Introduction
Clostridioides difficile, formerly Clostridium difficile, is a gram-positive and spore-forming bacterium. This obligate anaerobic bacillus is recognized for its ability to produce toxins and is the leading cause of antibiotic-associated diarrhea worldwide. C difficile infections can range from an asymptomatic carrier to diarrhea, progressing to severe conditions such as pseudomembranous colitis and toxic megacolon with septic shock, often resulting in a high mortality rate. Although traditionally considered a primary healthcare-associated infection, an increasing incidence of community-acquired C difficile infections also exists.[1] The emergence of a newer hypervirulent strain, the North American pulsed-field gel electrophoresis type 1 (NAP1), has been attributed to increased incidence and severity of C difficile infections over the last 2 decades.[2][3]
C difficile can colonize the intestines of healthy people without causing infection and survive on surfaces in the hospital and soil for extended periods. Contaminated surfaces and medical equipment in healthcare facilities can become reservoirs for C difficile spores, potentially transmitting to patients if proper cleaning protocols are not routinely implemented.[4] The most significant risk factor for C difficile infection is the use of antibiotics, particularly broad-spectrum antibiotics.[5][6] Patients can be colonized with C difficile without symptoms, but antibiotic use disturbs the balance of gut flora, enabling C difficile overgrowth and infection. Additional risk factors for C difficile infection include proton pump inhibitors, advanced age, immunosuppression, underlying health conditions, prior C difficile infection, recent hospitalization, prolonged hospital stays, inadequate infection control, and suboptimal antibiotic stewardship in healthcare settings, facilitating C difficile transmission among patients.[7][8]
While C difficile infection is primarily associated with healthcare settings, a growing recognition of community-acquired cases exists, particularly among younger and healthier individuals with minimal recent healthcare exposure. The emergence of whole genome sequencing has revealed that reservoirs for C difficile exist within healthcare systems, environment, food, soil, and animals, thereby comprising a "One Health" continuum.[9]
Managing C difficile infections remains challenging due to its increasing global burden, emergence in community settings, complexities in handling severe and recurrent cases, and rising morbidity and mortality rates. Understanding the epidemiology of C difficile infection is crucial for implementing effective prevention and control measures in both healthcare settings and the community. These strategies include prudent antibiotic use, hand hygiene practices, environmental cleaning, and early detection and management of cases. [4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
C difficile is a gram-positive, anaerobic, spore-forming, and toxin-producing bacterium, which is also a significant cause of antibiotic-associated colitis worldwide (see Image. Micrograph of Clostridioides difficile Showing Colitis). C difficile infection ranks among the most prevalent healthcare-associated infections, easily transmitted between patients and originating from both healthcare and community reservoirs. Clinical symptoms vary from asymptomatic carriage to mild diarrhea to severe conditions such as pseudomembranous colitis and toxic megacolon with septic shock. The potential of C difficile to cause healthcare-associated outbreaks, along with high morbidity and recurrent infections, poses treatment challenges, associated costs, and, in severe cases, mortality.[10]
The most significant risk factor for C difficile infection is antibiotic use, particularly broad-spectrum antibiotics. Various antibiotic classes, including penicillins, cephalosporins, fluoroquinolones, and clindamycin, have been associated with the development of the disease.[5][6] Advancements in our comprehension of the gut microbiome's role contribute to a deeper understanding of the disease's pathophysiology.
Patients may carry C difficile without symptoms, and antibiotic use can disturb the balance of the gut microbiome, leading to dysbiosis and enabling C difficile proliferation and infection.[11] Other risk factors for developing C difficile infection include proton pump inhibitors, advanced age, immunosuppression, underlying comorbidities, previous C difficile infection, kidney and hepatic failure, recent hospitalization, and prolonged hospital stays.[7][8] Incomplete infection control and antibiotic stewardship practices in healthcare institutions allow C difficile transmission among patients.
C difficile produces 2 types of toxins—toxins A and B—which are virulent factors in its pathogenicity. Most pathogenic strains associated with C difficile infection produce both toxins A and B. However, globally, there are reports of strains producing only toxin B.[12] In 2005, due to the increasing incidence and virulence of C difficile–associated disease, a newer strain was identified that contained a binary toxin (referred to as binary toxin CDT) in addition to toxins A and B. Deletions in the gene tcd, which serves as a negative regulator of toxins A and B, have been identified, potentially leading to overproduction of these virulence factors. The emergence of the newer, hypervirulent, antibiotic-resistant, epidemic strain—ribotype 027, commonly known as NAP1/B1/027 or the North American pulsed-field gel electrophoresis type 1 strain, restriction endonuclease analysis type B1, or polymerase chain reaction ribotype 027—is characterized by increased production of toxins A and B, as well as the production of a binary toxin CDT, and fluoroquinolone resistance.[11]
Epidemiology
C difficile is the primary causative agent of healthcare-associated post-antibiotic colitis, posing a significant public health challenge due to its transmissibility and associated morbidity and mortality.[13] C difficile is ubiquitous and is capable of colonizing the intestines of up to 3% to 5% of healthy individuals without causing any infections.[14] Although transmission of C difficile primarily occurs fecal-orally, it can also stem from various other environmental sources, such as soil. However, the bacterium is more commonly transmitted via contaminated surfaces in hospitals, often in the form of spores. Contaminated surfaces and medical equipment in healthcare facilities can become reservoirs for C difficile spores, potentially transmitting to patients if proper infection prevention and control practices, including appropriate cleaning protocols, are not enforced.[4] The healthcare environment presents a conducive setting for C difficile transmission, owing to the challenge of eradicating spores and the prevalent use of antimicrobials, which fosters the development of C difficile infections.
