Introduction
Foville syndrome is a rare inferior medial pontine syndrome first characterized in 1858 by anatomist and psychiatrist Achille Louis Francois Foville. In his paper “Notes on a Little-known Paralysis of Eye Muscles and Its Relation to the Anatomy and Physiology of the Pons,” Foville posed a question: Does the analysis of paralytic symptoms provide a basis for the exact localization of cerebral disease? He included a description of a 43-year-old man who presented with unilateral conjugate horizontal gaze paralysis and facial nerve paralysis on the same side with crossed hemiparesis. Though several clinical variants have emerged, classic Foville syndrome presents with ipsilateral 6th nerve palsy, facial palsy, and contralateral hemiparesis. Reports exist of other features such as facial hypoesthesia, peripheral deafness, Horner syndrome, ataxia, pain, and thermal hypoesthesia.[1][2][3]
Pontine Anatomy
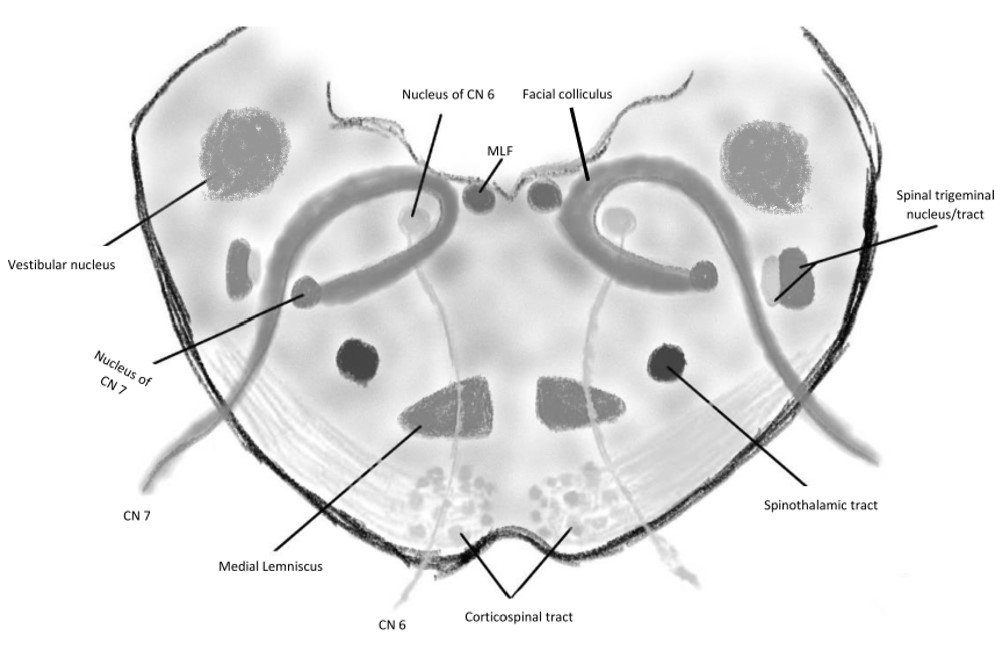
The pons is a large tissue segment projecting superior to the medulla (see Image. Hindbrain, Superficial Dissection). Thick transverse fibers comprise the pons proper. The cerebral peduncles attach to the pons superoanteriorly, while the pyramids arise from the pons' inferior aspect. The paired abducens nerves (cranial nerve VI, CN VI, or 6th cranial nerve) emerge from the inferior pontine sulcus near the pyramids.
The transverse pontine fibers fuse laterally to form the middle cerebellar peduncles connecting the pons to the posteriorly located cerebellum. The cerebellopontine angle is the triangular space between the middle cerebellar peduncle's inferior edge, cerebellum, and superior medullary margin. The facial (CN VII or 7th cranial nerve) and vestibulocochlear (CN VIII or 8th cranial nerve) nerves arise from the cerebellopontine angle. The trigeminal nerves (CN V or 5th cranial nerve) pierce the middle cerebellar peduncles on the pons' superolateral side.
