 Anatomy, Abdomen and Pelvis, Ovary Corpus Luteum
Anatomy, Abdomen and Pelvis, Ovary Corpus Luteum
Introduction
The corpus luteum is a vital yet temporary organ that plays a crucial role in fertility during the luteal phase. It is an endocrine structure in females existing within the ovary once the ovarian follicle has released a mature ovum during ovulation. See Image. Anatomy of the Internal Structures of the Ovary. The secretion of hormones from the corpus luteum will stop within 14 days after ovulation if the oocyte is not fertilized. It then degenerates into a scar within the ovary, known as a corpus albicans. The role of the corpus luteum is the maintenance of a uterine environment that allows for implementation and pregnancy. This occurs by the release of pregnancy-related hormones and regulation of the hypothalamic-pituitary access through inhibition of gonadotropin-releasing hormone from the hypothalamus, which in turn decreases the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) released from the anterior pituitary. The primary hormone produced by the corpus luteum is progesterone, but it also produces inhibin A and estradiol. In the absence of fertilization, the corpus luteum will regress over time. A corpus luteum develops each time a woman ovulates so that a woman will produce a corpus luteum numerous times throughout her lifetime.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The structure of the corpus luteum consists of parenchymal and nonparenchymal cells. Due to the vast diversity of cell types of the corpus luteum, gap junctions play an important role in intercellular communication allowing for the coordination of the function of these different cell types.[1] The corpus luteum is made up of follicular theca cells and follicular granulosa cells, and before becoming the corpus luteum, the follicle forms a corpus hemorhagicum. Since the corpus luteum is transient, its regulation is via interactions between stimulatory (luteotrophic) and inhibitory (luteolytic) mediators. Little is known about this topic, but studies show that prolactin is an important luteotrophic hormone.[2][3]
The corpus luteum has two fates depending on if there is a fertilized egg or not. If fertilization and implantation occur, by day nine, the syncytiotrophoblast cells of the blastocyst secrete human chorionic gonadotropin (HCG), the same hormone tested for the ascertainment of pregnancy. HCG is vital in the continuation of progesterone secretion from the corpus luteum. Progesterone's presence is critical in maintaining the lining of the endometrium, which is necessary for the implantation and growth of the embryo. The corpus luteum is then known as the corpus luteum graviditatis. The corpus luteum will not have this job for the remainder of the pregnancy. Instead, the placenta will assume the role of maintaining the pregnancy through progesterone secretion, and the corpus luteum will degenerate around week 12. The alternate fate of the corpus luteum occurs if the egg does not undergo fertilization. It will stop secreting progesterone and will decay and turn into a corpus albicans. Without progesterone maintaining the endometrium, females will shed the lining resulting in menstruation.
Embryology
Oogenesis occurs in the female embryo before birth but does not complete until puberty. Therefore, an embryo will not form a corpus luteum. Once a female begins ovulating and menstruating, a corpus luteum will form in the ovary once the secondary oocyte is released from the follicle during monthly ovulation.
Blood Supply and Lymphatics
The blood supply of the mature corpus luteum is the highest per-unit tissue of any organ in the body, which is why adequate blood supply, achieved through the recruitment of blood vessels, is an essential part of corpus luteum development. This critical process involves the breakdown of the follicular basement membrane, endothelial cell proliferation and migration, and capillary lumina development. Angiogenic growth factors are vital to this process.[1][4] The ovarian artery provides branches that supply the cortex and medulla. These branches are responsible for supplying the corpus luteum within the ovary.[5]
Nerves
The suspensory ligament of the ovary carries the sympathetic and parasympathetic nerves of the pelvic plexus that innervate the ovaries.
Muscles
Although the corpus luteum has no muscle attachments, it affects an essential muscular pelvic organ, the uterus. As discussed previously, the corpus luteum plays an important role in producing hormones responsible for the decidualization of the endometrium. The hormones released from the corpus luteum cause the ovary to enter a luteal phase and the uterus to enter a secretory phase. During this time, the uterus is preparing for implantation and growth of the fertilized egg.[6]
Physiologic Variants
It is crucial that the uterus can respond to hormones released from the corpus luteum. In some individuals, a luteal-phase dysfunction can induce a premature regression of the corpus luteum. This state then disrupts the ovulatory cycle. Steroidogenesis of the corpus luteum relies on the availability of cholesterol, achieved by the transference of cholesterol molecules to the site of steroid production. A dysfunction in this rate-limiting step can decrease the amount of steroid made, limiting what can be released. Steroidogenic acute regulatory protein is an integral part of this process that plays an important role in progesterone concentrations during the early and mid-luteal phases.[7] A luteal phase defect is a common cause of infertility in women. Etiologies that lead to delayed endometrial maturation associated with luteal phase dysfunction include defective corpus luteum function, disordered folliculogenesis, and abnormal luteal rescue. Weight loss, Hyperprolactinemia, stress, hyperandrogenism, and athletic training may contribute to dysfunction.[8]