C difficile infection has primarily been associated with healthcare settings. However, recent reports indicate a surge in C difficile infections in the community among individuals with no healthcare system affiliation or exposure to antibiotics.[15][16] Consequently, concerted efforts have been directed toward reducing the incidence of C difficile to enhance patient safety. This involves enhancing detection, surveillance, and infection prevention and control practices to mitigate the estimated burden of C difficile. Antibiotic misuse significantly heightens the risk of C difficile infections, with over half of hospitalized patients receiving antibiotics during their stay and 30% to 50% of prescribed antibiotics being unnecessary or incorrect.[17]
The last 2 decades have been crucial in enhancing the detection, surveillance, and estimation of the burden of C difficile infections globally, including in the United States and Europe. Countries have made concerted efforts to implement diagnostic, surveillance, and infection prevention and control protocols and tools, particularly in hospital settings. However, considering differences in practices and disparities in financial and staff resources across different regions is essential, which may influence the accuracy of estimated data concerning the burden of C difficile infections.[18]
Reviewing data from the early 2000s, a notable increase was seen in the incidence of C difficile infections and related hospitalizations, attributed to the emergence of the epidemic strain C difficile NAP1/B1/027.[19] In a 2007 study by Hall et al, a C difficile infection emerged as the leading cause of gastroenteritis, correlating with a 5-fold increase in mortality.[20] In addition, Dubberke et al estimated that in acute-care facilities in the United States, C difficile infections may have been responsible for $4.8 billion in excess costs.[21]
Furthermore, data from a 2011 article published by the Centers for Disease Control and Prevention (CDC) and authored by Lessa et al demonstrated a rise in C difficile incidence across 10 geographic areas in the United States compared to the previous decade, with a higher occurrence observed among females and individuals aged 65 and older.[22] This increase may have been associated with the adoption of newer, more sensitive C difficile assays, such as nucleic acid amplification tests (NAATs).[22] The data indicated that approximately half a million Americans are infected by C difficile infections annually. Among those infected, about 29,000 patients experienced fatal outcomes within a month of diagnosis, and 15,000 of these deaths were directly linked to C difficile infection. Moreover, approximately 83,000 patients experienced at least a recurrence of C difficile infection, and 29,000 of them succumbed within 30 days of the initial diagnosis.[22]
However, more recent data from the United States [1][23][19] and Europe [24] show a decreasing prevalence and trend of C difficile infections within healthcare systems, particularly notable in the prevalence of NAP1/B1/027 strain. This decrease may be attributed to a multifaceted approach aimed at reducing unnecessary antimicrobial usage, implementing antibiotic stewardship practices, and enhancing infection control procedures. Nevertheless, heterogeneity in testing, surveillance, infection prevention, and control practices prevails among hospitals globally and between countries.[25]
Pathophysiology
The human gastrointestinal tract harbors a diverse array of bacteria, collectively forming a microbial community known as the microbiome, comprising approximately 100 trillion microorganisms.[26] This microbiome is vital for maintaining homeostasis and serves various functions, such as food fermentation, acting as a barrier against pathogens, synthesizing vitamins, and regulating metabolism and immunity.[26] Reductions in both the diversity and abundance of these bacteria, along with perturbations in the composition of the microbiome, can disrupt interactions with the host and the immune system. In the case of C difficile, such disruptions can facilitate its colonization of the human gut, even in healthy individuals. The link between antibiotics and the onset of C difficile infections stems from the dysbiosis within the gut microbiome ecosystem induced by antibiotic use.[27]
The most significant risk factor for developing C difficile infection is the use of antibiotics, particularly broad-spectrum antibiotics. Several classes of antibiotics, including penicillins, cephalosporins, fluoroquinolones, and clindamycin, are associated with the onset of the disease, although any antibiotic use can potentially lead to C difficile infections.[5][6] Our understanding of the role of the gut microbiome facilitates a better understanding of the pathophysiology of the disease.
C difficile is ubiquitous and can colonize the intestines of up to 3% to 5% of healthy individuals without causing infections.[14] Even healthy adults with adequate immune responses can become asymptomatic carriers of the C difficile. Neonates, lacking intestinal receptors for C difficile, exhibit a high asymptomatic carrier rate.[28] Antibiotics alter the microbial balance in the large intestine, increasing susceptibility to C difficile infection, which is transmitted through the fecal-oral route. Diarrhea and pseudomembranous colitis, characteristic symptoms of C difficile infection, result from clostridial glycosylation exotoxins—toxin A (TcdA), an enterotoxin, and toxin B (TcdB), which is cytotoxic.[29] Toxin A has a carbohydrate receptor binding site that facilitates the intracellular transport of toxins A and B.[30] After becoming intracellular, both toxins cause the inactivation of pathways mediated by the Rho family of proteins, resulting in damage to colonocytes, disruption of intercellular tight junctions, and colitis.[31] Furthermore, toxin A directly activates neutrophils, while both toxins A and B contribute to neutrophil chemotaxis.[32]
A newer strain was identified in 2005, featuring a binary toxin (CDT) alongside toxins A and B. Additionally, deletions in the gene tcd, acting as a negative regulator of toxins A and B, were identified, potentially leading to an overproduction of these virulence factors. This new epidemic strain—ribotype 027, commonly known as NAP1/B1/027 or North American pulsed-field gel electrophoresis type 1, restriction endonuclease analysis type B1, or polymerase chain reaction ribotype 027—is characterized by increased production of toxins A and B, as well as binary toxin CDT, and demonstrates resistance to fluoroquinolones.[11]
History and Physical
Clinical presentations of C difficile infections vary from being asymptomatic carriers to symptomatic patients experiencing mild diarrhea to severe manifestations such as fever, abdominal pain, pseudomembranous colitis, toxic megacolon, and septic shock. The severity of clinical signs and symptoms varies depending on factors such as the patient's overall health status and the specific strain of C difficile involved. Common symptoms associated with diarrhea and colitis caused by C difficile include watery diarrhea with mucus or occult blood, anorexia, nausea, vomiting, low-grade fever, and lower abdominal pain.[33]
In some cases, patients may progress to fulminant colitis, characterized by diarrhea, diffuse abdominal pain, abdominal distension, hypovolemia, toxic megacolon, and perforated bowel with peritonitis.[34] Other manifestations of the disease include protein-losing enteropathy and extra-colonic symptoms such as appendicitis and reactive arthritis.[35][36] Recurrent infections caused by C difficile are defined by the complete resolution of symptoms after treatment, followed by a recurrence of symptoms.[37] Risk factors for recurrence include recent hospitalization, antibiotic use, or close contact with individuals who have experienced diarrhea.