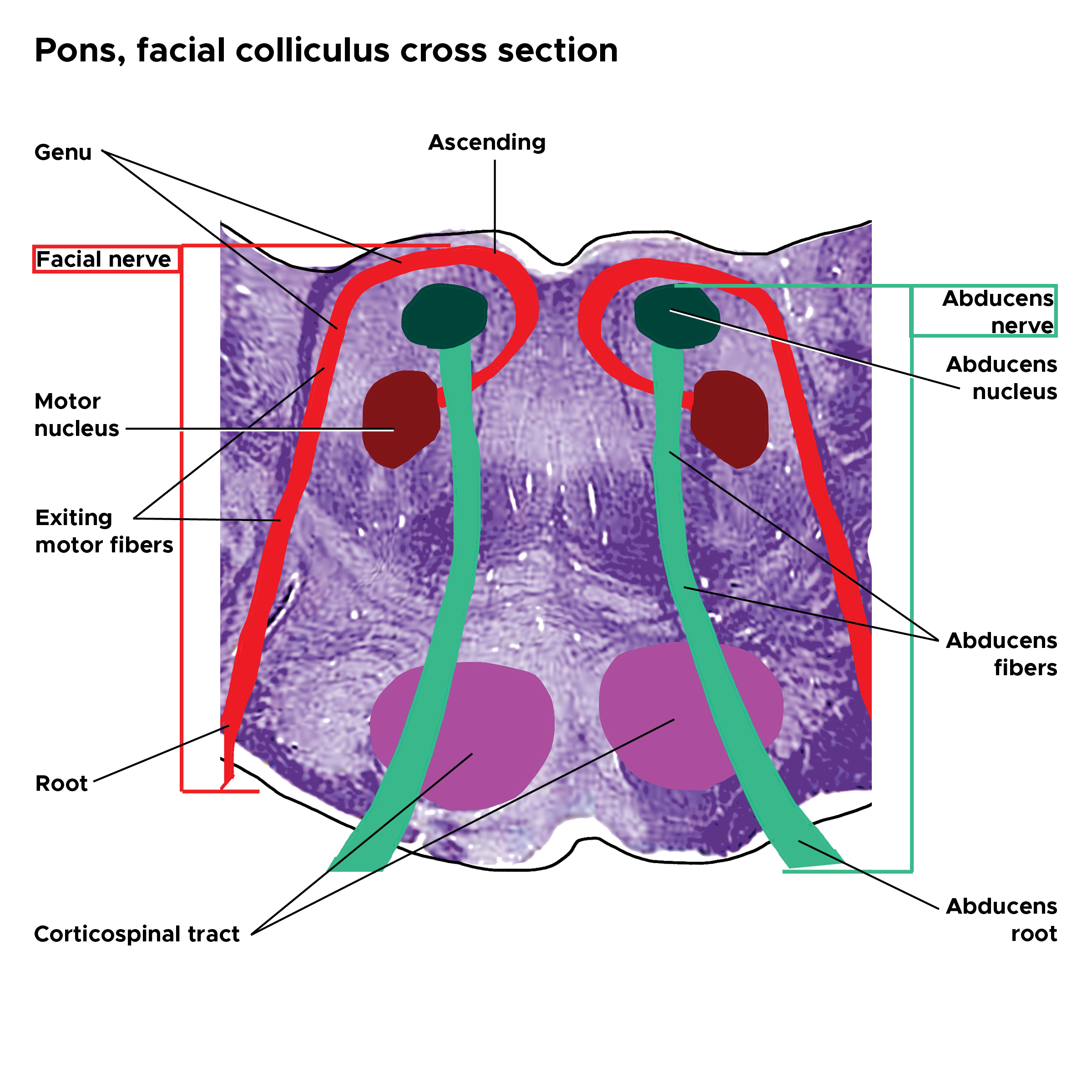
The posterior pontine margin serves as the superior surface of the fourth ventricle's floor. The pontomedullary junction and lateral recesses of the 4th ventricle lie in the widest area of this triangular region. The facial colliculus arises from the CN VI nucleus and CN VII fibers and lies superior to the lateral recesses. Completing the fourth ventricle boundaries are the superior cerebellar peduncles (walls), cerebellar vermis (roof), and anterior medullary velum (roof).
Histologically, the pons is structurally divided into the basilar portion (anterior) and tegmentum (posterior). The pons may also be divided functionally, with the inferior and superior pontine sections carrying different cranial nerve nuclei.
