lactating women.[16](B3)
Treatment failure can occur. Although the etiology is unclear, repeated use of the same medication raises the question of drug resistance. Pyrantel pamoate and levamisole are alternative treatments, but neither has equal efficacy with albendazole.
Cutaneous larvae migrans is usually self-limited and confined to the skin. However, treatment is sometimes needed, and it responds well to oral albendazole or ivermectin.
Coadministration of deworming and iron supplementation has a greater impact on anemia, especially in nutritionally deficient populations. In a study of 746 school children, multi-nutrient supplements along with anthelminthic medications increased Hb, irrespective of initial Hb and nutritional status.[16][17](B3)
Blood transfusion might be necessary for patients with severe anemia. Nutritional support and frequent monitoring of response are indicated in severely affected individuals.
There is no sufficient available data to guide long term monitoring of treatment. As the failure of treatment and reinfection are real threats, we suggest that follow up of clinical symptoms and anemia and stool testing would be advised. A follow up at 1, 4, and 12 months seems to be appropriate in this context.[1][18]
Differential Diagnosis
Other intestinal causes of iron deficiency anemia have to be excluded, such as:
- Malabsorption
- Gastric or esophageal erosions
- Peptic ulcer disease
- Gastrointestinal malignancies
Moreover, the differential diagnosis includes other helminthic infestations that share common features with hookworm infections, such as:
- Ascariasis
- Schistosomiasis
- Strongyloidiasis
Cutaneous manifestations require differentiation from other similar conditions like contact dermatitis, migratory myiasis, scabies infection, and cercarial dermatitis.[5]
Prognosis
Hookworm infection tends to cause morbidity rather than mortality. In adults, anemia and malnourishment lead to reduced productivity with subsequently increased poverty.
In pregnancy, demand increases for iron, and subsequently, the risk is higher in this class of patients with an effect on both the mother and her fetus's wellbeing. School children are at risk of a decline in cognitive function and school achievement. In contrast, preschool children suffer less from the threat of anemia and malnutrition.
There are growing concerns regarding treatment failure, especially after mass drug administration campaigns. There is no sufficient data to illustrate the effect of deworming interventions on quality of life. Additionally, there is a need to develop a new generation of broad-spectrum agents and further assess the efficacy of combination therapy on the outcome.
Reinfection is another challenge in the treatment of hookworms. Moderate reinfection rates post-treatment support the concept of repeated drug regimens in highly endemic areas. A study of 405 school children, at 18 weeks follow-up post-treatment, showed that the reinfection rate was 25.0 % for hookworms (95 % CI: 15.5–36.6).[1][5][19]
Complications
Adult hookworm infection complications often include iron deficiency anemia; however, rarely, it may include an overt gastrointestinal bleed. Other associated complications include cutaneous larvae migrans and eosinophilic pneumonia.[18]
Deterrence and Patient Education
Mass drug administration campaigns are effective in reducing the prevalence and burden of the infection. However, after stopping the medication, reinfection rates are usually high.
Prevention is mainly through health education campaigns regarding food sanitation, safe drinking water, hand washing, and footwear.[10][20]
Vaccine production still under development and clinical trials. It is intended to prevent infections in highly endemic areas with the worst burden of the disease. The vaccine elicits neutralizing antibodies that interfere with adult worms’ survival in the gut and its ability to consume the host's blood.[1][15]
Reduction of poverty and increased economic development have done more to eliminate hookworm infection than any other factor, but obviously, that would not be easy.[7]
Pearls and Other Issues
- Hookworm infections are currently considered one of the most underfunded neglected tropical diseases.
- Millions of people get infected with the parasite around the world, especially in poorer tropical countries.
- Ancylostoma duodenale and Necator americanus are the main species that infect humans. A. ceylanicum, A. caninum, and A. braziliense are other minor causes.
- Hookworm infections are usually asymptomatic; symptoms vary depending on the stage of the life cycle, from cutaneous ground itch to respiratory symptoms until the main feature of iron deficiency anemia and, on rare occasions, intestinal bleeding.
- Diagnosis depends on both epidemiological, clinical, and microscopic evaluation.
- Management is mainly by either single-dose albendazole or multi-dose mebendazole. Mass treatment is useful in endemic areas.
- Health education and sanitation are the mainstays of preventing the disease. The vaccine is not available currently but under ongoing research and development.
Enhancing Healthcare Team Outcomes
The diagnosis and management of hookworm infections necessitate an interprofessional approach, including histopathologist, infectious disease specialist, gastroenterologist, and general practitioner. Patients usually present to their general practitioner with general non-specific symptoms or anemia. Diagnosis requires a high index of suspicion with careful history and examination, especially in non-endemic areas, besides a thorough analysis of stool samples from experienced histopathologists. Referrals might be necessary for both infectious disease specialists or gastroenterologists, and both have to be mindful of such a diagnosis for suggestive presentations. Treatment requires collaboration between pharmacists, nurses, and physicians.
The pharmacist has to educate the patient about drug compliance. Ideally, an infectious disease specialty pharmacist can consult on the medication regimen, verifying dosing, offering alternative agents in the event of treatment failure, and ensuring there are no drug interactions that could compromise therapeutic effectiveness. Nurses should also educate travelers about hygiene and other safety issues when entering shallow waters in the tropics. Nursing can also assess the effectiveness of medical treatment on subsequent patient encounters and inform the clinical team members if a potential therapy change is necessary.