Evaluation
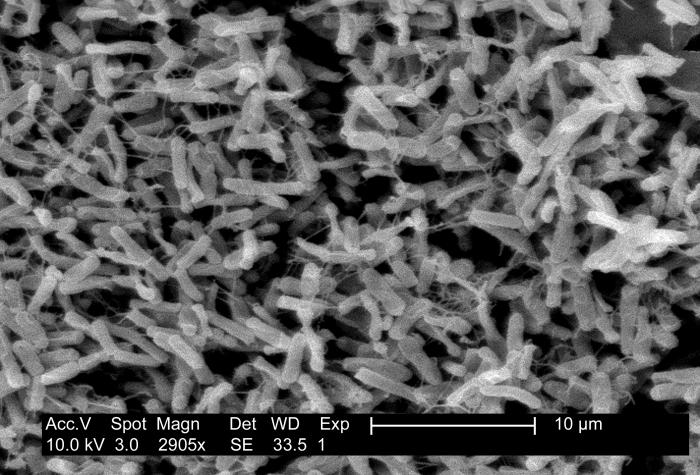
C difficile should be considered in any patient with 3 or more loose stools within 24 hours, particularly those with a recent history of hospitalization and antibiotic use within the last 3 months. Additionally, diarrhea persisting for 48 hours or longer after hospital admission warrants consideration. Evaluating for community-acquired cases is important, even in patients without a history of such exposures (see Image. Clostridioides difficile Pathology and Diagnosis).
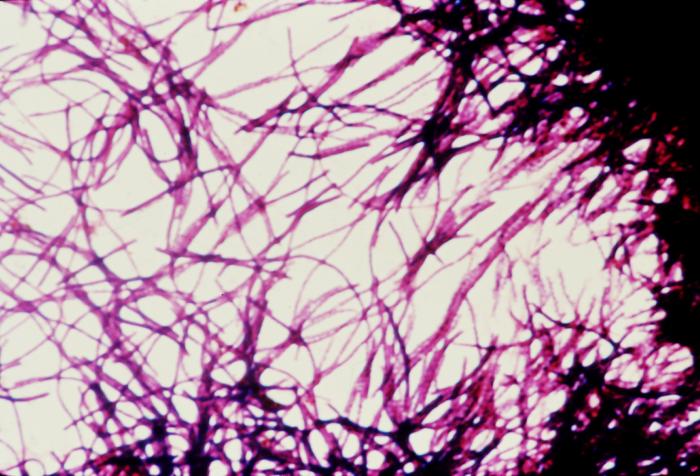
Diagnostic tests for C difficile infections include an enzyme immunoassay for glutamate dehydrogenase tests, toxigenic cultures, cell culture cytotoxicity neutralization tests, toxins A and B enzyme immunoassays (EIAs), and NAATs. A singular test lacks sufficient informativeness, thus necessitating a 2-step, sequential testing approach.[38] In the diagnostic process, the primary focus is on detecting the presence of C difficile antigen, achieved through either GDH testing for glutamate dehydrogenase produced by C difficile or NAATs. Negative results from these tests typically conclude the testing process. However, if positive, they do not indicate toxin presence. Therefore, EIAs should be used subsequently to detect and confirm toxin production (see Image. Impression Smear of Clostridioides difficile).
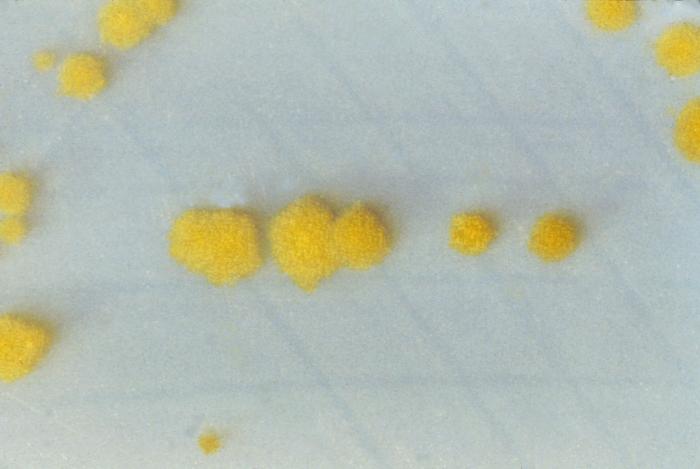
Most guidelines recommend a 2-step method for diagnosing C difficile infections, although using a NAAT alone or in conjunction with GDH in a 2-step process is also feasible. While selective anaerobic culture is an option for diagnosis, it is time-consuming (see Image. Clostridioides difficile Cultures on Cycloserine Mannitol Agar).[39][40][38] Studies have demonstrated that PCR-based diagnostic approaches, whether single or multistep, offer the highest sensitivity and specificity for diagnosing C difficile.[33] In certain scenarios, additional diagnostic modalities, such as radiographic imaging of the abdomen and pelvis or lower gastrointestinal endoscopy, may be warranted, particularly when patients present with fulminant colitis or alternative diagnoses are under consideration.[40]
Treatment / Management
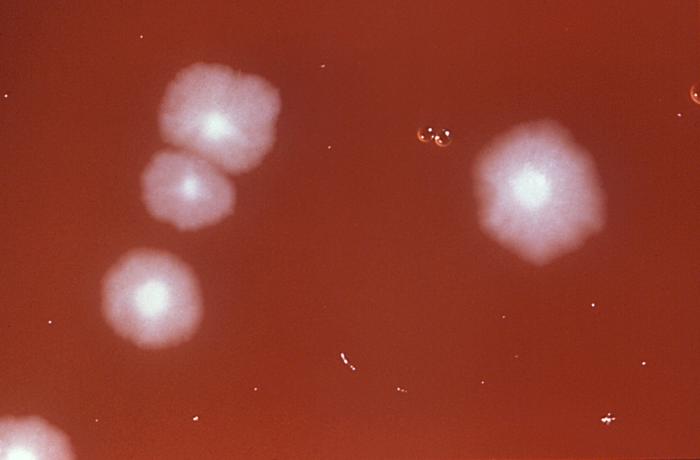
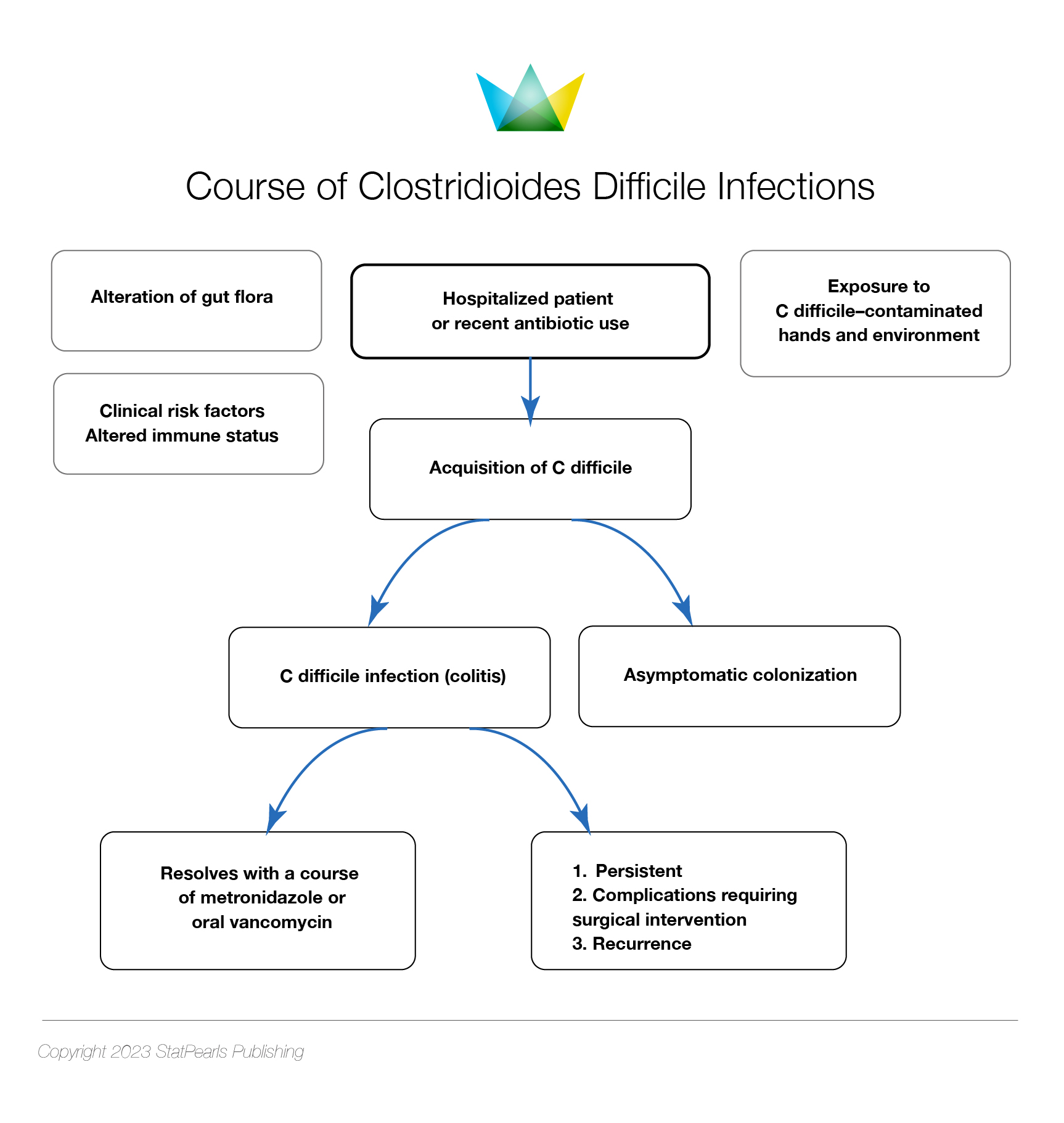