In the inferior (caudal) pons, the corticospinal tracts (CST) emerge at the basilar portion's center. These tracts control voluntary movement. Pontine nuclear fibers (transverse fibers) cross from one side to the other, giving rise to the contralateral middle cerebellar peduncle. The pontine fibers receive signals from the ipsilateral cerebral cortex, subsequently relayed to the cerebellum through the middle cerebellar peduncles.
The medial lemniscus passes from the medulla through the pontine tegmentum. This nerve tract transmits proprioceptive and tactile sensations from the body to the brain. The medial longitudinal fasciculus (MLF) crosses near the midline in the fourth ventricle's floor. The MLF consists of ascending fibers arising from the vestibular nuclei and projecting to the cranial nerve nuclei controlling the extraocular muscles.
The trapezoid body lies in the tegmentum's anterior aspect. Decussating fibers from this area are involved in audition. The superior olive is a small nucleus situated posterolaterally to the trapezoid body. This structure is also involved in auditory function.
The central tegmental tract is a pontine nerve collection that contributes to the ascending reticular formation, which sends thalamic and hypothalamic projections critical to wakefulness and attention. The paramedian pontine reticular formation (PPRF) also arises from the pontine tegmentum. The PPRF processes visual signals and sends projections to the abducens and oculomotor (CN III or 3rd cranial nerve) nerves, which control the eyes' horizontal movements. Ascending taste fibers also lie within the central tegmental tract and travel to the thalamus.
The facial nerve's motor nucleus lies medial to the trigeminal nerve's spinal tract and posterior to the superior olive. The internal genu is an internal loop formed by the facial nerve tracts, passing by the abducens nerve at this level (see Image. Pons Facial Colliculus Cross-Section). The abducens nerve arises from the abducens nucleus, passes through the pontine tegmentum, transits lateral to the pyramidal tract, and exits the brainstem to innervate the lateral rectus muscle. The vestibular nuclei are situated lateral to the fourth ventricle floor.
In the superior pons, the corticopontine fibers descend from the cerebral lobes to the pontine nuclei. These nerves are involved in controlling voluntary movement. The motor (medial) and principal sensory (lateral) trigeminal nerve nuclei are also found in this region. The mesencephalic trigeminal nerve tract passes in this region, carrying stretch receptor signals from the mastication muscles.
The basilar artery is the pons' main arterial source. This artery forms from the union of the vertebral arteries inferiorly and ascends through the ventral median sulcus (basal sulcus) to the superior pontine margin. Smaller paramedian perforating branches of the basilar artery supply each side of the paramedian caudal pons. The blood vessel bifurcates into the posterior cerebral arteries superior to the pons.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Foville syndrome's clinical presentation is primarily the result of pontine infarction. However, other etiologies, such as hemorrhage, granulomas, and pontine tumors, have also been reported.
Isolated pontine infarcts comprise 3% of all ischemic strokes and are usually caused by atheromatous branch occlusive disease. Atheromatous plaques in the basilar artery can encroach into this vessel's smaller paramedian pontine branches through its perforator ostia, leading to occlusion and infarction.[4][5] Other possible causes of infarction include small vessel disease and cardioembolism.[6]
Paramedian pontine infarction manifests as dysfunction of the nerves in this area. From dorsal to ventral, these structures include the abducens nucleus, intrapontine facial nerve fibers, and CST (see Image. Foville Syndrome Anatomy). Other structures that may be involved in severe cases include the MLF, PPRF, spinal trigeminal nucleus, medial lemniscus, vestibuloocular nucleus, middle cerebellar peduncle afferent fibers, and descending sympathetic fibers.
Epidemiology
Foville syndrome is a brainstem stroke syndrome with few reported cases worldwide. Classic Foville syndrome may be confused with Raymond-Cestan syndrome, sometimes called "superior Foville syndrome." Classic Foville syndrome is sometimes called "inferior Foville syndrome" in the literature.