Surgical Considerations
It is possible, although rare, to develop a corpus luteum cyst or hematoma. These cysts are characterized by intense endocrine activity and often produce excess progesterone. They can take up to three months to disappear, but they usually regress. They can enlarge and rupture, causing hemoperitoneum.[9] Patients on blood thinners may be more likely to develop a life-threatening bleed from a ruptured corpus luteum.[10]
Clinical Significance
The corpus luteum is very important clinically. What is termed luteal support involves the administration of progestins to encourage the uterine lining to support an implanted fertilized egg. These progestins complement the corpus luteum. A luteal phase defect results from the inability of the lining of the uterus to respond to hormones produced by the corpus luteum, which is a common cause of infertility. Another clinically significant topic concerning the corpus luteum is the use of oral contraceptives. Combined oral contraceptive pills contain two hormones, estrogen, and progesterone, which suppress FSH and LH, thus inhibiting ovulation. Additionally, this suppression will cause degeneration of the corpus luteum resulting in a drop in progesterone levels, which inhibits normal implantation of the fertilized ova and placental attachment. In addition to the importance of progesterone in maintaining pregnancy, the corpus luteum also releases relaxin, which softens the pubic symphysis for parturition. Another clinically significant role of the corpus luteum is how exogenous hormones may manipulate it. The belief is that the supraphysiologic levels of steroids secreted during the luteal phase in patients undergoing IVF cause a corpus luteum dysfunction; this is because of the inhibition of LH release secondary to the secretion of these hormones that act via negative feedback actions at the hypothalamic-pituitary axis level, thus suppressing stimulation of progesterone. Exogenous progesterone or HCG can provide essential luteal support in patients undergoing IVF.[7]
Other Issues
It is essential to consider radiologic findings of the corpus luteum because they may be interpreted as a pathologic findings. Differential diagnoses to consider include endometrioma, ectopic pregnancy, tubo-ovarian abscess, degeneration of a fibroid, and ovarian neoplasia. The normal radiological findings of the corpus luteum should be a thick-walled cysts crenulated inner margin and internal echoes with a “ring of fire” peripheral vascularity.[11]
Media
(Click Image to Enlarge)
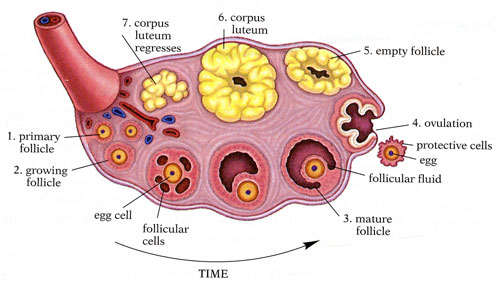
Anatomy of the Internal Structures of the Ovary. The internal structures of the ovary include the primary follicle, growing follicle, egg cell, follicular cells, mature follicle, follicular fluid, egg, ovulation, empty follicle, corpus luteum, and regressing corpus luteum.
Kimanh Nguyen, Public Domain, via Wikimedia Commons
References
Reynolds LP, Redmer DA. Growth and development of the corpus luteum. Journal of reproduction and fertility. Supplement. 1999:54():181-91 [PubMed PMID: 10692854]
Level 3 (low-level) evidenceBachelot A, Binart N. Corpus luteum development: lessons from genetic models in mice. Current topics in developmental biology. 2005:68():49-84 [PubMed PMID: 16124996]
Level 3 (low-level) evidenceMurphy BD, Rajkumar K. Prolactin as a luteotrophin. Canadian journal of physiology and pharmacology. 1985 Mar:63(3):257-64 [PubMed PMID: 2985224]
Level 3 (low-level) evidenceSmith MF,McIntush EW,Smith GW, Mechanisms associated with corpus luteum development. Journal of animal science. 1994 Jul; [PubMed PMID: 7928766]
Level 3 (low-level) evidenceHossain MI, O'Shea JD. The vascular anatomy of the ovary and the relative contribution of the ovarian and uterine arteries to the blood supply of the ovary in the guinea-pig. Journal of anatomy. 1983 Oct:137 (Pt 3)(Pt 3):457-66 [PubMed PMID: 6654738]
Level 3 (low-level) evidenceWalker HK, Hall WD, Hurst JW, Hatcher RA, Kowal D. Birth Control. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250126]
Devoto L, Kohen P, Muñoz A, Strauss JF 3rd. Human corpus luteum physiology and the luteal-phase dysfunction associated with ovarian stimulation. Reproductive biomedicine online. 2009:18 Suppl 2():19-24 [PubMed PMID: 19406027]
Ginsburg KA, Luteal phase defect. Etiology, diagnosis, and management. Endocrinology and metabolism clinics of North America. 1992 Mar; [PubMed PMID: 1576984]
Lee MS, Moon MH, Woo H, Sung CK, Jeon HW, Lee TS. Ruptured Corpus Luteal Cyst: Prediction of Clinical Outcomes with CT. Korean journal of radiology. 2017 Jul-Aug:18(4):607-614. doi: 10.3348/kjr.2017.18.4.607. Epub 2017 May 19 [PubMed PMID: 28670155]
Level 2 (mid-level) evidenceBarbuscia M, De Luca M, Ilaqua A, Cingari E, Lemma G, Querci A, Lentini M, Gorgone S. [Etiopathogenetic and clinical considerations of corpus luteum cysts]. Il Giornale di chirurgia. 2010 Mar:31(3):103-7 [PubMed PMID: 20426922]
Level 3 (low-level) evidenceBonde AA, Korngold EK, Foster BR, Fung AW, Sohaey R, Pettersson DR, Guimaraes AR, Coakley FV. Radiological appearances of corpus luteum cysts and their imaging mimics. Abdominal radiology (New York). 2016 Nov:41(11):2270-2282 [PubMed PMID: 27472937]