Managing C difficile infections involves a multistep approach, including implementing infection prevention and control protocols for suspected or confirmed cases, making a definite diagnosis, discontinuing unnecessary antibiotics if possible, and administering appropriate medication following evidence-based guidelines. Testing for C difficile infection is unnecessary in asymptomatic patients who do not have diarrhea, and asymptomatic individuals with positive stool toxin tests do not require treatment (see Image. Course of Clostridioides difficile Infections).
Response to treatment is defined as the resolution of diarrhea or the presence of formed stool for at least 48 hours after completing treatment. Refractory C difficile infections occur when there is no response to treatment after a specified duration. Recurrence refers to a new episode within 8 weeks following the complete resolution of a previous C difficile infection.[41]
Severe C difficile infection is characterized by a white blood cell count exceeding 15,000 cells/μL, serum albumin below 3 g/dL, and serum creatinine elevated to more than 1.5 times the premorbid level.
Multiple recommendations and guidelines from different societies provide therapeutic guidance for C difficile infections, with minor variations. The fundamental approach to treatment is generally consistent.[42][41][43] Previously, 3 antibiotics—oral vancomycin, oral or intravenous metronidazole, and oral fidaxomicin—were commonly used to treat C difficile infections.
- Vancomycin and fidaxomicin are now considered standard-of-care (SoC) antibiotics for C difficile infections, with specific treatment recommendations tailored to first episodes, recurrences, or severe infections.
- Metronidazole is no longer recommended as first-line therapy for C difficile infections and should only be considered in cases where vancomycin or fidaxomicin are unavailable. Alternatively, intravenous metronidazole may be necessary if ileus is present, as oral vancomycin inadequately secretes into the colon.
Bezlotoxumab, a recently approved human monoclonal antibody targeting C difficile toxin, has shown promising results in managing recurrent infections. Clinical studies have demonstrated its efficacy and revealed positive outcomes.[44] Fecal transplantation, involving the introduction of fecal matter from a healthy donor into the patient's gastrointestinal tract, has reported an 80% to 90% success rate in reducing C difficile recurrence.[45]
Initial Treatment for C difficile Infections
- If fidaxomicin is available, it should be administered 200 mg PO bid for 10 days instead of vancomycin to treat patients initially.