Pathophysiology
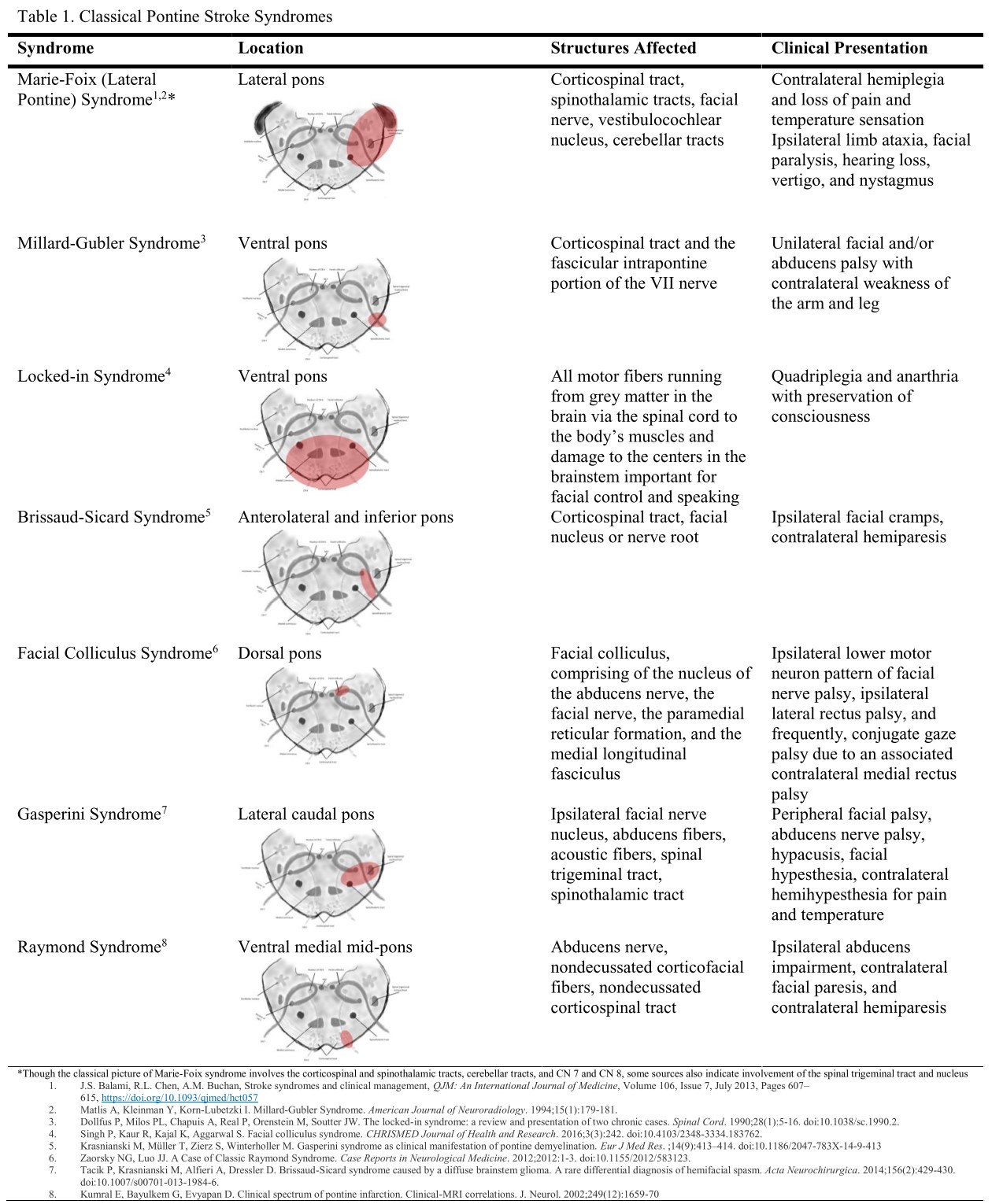
Damage to different parts of the pons produces different manifestations. However, the constellation of symptoms indicates the pontine injury location. Foville syndrome is a stroke syndrome arising from the inferior dorsomedial pontine area. (see Image. Pontine Stroke Syndromes).