Prevention requires cooperation between public health specialists, general practitioners, and local authorities to improve sanitation and living conditions while raising awareness between affected patients and populations at risk. In the case of hookworm infections, the interprofessional team can expand beyond the usual players since it can present a public health issue in endemic areas, which will require even more broad-based collaboration among different healthcare team members as an interprofessional functional unit to drive individual and population outcomes effectively. [Level 5]
Media
References
Loukas A,Hotez PJ,Diemert D,Yazdanbakhsh M,McCarthy JS,Correa-Oliveira R,Croese J,Bethony JM, Hookworm infection. Nature reviews. Disease primers. 2016 Dec 8; [PubMed PMID: 27929101]
Wei KY,Yan Q,Tang B,Yang SM,Zhang PB,Deng MM,Lü MH, Hookworm Infection: A Neglected Cause of Overt Obscure Gastrointestinal Bleeding. The Korean journal of parasitology. 2017 Aug; [PubMed PMID: 28877570]
Periago MV,Bethony JM, Hookworm virulence factors: making the most of the host. Microbes and infection. 2012 Dec; [PubMed PMID: 23006854]
Level 3 (low-level) evidenceAlbonico M,Savioli L, Hookworm: a neglected resurgent infection. BMJ (Clinical research ed.). 2017 Oct 24; [PubMed PMID: 29066626]
Brooker S,Bethony J,Hotez PJ, Human hookworm infection in the 21st century. Advances in parasitology. 2004; [PubMed PMID: 15603764]
Level 3 (low-level) evidenceParija SC,Chidambaram M,Mandal J, Epidemiology and clinical features of soil-transmitted helminths. Tropical parasitology. 2017 Jul-Dec; [PubMed PMID: 29114484]
Hotez PJ,Brooker S,Bethony JM,Bottazzi ME,Loukas A,Xiao S, Hookworm infection. The New England journal of medicine. 2004 Aug 19; [PubMed PMID: 15317893]
Level 3 (low-level) evidenceJiraanankul V,Aphijirawat W,Mungthin M,Khositnithikul R,Rangsin R,Traub RJ,Piyaraj P,Naaglor T,Taamasri P,Leelayoova S, Incidence and risk factors of hookworm infection in a rural community of central Thailand. The American journal of tropical medicine and hygiene. 2011 Apr [PubMed PMID: 21460016]
Pearson MS,Tribolet L,Cantacessi C,Periago MV,Valero MA,Jariwala AR,Hotez P,Diemert D,Loukas A,Bethony J, Molecular mechanisms of hookworm disease: stealth, virulence, and vaccines. The Journal of allergy and clinical immunology. 2012 Jul; [PubMed PMID: 22742835]
Level 3 (low-level) evidenceJourdan PM,Lamberton PHL,Fenwick A,Addiss DG, Soil-transmitted helminth infections. Lancet (London, England). 2018 Jan 20; [PubMed PMID: 28882382]
Feldmeier H,Schuster A, Mini review: Hookworm-related cutaneous larva migrans. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2012 Jun [PubMed PMID: 21922198]
Level 3 (low-level) evidenceLoukas A,Prociv P, Immune responses in hookworm infections. Clinical microbiology reviews. 2001 Oct; [PubMed PMID: 11585781]
Level 3 (low-level) evidenceWu X,Chen H,Gan T,Chen J,Ngo CW,Peng Q, Automatic Hookworm Detection in Wireless Capsule Endoscopy Images. IEEE transactions on medical imaging. 2016 Jul [PubMed PMID: 26886971]
Keiser J,Utzinger J, Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008 Apr 23 [PubMed PMID: 18430913]
Level 3 (low-level) evidenceHotez PJ,Beaumier CM,Gillespie PM,Strych U,Hayward T,Bottazzi ME, Advancing a vaccine to prevent hookworm disease and anemia. Vaccine. 2016 Jun 3; [PubMed PMID: 27040400]
Brooker S,Hotez PJ,Bundy DA, Hookworm-related anaemia among pregnant women: a systematic review. PLoS neglected tropical diseases. 2008 Sep 17 [PubMed PMID: 18820740]
Level 3 (low-level) evidenceFriis H,Mwaniki D,Omondi B,Muniu E,Thiong'o F,Ouma J,Magnussen P,Geissler PW,Michaelsen KF, Effects on haemoglobin of multi-micronutrient supplementation and multi-helminth chemotherapy: a randomized, controlled trial in Kenyan school children. European journal of clinical nutrition. 2003 Apr [PubMed PMID: 12700619]
Level 3 (low-level) evidenceSharma V,Gunjan D,Chhabra P,Sharma R,Rana SS,Bhasin DK, Gastrointestinal bleeding in the tropics: Look for the hookworm. Tropical doctor. 2017 Jan; [PubMed PMID: 27075012]
Speich B,Moser W,Ali SM,Ame SM,Albonico M,Hattendorf J,Keiser J, Efficacy and reinfection with soil-transmitted helminths 18-weeks post-treatment with albendazole-ivermectin, albendazole-mebendazole, albendazole-oxantel pamoate and mebendazole. Parasites & vectors. 2016 Mar 2 [PubMed PMID: 26935065]
Bieri FA,Gray DJ,Williams GM,Raso G,Li YS,Yuan L,He Y,Li RS,Guo FY,Li SM,McManus DP, Health-education package to prevent worm infections in Chinese schoolchildren. The New England journal of medicine. 2013 Apr 25; [PubMed PMID: 23614586]
Level 1 (high-level) evidence