- If fidaxomicin is unavailable, oral vancomycin should be given at 125 mg every 6 hours for 10 days.[41][46] (A1)
- If neither fidaxomicin nor vancomycin are available, metronidazole should be administered orally.[41][46] (A1)
- The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommends fidaxomicin for subsequent episodes if it was not administered during the initial episode. If fidaxomicin was previously used, it can be administered again with bezlotoxumab. Bezlotuxumab should be incorporated into oral SoC treatment for C difficile infections with an elevated risk of recurrence when fidaxomicin is inaccessible.[41]
Recommendations and Options for Recurrent C difficile Infections
- For patients with recurrent C difficile infections, fidaxomicin is the preferred treatment option, either in a standard or extended-pulsed regimen, if available, as recommended by ESCMID.[41][46] (A1)
- In cases where fidaxomicin is unavailable, vancomycin serves as an alternative treatment for recurrent C difficile infections. This drug can be administered in a tapered and pulsed regimen or a standard course, with doses of oral vancomycin 125 mg every 6 hours for 10 to 14 days, followed by 125 mg every 12 hours for 7 days, then once daily for 7 days, and finally once every 2 to 3 days for 2 to 8 weeks. Alternatively, fidaxomicin 200 mg every 12 hours for 10 days can be considered if vancomycin was used for the initial episode.[46] (A1)
- According to the Infectious Diseases Society of America (IDSA), if recurrence occurs within 6 months of the initial infection, bezlotoxumab should be administered as a co-intervention with SoC antibiotics (fidaxomicin or vancomycin).[46] (A1)
- ESCMID recommends using bezlotoxumab along with SoC for the initial, recurrent episode of C difficile infections.[41]
Second Subsequent Recurrence of C difficile Infections
Treatment options for a second or subsequent recurrence of C difficile infections include a taper and pulsed vancomycin regimen. This involves oral vancomycin 125 mg every 6 hours for 10 to 14 days, followed by rifaximin 400 mg orally 3 times daily for 20 days, fidaxomicin 200 mg twice daily for 10 days, or fecal transplantation.[41][46](A1)
Initial Severe Infections
For an initial fulminant episode with hypotension, shock, ileus, or megacolon, the treatment options include:[41]
- Oral fidaxomicin 200 mg PO bid or oral vancomycin 500 mg every 6 hours orally or via a nasogastric tube if ileus is present. In addition, rectal vancomycin enema (500 mg in 100 ml normal saline per rectum every 6 hours) can be administered.
- Additionally, adjunctive therapy with intravenous metronidazole 500 mg every 8 hours is recommended.
- Surgical intervention, such as subtotal colectomy with preservation of the rectum, may be necessary for patients with fulminant colitis, particularly those at risk of complications such as toxic megacolon, intestinal perforation, and necrotizing colitis.[47]
Differential Diagnosis
Differential diagnoses for C difficile infections include Crohn disease, diverticulitis, irritable bowel syndrome, malabsorption, peritonitis, Salmonella infections, shigellosis, ulcerative colitis, vibrio infections, and viral gastroenteritis.
Prognosis
C difficile infections cause a significant burden on healthcare systems, leading to prolonged hospital stays, escalated healthcare expenses, elevated morbidity and mortality rates, recurrence of the disease, and increased disability-adjusted life years (DALYs). DALYs, a composite health metric, estimate the years lived with disabilities (YLDs) following disease onset and years of life lost due to premature mortality (YLLs) compared to a standardized life expectancy.[1][22][48][49][50][51]
Complications
C difficile infection continues to be a significant cause of morbidity and mortality among hospitalized patients, with severe cases potentially resulting in toxic megacolon, colonic perforation, and sepsis. The infection is associated with heightened morbidity, mortality, prolonged hospitalization, and increased recurrence rates. In cases of fulminant colitis, surgical intervention such as subtotal colectomy with rectal preservation may be necessary, posing risks of complications such as toxic megacolon, intestinal perforation, and necrotizing colitis.[1][22][48][49][50][51]
Consultations
When patients are suspected or confirmed to have C difficile infection, consultation with various medical specialists may be necessary, particularly in cases of recurrence or severe illness. Primary care and emergency physicians often serve as the initial point of contact for these patients. Maintaining a high index of suspicion is crucial for considering C difficile in the differential diagnosis of diarrhea, especially in individuals with recent healthcare facility exposure, antibiotic use, or a history of C difficile infection.
Accurate stool testing is essential for diagnosing C difficile infection, and prompt treatment in accordance with guidelines is imperative. Infectious disease specialists may be consulted to aid in diagnosis and management, facilitating communication with the microbiology laboratory, particularly in cases of recurrent or severe infections. Additionally, clinical pharmacists may offer valuable guidance in treating C difficile infections.
In cases of severe diarrhea with systemic symptoms such as dehydration, septic shock, acute colitis, or unresponsive toxic megacolon despite medical therapy, consultation with gastroenterologists, intensive care physicians, and surgeons may be necessary. Such patients may require admission to the intensive care unit, and, in severe instances, emergency colectomy may be warranted. These specialists work collaboratively to provide comprehensive care for patients with C difficile diarrhea, addressing various aspects of the infection, its treatment, and associated complications.
Deterrence and Patient Education
Patients should receive comprehensive education regarding the course and treatment of C difficile infection, potential recurrence, the judicious use of antibiotics, and preventive measures to contain its spread within households. Adhering to the prescribed treatment duration and abstaining from other antibiotics during C difficile treatment are crucial. Patients must understand that symptoms should gradually resolve and prompt reporting to their clinician is warranted if symptoms persist, worsen, or reappear, as this may indicate recurrence or exacerbation of the original episode.
Additionally, patients should be instructed to wash their hands thoroughly with soap and water, as hand antiseptics may not effectively eliminate C difficile spores. This practice is especially vital after using the bathroom to mitigate the transmission of C difficile within their household.
Pearls and Other Issues
Prevention of C difficile infections
Effective prevention of C difficile infections includes various generalized and specific targeted strategies. Generalized approaches involve early disease detection, isolating patients with dedicated facilities and using contact precautions, and promoting hygiene practices such as thorough handwashing with soap and water. Additionally, comprehensive environmental cleaning, including terminal room cleaning, is crucial. These infection control measures should be applied in both healthcare and community settings. Moreover, prudent antibiotic use and the adoption of antibiotic stewardship programs play a pivotal role in preventing the onset and dissemination of C difficile infections within healthcare systems.[52]
Ongoing Research
Current research efforts are exploring newer antibiotics, microbiome restoration therapies, and vaccine development for the treatment and prevention of C difficile infections. Ridinilazole, granted Fast Track Status and designated as a Qualified Infectious Disease Product by the U.S. Food and Drug Administration (FDA), holds promise as a potential treatment option.[53] Ribaxamase, an oral beta-lactamase, is under investigation for its ability to preserve gut bacteria and prevent C difficile infections and antibiotic-associated diarrhea. Furthermore, microbiome-based therapies are being studied as an alternative approach to preventing antibiotic-associated diarrhea.[54] Pharmaceutical companies are also actively developing a vaccine to prevent C difficile infections.[55]
Enhancing Healthcare Team Outcomes
Treating C difficile infections necessitates collaboration among all members of the interprofessional healthcare team, including infectious disease physicians, clinical pharmacists, microbiologists, gastroenterologists, intensivists, and surgeons. This collaborative approach ensures efficient, comprehensive, and coordinated care, thereby enhancing outcomes for patients with C difficile infections.