As mentioned, the pons' blood supply arises from the vertebrobasilar system's branches. Paramedial caudal pontine ischemia is the primary cause of Foville syndrome. The classic presentation has the following symptoms arising from injury to different parts of the pons:
- Horizontal gaze palsy due to ipsilateral CN VI nucleus injury
- Facial weakness or paralysis due to CN VII nerve damage in the ipsilateral pontine region
- Hemiparesis or hemiplegia contralateral to the side of pontine injury due to CST damage above the inferior medullary decussation
While Foville syndrome has these primary manifestations, additional deficits may accompany the syndrome if the neurologic injury is extensive. For example, internuclear ophthalmoplegia due to MLF damage often accompanies CN VI palsy, causing ipsilateral adduction impairment and contralateral abduction nystagmus. A comprehensive understanding of pontine anatomy and this condition's pathophysiology is crucial for thorough evaluation and tailored management.
History and Physical
Foville syndrome's clinical presentation is consistent with most brainstem strokes, which present as cranial nerve paresis with crossed motor or sensory signs affecting the extremities. Different clinical variants of this condition exist due to its diverse etiologies presenting with different severity levels. The classic syndrome involves the inferior medial pons, damage to which can produce dysfunction of CNs VI and VII and the CST. Larger lesions may also involve other pontine structures like CNs V and VIII, the MLF, and the PPRF. Ataxia and Horner syndrome may also arise due to damage to the afferent white matter fibers to the middle cerebellar peduncle and sympathetic fibers, respectively.
Foville syndrome is strongly associated with atherosclerosis and cardiovascular disorders. Patients may report a history of hypertension, hyperlipidemia, chronic smoking, and diabetes mellitus. A history of atrial fibrillation and anticoagulant therapy indicate a possible embolic or hemorrhagic etiology.
Patients warrant a complete physical examination with detailed cardiovascular and neurologic assessment. Vital signs measurement may reveal high blood pressure and cardiac rate or rhythm abnormalities. Funduscopy may reveal vascular disease signs like cotton-wool spots, papilledema, and flame-shaped hemorrhages. Arrhythmias or murmurs may be appreciated on auscultation in patients with chronic heart disease.
Neurologic examination may reveal the following cranial nerve findings:
- Abducens nerve palsy: Characterized by the inability to abduct the ipsilateral eye. CN VI nucleus or PPRF involvement presents with conjugate gaze palsy or inability to move the eyes toward the affected side. Conjugate gaze palsy is an important sign that can localize the lesion to more than one area in the neuroaxis. This condition impairs the eyes' ability to cross the midline with vestibuloocular reflex testing due to nuclear involvement.
- Facial nerve palsy: Presents with ipsilateral upper and lower facial weakness, signifying lower motor neuron involvement. The lesion is located in CN VII's internal genu that winds around the abducens nucleus at the inferomedial pontine aspect.
- Vestibulocochlear nuclear palsy: Presents with ipsilateral sensorineural hearing loss. This symptom indicates greater lateral involvement.
- Trigeminal nuclear palsy: Characterized by ipsilateral loss of facial crude touch, pain, and temperature sensations. The neurologic injury can extend to the spinal tract of the trigeminal nucleus, which runs caudally from the pons.
On the other hand, the following long-tract signs may be elicited:
- CST damage: Presents with contralateral hemiplegia, as these fibers cross at the inferior medulla and not at the inferior pons.
- Medial lemniscus injury: Characterized by contralateral hemisensory loss (fine touch, vibration, and proprioception), as these fibers are ascending and decussating at this point.
- Descending sympathetic nerve damage: May present as Horner syndrome, with ptosis, miosis, and anhidrosis ipsilateral to the lesion.
Middle cerebellar peduncular afferent fiber dysfunction may manifest with ipsilateral appendicular ataxia. Corticopontocerebellar pathways are involved in movement coordination and planning.
Evaluation
The workup includes imaging, physiologic, and laboratory studies. Brain computed tomography (CT) is a good initial imaging test for patients presenting with stroke symptoms. Stroke is a time-sensitive condition, and CT images may be quickly obtained. CT should reveal the lesion type and location. CT can differentiate between an ischemic and a hemorrhagic stroke and enable suitability assessment for thrombolytic therapy. The lesion should be located in the inferomedial pons.