Hand hygiene should be conducted using soap and water rather than alcohol-based products when caring for patients with C difficile infection due to the superior efficacy of soap and water in spore removal. To prevent C difficile infections, adhere to infection control recommendations and practice judicious antibiotic use. In addition, it is advisable to maintain contact precautions for a minimum of 2 days after diarrhea resolution and to continue such precautions until discharge if infection rates persist despite standard infection control measures.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, Wilson LE, Holzbauer SM, Phipps EC, Dumyati GK, Beldavs ZG, Kainer MA, Karlsson M, Gerding DN, McDonald LC, Emerging Infections Program Clostridioides difficile Infection Working Group. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. The New England journal of medicine. 2020 Apr 2:382(14):1320-1330. doi: 10.1056/NEJMoa1910215. Epub [PubMed PMID: 32242357]
See I, Mu Y, Cohen J, Beldavs ZG, Winston LG, Dumyati G, Holzbauer S, Dunn J, Farley MM, Lyons C, Johnston H, Phipps E, Perlmutter R, Anderson L, Gerding DN, Lessa FC. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 May:58(10):1394-400. doi: 10.1093/cid/ciu125. Epub 2014 Mar 5 [PubMed PMID: 24604900]
McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. The New England journal of medicine. 2005 Dec 8:353(23):2433-41 [PubMed PMID: 16322603]
Carling PC, Parry MF, Olmstead R. Environmental approaches to controlling Clostridioides difficile infection in healthcare settings. Antimicrobial resistance and infection control. 2023 Sep 7:12(1):94. doi: 10.1186/s13756-023-01295-z. Epub 2023 Sep 7 [PubMed PMID: 37679758]
Gorbach SL. John G. Bartlett: Contributions to the discovery of Clostridium difficile antibiotic-associated diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Sep 15:59 Suppl 2():S66-70. doi: 10.1093/cid/ciu419. Epub [PubMed PMID: 25151480]
Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. The New England journal of medicine. 1978 Mar 9:298(10):531-4 [PubMed PMID: 625309]
Khanafer N, Vanhems P, Barbut F, Luxemburger C, CDI01 Study group, Demont C, Hulin M, Dauwalder O, Vandenesch F, Teams of:, Argaud L, Badet L, Barth X, Bertrand M Dr, Burillon C, Chapurlat R, Chuzeville M, Comte B, Disant F, Fessy MH, Gouillat C, Juillard L, Lermusiaux P, Monneuse O, Morelon E, Ninet J, Ponchon T, Poulet E, Rimmele T, Tazarourte K. Factors associated with Clostridium difficile infection: A nested case-control study in a three year prospective cohort. Anaerobe. 2017 Apr:44():117-123. doi: 10.1016/j.anaerobe.2017.03.003. Epub 2017 Mar 6 [PubMed PMID: 28279859]
Level 2 (mid-level) evidenceEeuwijk J, Ferreira G, Yarzabal JP, Robert-Du Ry van Beest Holle M. A Systematic Literature Review on Risk Factors for and Timing of Clostridioides difficile Infection in the United States. Infectious diseases and therapy. 2024 Feb:13(2):273-298. doi: 10.1007/s40121-024-00919-0. Epub 2024 Feb 13 [PubMed PMID: 38349594]
Level 1 (high-level) evidenceLim SC, Knight DR, Riley TV. Clostridium difficile and One Health. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020 Jul:26(7):857-863. doi: 10.1016/j.cmi.2019.10.023. Epub 2019 Nov 1 [PubMed PMID: 31682985]
Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Feb 1:34(3):346-53 [PubMed PMID: 11774082]
Kelly CR, Allegretti JR. Review Article: Gastroenterology and Clostridium difficile Infection: Past, Present, and Future. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2023 Dec 5:77(Suppl 6):S463-S470. doi: 10.1093/cid/ciad644. Epub [PubMed PMID: 38051967]
Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010 Oct 7:467(7316):711-3. doi: 10.1038/nature09397. Epub 2010 Sep 15 [PubMed PMID: 20844489]
Level 3 (low-level) evidenceGhose C. Clostridium difficile infection in the twenty-first century. Emerging microbes & infections. 2013 Sep:2(9):e62. doi: 10.1038/emi.2013.62. Epub 2013 Sep 18 [PubMed PMID: 26038491]
Bouza E, Muñoz P, Alonso R. Clinical manifestations, treatment and control of infections caused by Clostridium difficile. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2005 Jul:11 Suppl 4():57-64 [PubMed PMID: 15997485]
Lessa FC, Winston LG, McDonald LC, Emerging Infections Program C. difficile Surveillance Team. Burden of Clostridium difficile infection in the United States. The New England journal of medicine. 2015 Jun 11:372(24):2369-70. doi: 10.1056/NEJMc1505190. Epub [PubMed PMID: 26061850]
Khanna S, Baddour LM, Huskins WC, Kammer PP, Faubion WA, Zinsmeister AR, Harmsen WS, Pardi DS. The epidemiology of Clostridium difficile infection in children: a population-based study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 May:56(10):1401-6. doi: 10.1093/cid/cit075. Epub 2013 Feb 13 [PubMed PMID: 23408679]
Level 2 (mid-level) evidenceDavey P, Brown E, Fenelon L, Finch R, Gould I, Holmes A, Ramsay C, Taylor E, Wiffen P, Wilcox M. Systematic review of antimicrobial drug prescribing in hospitals. Emerging infectious diseases. 2006 Feb:12(2):211-6 [PubMed PMID: 16494744]
Level 1 (high-level) evidencevan Dorp SM, Notermans DW, Alblas J, Gastmeier P, Mentula S, Nagy E, Spigaglia P, Ivanova K, Fitzpatrick F, Barbut F, Morris T, Wilcox MH, Kinross P, Suetens C, Kuijper EJ, European Clostridium difficile Infection Surveillance Network (ECDIS-Net) project on behalf of all participants. Survey of diagnostic and typing capacity for Clostridium difficile infection in Europe, 2011 and 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2016 Jul 21:21(29):. doi: 10.2807/1560-7917.ES.2016.21.29.30292. Epub [PubMed PMID: 27469624]
Level 3 (low-level) evidenceGiancola SE, Williams RJ 2nd, Gentry CA. Prevalence of the Clostridium difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2018 Aug:24(8):877-881. doi: 10.1016/j.cmi.2017.11.011. Epub 2017 Nov 22 [PubMed PMID: 29174729]
Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999-2007. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Jul:55(2):216-23. doi: 10.1093/cid/cis386. Epub 2012 Apr 4 [PubMed PMID: 22491338]
Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Aug:55 Suppl 2(Suppl 2):S88-92. doi: 10.1093/cid/cis335. Epub [PubMed PMID: 22752870]
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. Burden of Clostridium difficile infection in the United States. The New England journal of medicine. 2015 Feb 26:372(9):825-34. doi: 10.1056/NEJMoa1408913. Epub [PubMed PMID: 25714160]
Gentry CA, Williams RJ 2nd, Campbell D. Continued decline in the prevalence of the Clostridioides difficile BI/NAP1/027 strain across the United States Veterans Health Administration. Diagnostic microbiology and infectious disease. 2021 Jun:100(2):115308. doi: 10.1016/j.diagmicrobio.2021.115308. Epub 2021 Jan 22 [PubMed PMID: 33626478]
Dingle KE, Didelot X, Quan TP, Eyre DW, Stoesser N, Golubchik T, Harding RM, Wilson DJ, Griffiths D, Vaughan A, Finney JM, Wyllie DH, Oakley SJ, Fawley WN, Freeman J, Morris K, Martin J, Howard P, Gorbach S, Goldstein EJC, Citron DM, Hopkins S, Hope R, Johnson AP, Wilcox MH, Peto TEA, Walker AS, Crook DW, Modernising Medical Microbiology Informatics Group. Effects of control interventions on Clostridium difficile infection in England: an observational study. The Lancet. Infectious diseases. 2017 Apr:17(4):411-421. doi: 10.1016/S1473-3099(16)30514-X. Epub 2017 Jan 25 [PubMed PMID: 28130063]
Level 2 (mid-level) evidenceViprey VF, Granata G, Vendrik KEW, Davis GL, Petrosillo N, Kuijper EJ, Vilken T, Lammens C, Schotsman JJ, Benson AD, Cataldo MA, van der Kooi TII, Wilcox MH, Davies KA, COMBACTE-CDI consortium. European survey on the current surveillance practices, management guidelines, treatment pathways and heterogeneity of testing of Clostridioides difficile, 2018-2019: results from The Combatting Bacterial Resistance in Europe CDI (COMBACTE-CDI). The Journal of hospital infection. 2023 Jan:131():213-220. doi: 10.1016/j.jhin.2022.11.011. Epub 2022 Dec 1 [PubMed PMID: 36462673]
Level 3 (low-level) evidenceHou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Signal transduction and targeted therapy. 2022 Apr 23:7(1):135. doi: 10.1038/s41392-022-00974-4. Epub 2022 Apr 23 [PubMed PMID: 35461318]
Sehgal K, Khanna S. Gut microbiome and Clostridioides difficile infection: a closer look at the microscopic interface. Therapeutic advances in gastroenterology. 2021:14():1756284821994736. doi: 10.1177/1756284821994736. Epub 2021 Feb 23 [PubMed PMID: 33747125]
Level 3 (low-level) evidenceJangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. Journal of pediatric gastroenterology and nutrition. 2010 Jul:51(1):2-7. doi: 10.1097/MPG.0b013e3181d29767. Epub [PubMed PMID: 20512057]
Oka K, Osaki T, Hanawa T, Kurata S, Okazaki M, Manzoku T, Takahashi M, Tanaka M, Taguchi H, Watanabe T, Inamatsu T, Kamiya S. Molecular and microbiological characterization of Clostridium difficile isolates from single, relapse, and reinfection cases. Journal of clinical microbiology. 2012 Mar:50(3):915-21. doi: 10.1128/JCM.05588-11. Epub 2012 Jan 11 [PubMed PMID: 22205786]
Level 3 (low-level) evidenceDove CH, Wang SZ, Price SB, Phelps CJ, Lyerly DM, Wilkins TD, Johnson JL. Molecular characterization of the Clostridium difficile toxin A gene. Infection and immunity. 1990 Feb:58(2):480-8 [PubMed PMID: 2105276]
Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995 Jun 8:375(6531):500-3 [PubMed PMID: 7777059]
Level 3 (low-level) evidenceSouza MH, Melo-Filho AA, Rocha MF, Lyerly DM, Cunha FQ, Lima AA, Ribeiro RA. The involvement of macrophage-derived tumour necrosis factor and lipoxygenase products on the neutrophil recruitment induced by Clostridium difficile toxin B. Immunology. 1997 Jun:91(2):281-8 [PubMed PMID: 9227329]
Level 3 (low-level) evidenceBagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015 Jan 27:313(4):398-408. doi: 10.1001/jama.2014.17103. Epub [PubMed PMID: 25626036]
Level 1 (high-level) evidenceRubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Diseases of the colon and rectum. 1995 Apr:38(4):350-4 [PubMed PMID: 7720439]
Level 2 (mid-level) evidenceRybolt AH, Bennett RG, Laughon BE, Thomas DR, Greenough WB 3rd, Bartlett JG. Protein-losing enteropathy associated with Clostridium difficile infection. Lancet (London, England). 1989 Jun 17:1(8651):1353-5 [PubMed PMID: 2567373]
Mattila E, Arkkila P, Mattila PS, Tarkka E, Tissari P, Anttila VJ. Extraintestinal Clostridium difficile infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Sep:57(6):e148-53. doi: 10.1093/cid/cit392. Epub 2013 Jun 13 [PubMed PMID: 23771984]
Level 2 (mid-level) evidenceFekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997 Mar:24(3):324-33 [PubMed PMID: 9114180]