A brain magnetic resonance imaging (MRI) helps evaluate for other brainstem structural etiologies such as tumors and inflammatory lesions owing to better visualization of the soft tissues. An MRI or CT angiography should be performed if a vascular lesion is suspected. Atherosclerosis can involve the basilar artery or its branches.[6][7]
Cardiac evaluation is indicated to rule out any sources of cardioembolism, especially if the brain vascular evaluation is unremarkable. Recommendations include assessment and screening for cardiovascular risk factors per the American Heart Association (AHA) guidelines. An electrocardiogram can show atrial fibrillation or other arrhythmias predisposing to embolic disease. Cardiac enzymes may be obtained for new-onset arrhythmias, as acute coronary syndrome may occur simultaneously with cerebrovascular accidents. Coagulation studies may reveal abnormally high prothrombin, partial thromboplastin, or bleeding time in patients with hemorrhagic diathesis. Fasting lipid profile and blood glucose or HbA1c levels may reveal metabolic conditions predisposing to arterial disease. Doppler ultrasound of the lower limbs may show peripheral arterial disease.
Treatment / Management
As discussed previously, Foville syndrome has various etiologies, though the most common is arterial vascular disease. The treatment approach is similar to other acute strokes, depending on the presentation time. Thrombolytic therapy is generally indicated for patients presenting within 4.5 hours from symptom onset or the last known normal time. Endovascular treatments usually are not recommended for small-vessel ischemic disease.
Long-term lacunar-stroke treatment mainstays include antiplatelet therapy and controlling vascular risk factors for secondary prevention. Antiplatelet and statin therapy should commence on admission. Smoking cessation and hypertension, diabetes, and hyperlipidemia management must be initiated as per the AHA's stroke guidelines.[8] Patients in postacute stroke rehabilitation typically undergo evaluation by physical and occupational therapists and rehabilitation physicians. Postdischarge follow-up and complications require attention in the outpatient setting.
Advances in acute stroke treatment, intravenous thrombolytic therapy, and endovascular capabilities have improved stroke outcomes. However, stroke largely remains a chronic condition with a limited evidence base for recovery measures. Stroke rehabilitation provides a conduit after acute hospitalization to address residual functional deficits that most stroke survivors develop. AHA guidelines suggest rehabilitation programs with different care and intervention levels, comorbidity management, and disability assessments. Outpatient stroke prevention clinics provide an opportunity to address care transitions and provide patient-centered care for stroke survivors.[9](B2)
Differential Diagnosis
Etiology-wise, the differential diagnosis of Foville syndrome includes conditions that can damage the inferomedial pontine area, such as neoplastic processes, hemorrhages, inflammatory disorders, and vascular anomalies like arteriovenous malformations.[10] In terms of symptomatology, conditions that may present with both cranial nerve and sensorimotor deficits include Guillain-Barré Syndrome, neurotoxin exposure, multiple sclerosis, Wernicke encephalopathy, malingering, and other pontine stroke syndromes like Brissaud-Sicard syndrome.
Foville syndrome diagnosis requires a comprehensive evaluation, including detailed history-taking, neurological examination, imaging studies, and laboratory investigations, to identify the underlying cause and guide appropriate management. Collaboration with neurologists and other specialists may be necessary for optimal patient care and outcomes.
Prognosis
The evidence for long-term outcomes has been a research topic in a few prospective studies. Zhao et al's study involving 281 patients with pontine infarcts revealed that most patients who have had a stroke involving the paramedian arteries had a good prognosis, defined as a modified Rankin score of less than 2.[11] Factors that may impact patient recovery include severity, etiology (hemorrhagic strokes generally have a worse prognosis than ischemic lesions), time to intervention, treatment response, and adherence to poststroke treatment and rehabilitation regimens.
Complications
Medical complications reported after stroke include recurrent strokes, falls, deep vein thrombosis, pulmonary embolism, chest and urinary tract infections, pain, pressure sores, and depression or anxiety.[12] Preventing and managing these complications are essential components of stroke rehabilitation and long-term care.
Deterrence and Patient Education
Primary preventive measures for Foville syndrome include maintenance of healthy habits, adherence to hypertension and diabetes treatments, smoking cessation, alcohol intake limitation, stroke education, and regular health checkups. Patient health monitoring allows for timely interventions for conditions like hypertension, malignancies, atrial fibrillation, and other conditions predisposing to Foville syndrome. Secondary preventive measures include antiplatelet and statin therapy, continued smoking abstinence and lifestyle modifications, and management of hypertension, diabetes, and hyperlipidemia.