Level 2 (mid-level) evidenceMcDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Mar 19:66(7):e1-e48. doi: 10.1093/cid/cix1085. Epub [PubMed PMID: 29462280]
Level 1 (high-level) evidenceCohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare Epidemiology of America, Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infection control and hospital epidemiology. 2010 May:31(5):431-55. doi: 10.1086/651706. Epub [PubMed PMID: 20307191]
Level 1 (high-level) evidenceCrobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2016 Aug:22 Suppl 4():S63-81. doi: 10.1016/j.cmi.2016.03.010. Epub 2016 Jul 25 [PubMed PMID: 27460910]
van Prehn J, Reigadas E, Vogelzang EH, Bouza E, Hristea A, Guery B, Krutova M, Norén T, Allerberger F, Coia JE, Goorhuis A, van Rossen TM, Ooijevaar RE, Burns K, Scharvik Olesen BR, Tschudin-Sutter S, Wilcox MH, Vehreschild MJGT, Fitzpatrick F, Kuijper EJ, Guideline Committee of the European Study Group on Clostridioides difficile. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2021 Dec:27 Suppl 2():S1-S21. doi: 10.1016/j.cmi.2021.09.038. Epub 2021 Oct 20 [PubMed PMID: 34678515]
Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, Stollman NH. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. The American journal of gastroenterology. 2021 Jun 1:116(6):1124-1147. doi: 10.14309/ajg.0000000000001278. Epub [PubMed PMID: 34003176]
Bishop EJ, Tiruvoipati R. Management of Clostridioides difficile infection in adults and challenges in clinical practice: review and comparison of current IDSA/SHEA, ESCMID and ASID guidelines. The Journal of antimicrobial chemotherapy. 2022 Dec 23:78(1):21-30. doi: 10.1093/jac/dkac404. Epub [PubMed PMID: 36441203]
Bartlett JG. Bezlotoxumab - A New Agent for Clostridium difficile Infection. The New England journal of medicine. 2017 Jan 26:376(4):381-382. doi: 10.1056/NEJMe1614726. Epub [PubMed PMID: 28121509]
Kassam Z, Lee CH, Hunt RH. Review of the emerging treatment of Clostridium difficile infection with fecal microbiota transplantation and insights into future challenges. Clinics in laboratory medicine. 2014 Dec:34(4):787-98. doi: 10.1016/j.cll.2014.08.007. Epub 2014 Sep 30 [PubMed PMID: 25439277]
Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, Wilcox MH. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Sep 7:73(5):e1029-e1044. doi: 10.1093/cid/ciab549. Epub [PubMed PMID: 34164674]
Level 1 (high-level) evidenceSteele SR, McCormick J, Melton GB, Paquette I, Rivadeneira DE, Stewart D, Buie WD, Rafferty J. Practice parameters for the management of Clostridium difficile infection. Diseases of the colon and rectum. 2015 Jan:58(1):10-24. doi: 10.1097/DCR.0000000000000289. Epub [PubMed PMID: 25489690]
Marra AR, Perencevich EN, Nelson RE, Samore M, Khader K, Chiang HY, Chorazy ML, Herwaldt LA, Diekema DJ, Kuxhausen MF, Blevins A, Ward MA, McDanel JS, Nair R, Balkenende E, Schweizer ML. Incidence and Outcomes Associated With Clostridium difficile Infections: A Systematic Review and Meta-analysis. JAMA network open. 2020 Jan 3:3(1):e1917597. doi: 10.1001/jamanetworkopen.2019.17597. Epub 2020 Jan 3 [PubMed PMID: 31913488]
Level 1 (high-level) evidenceCassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducomble T, Haller S, Harder T, Klingeberg A, Sixtensson M, Velasco E, Weiß B, Kramarz P, Monnet DL, Kretzschmar ME, Suetens C. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS medicine. 2016 Oct:13(10):e1002150. doi: 10.1371/journal.pmed.1002150. Epub 2016 Oct 18 [PubMed PMID: 27755545]
Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. The New England journal of medicine. 2014 Mar 27:370(13):1198-208. doi: 10.1056/NEJMoa1306801. Epub [PubMed PMID: 24670166]
Level 3 (low-level) evidenceCioni G, Viale P, Frasson S, Cipollini F, Menichetti F, Petrosillo N, Brunati S, Spigaglia P, Vismara C, Bielli A, Barbanti F, Landini G, Panigada G, Gussoni G, Bonizzoni E, Gesu GP, Research Department of FADOI. Epidemiology and outcome of Clostridium difficile infections in patients hospitalized in Internal Medicine: findings from the nationwide FADOI-PRACTICE study. BMC infectious diseases. 2016 Nov 8:16(1):656 [PubMed PMID: 27825317]
Kociolek LK, Gerding DN, Carrico R, Carling P, Donskey CJ, Dumyati G, Kuhar DT, Loo VG, Maragakis LL, Pogorzelska-Maziarz M, Sandora TJ, Weber DJ, Yokoe D, Dubberke ER. Strategies to prevent Clostridioides difficile infections in acute-care hospitals: 2022 Update. Infection control and hospital epidemiology. 2023 Apr:44(4):527-549. doi: 10.1017/ice.2023.18. Epub [PubMed PMID: 37042243]
Gonzales-Luna AJ, Carlson TJ, Garey KW. Emerging Options for the Prevention and Management of Clostridioides difficile Infection. Drugs. 2023 Feb:83(2):105-116. doi: 10.1007/s40265-022-01832-x. Epub 2023 Jan 16 [PubMed PMID: 36645620]
Kokai-Kun JF, Le C, Trout K, Cope JL, Ajami NJ, Degar AJ, Connelly S. Ribaxamase, an Orally Administered β-Lactamase, Diminishes Changes to Acquired Antimicrobial Resistance of the Gut Resistome in Patients Treated with Ceftriaxone. Infection and drug resistance. 2020:13():2521-2535. doi: 10.2147/IDR.S260258. Epub 2020 Jul 22 [PubMed PMID: 32801790]
Heuler J, Chandra H, Sun X. Mucosal Vaccination Strategies against Clostridioides difficile Infection. Vaccines. 2023 Apr 23:11(5):. doi: 10.3390/vaccines11050887. Epub 2023 Apr 23 [PubMed PMID: 37242991]