Pearls and Other Issues
Foville syndrome typically presents with a triad of ipsilateral facial palsy, ipsilateral horizontal gaze palsy, and contralateral hemiparesis. This constellation of symptoms arises from lesions affecting the inferomedial pontine region. The most commonly involved structures are the abducens nucleus, facial nerve, and CST. The MLF, PPRF, spinal trigeminal tract, and other nearby structures may be involved in severe cases. The most common cause of Foville syndrome is ischemic stroke involving the paramedian pontine branches of the basilar artery. Other etiologies include brainstem tumors, hemorrhage, demyelinating diseases, and vascular malformations affecting the pontine region.
Neuroimaging studies are crucial for confirming the diagnosis of Foville syndrome. CT is quicker to obtain than MRI and can differentiate between an ischemic and a hemorrhagic stroke. CT is thus a good initial imaging test for patients being considered for immediate thrombolytic therapy. MRI can help search for other etiologies, such as pontine neoplasms and vascular anomalies. Other tests may be conducted if CT or MRI alone cannot establish the diagnosis.
Management of Foville syndrome involves treatment of the underlying cause, supportive care, and rehabilitation to optimize functional recovery. In cases of ischemic stroke, thrombolytic therapy or mechanical thrombectomy may be indicated within the appropriate time window. Prompt recognition and management of Foville syndrome and its underlying cause are essential for minimizing neurological deficits and improving outcomes for affected individuals.
Enhancing Healthcare Team Outcomes
Stroke is the 5th leading cause of death and the leading cause of severe long-term disability in the US. The incidence of stroke continues to rise, with 7 million stroke survivors currently in the US, driven by an aging population. The combined costs of stroke treatment and care are expected to double to $240.7 billion annually by 2030. Improvements in primary and secondary stroke prevention are necessary to reduce this condition's current and projected economic burden. Stroke survivors have physical and cognitive impairments and complex medical and social needs unmet by the current fragmented outpatient stroke care system. An interprofessional team dedicated to caring for patients who have had a stroke can help improve outcomes significantly.
Emergency physicians are often the first to diagnose and provide initial treatment to patients presenting with stroke symptoms in the hospital setting. Neurologists diagnose stroke and provide definitive care to patients who develop the condition. Intensivists may be involved in treating patients who require intensive care due to severe disease. Surgical specialists such as neurosurgeons and cardiovascular surgeons may lend their expertise if surgical interventions are warranted, eg, surgical mass resection and thrombectomy. Cardiologists may comanage cardiovascular conditions like hypertension, dysrhythmias, and acute coronary syndrome.
After hospital discharge, primary care providers should monitor patients' progress in the outpatient setting and educate them on preventing stroke recurrence. Pharmacists are tasked with medication reconciliation and dosage verification. Nursing staff can assess for progress and any adverse medication effects on follow-up. Physical, speech, and occupational therapists may prescribe personalized rehabilitation programs to help patients regain functional independence.
By working together collaboratively, the interprofessional team can provide comprehensive care that addresses the medical, physical, functional, emotional, and social aspects of Foville syndrome. These efforts ultimately optimize patient outcomes and quality of life.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
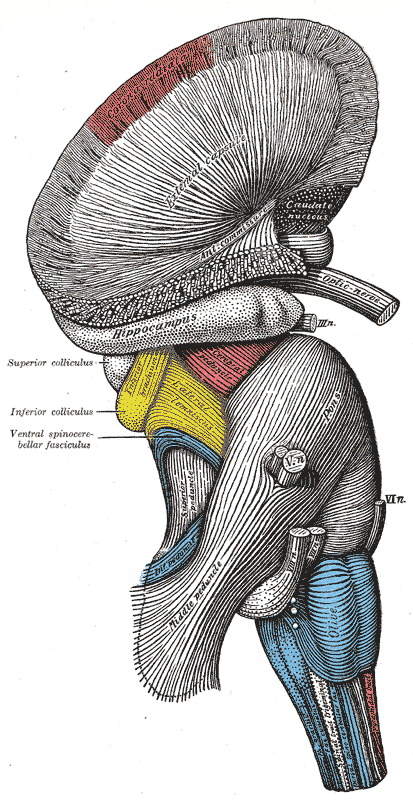
Hindbrain, Superficial Dissection. This lateral-view illustration shows the relationships between the hindbrain (rhombencephalon) structures. The structures included are the corona radiata, external capsule, anterior commissure, hippocampus, cerebral peduncle, superior and inferior colliculus, brachium of the inferior colliculus, lateral lemniscus, ventral spinocerebellar fasciculus, pons, superior, middle, and inferior peduncles, optic, oculomotor, trigeminal, abducens, facial, and vestibulocochlear nerves, olive, and medulla oblongata.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Canepa Raggio C, Dasgupta A. Three cases of Spontaneous Vertebral Artery Dissection (SVAD), resulting in two cases of Wallenberg syndrome and one case of Foville syndrome in young, healthy men. BMJ case reports. 2014 Apr 28:2014():. doi: 10.1136/bcr-2014-203945. Epub 2014 Apr 28 [PubMed PMID: 24777086]
Level 3 (low-level) evidenceMassi DG, Nyassinde J, Ndiaye MM. Superior Foville syndrome due to pontine hemorrhage: a case report. The Pan African medical journal. 2016:25():215. doi: 10.11604/pamj.2016.25.215.10648. Epub 2016 Dec 6 [PubMed PMID: 28292169]
Level 3 (low-level) evidenceBrogna C, Fiengo L, Türe U. Achille Louis Foville's atlas of brain anatomy and the Defoville syndrome. Neurosurgery. 2012 May:70(5):1265-73; discussion 1273. doi: 10.1227/NEU.0b013e31824008e7. Epub [PubMed PMID: 22072133]
Baran G, Gultekin TO, Baran O, Deniz C, Katar S, Yildiz GB, Asil T. Association between etiology and lesion site in ischemic brainstem infarcts: a retrospective observational study. Neuropsychiatric disease and treatment. 2018:14():757-766. doi: 10.2147/NDT.S154224. Epub 2018 Mar 13 [PubMed PMID: 29559783]
Level 2 (mid-level) evidenceKlein IF, Lavallée PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010 Jul:41(7):1405-9. doi: 10.1161/STROKEAHA.110.583534. Epub 2010 Jun 10 [PubMed PMID: 20538696]
Huang R, Zhang X, Chen W, Lin J, Chai Z, Yi X. Stroke Subtypes and Topographic Locations Associated with Neurological Deterioration in Acute Isolated Pontine Infarction. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2016 Jan:25(1):206-13. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.019. Epub 2015 Oct 21 [PubMed PMID: 26508683]
Evans MR, Weeks RA. Putting pontine anatomy into clinical practice: the 16 syndrome. Practical neurology. 2016 Dec:16(6):484-487. doi: 10.1136/practneurol-2016-001367. Epub 2016 Jun 27 [PubMed PMID: 27354565]
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Mar:49(3):e46-e110. doi: 10.1161/STR.0000000000000158. Epub 2018 Jan 24 [PubMed PMID: 29367334]
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016 Jun:47(6):e98-e169. doi: 10.1161/STR.0000000000000098. Epub 2016 May 4 [PubMed PMID: 27145936]
Level 2 (mid-level) evidenceBeucler N, Boissonneau S, Ruf A, Fuentes S, Carron R, Dufour H. Crossed brainstem syndrome revealing bleeding brainstem cavernous malformation: an illustrative case. BMC neurology. 2021 May 20:21(1):204. doi: 10.1186/s12883-021-02223-7. Epub 2021 May 20 [PubMed PMID: 34016062]
Level 3 (low-level) evidenceZhao FL, Mi DH, Zhang CQ, Song QH, Liu HS, Dai HL, Liu ZM, Ge CQ, Wang YJ, Liu LP, Guo L. A cohort study of isolated brainstem infarction based on head MR imaging and clinical findings. The Journal of international medical research. 2018 Dec:46(12):4974-4984. doi: 10.1177/0300060518788253. Epub 2018 Sep 23 [PubMed PMID: 30246581]
Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, Dick F, Taylor GS, Murray G. Medical complications after stroke: a multicenter study. Stroke. 2000 Jun:31(6):1223-9 [PubMed PMID: 10835436]
Level 2 (mid-level) evidence