Introduction
Blood donation is paramount in global healthcare, with over 100 million blood units contributed annually. Blood transfusion is crucial for patients undergoing surgery, coping with trauma, managing chronic illnesses, and battling cancer. This essential procedure serves as a lifeline, providing sustenance and saving lives. Furthermore, donating blood serves as therapeutic phlebotomy for individuals with hereditary hemochromatosis, polycythemia vera, and other rare conditions.[1] This activity provides a brief history of the origin of blood donation and testing and discusses donor eligibility and selection, the adverse effects of donation, pathogen reduction and inactivation methods for donated blood, and the significance of blood donation as a primary medical intervention.
In the early 20th century, Karl Landsteiner identified ABO blood groups. During that period, the practice of blood typing for individuals was beginning to be adopted as a universal standard practice.[2] Initially, blood transfusions encountered significant limitations because of the difficulty in clot prevention once the blood was extracted from donors. The transfusion involved directly transferring blood from the donor to the recipient without any intermediate storage or transport. This approach was only effective on a small scale for a limited number of patients, as donors and recipients had to be connected in time and space.
Lists of donors comprised individuals locally available for donation at any time. The imperative need for a more flexible donation and storage system arose with the onset of World War I. The concept of "on-demand" blood donation was not viable for such a large-scale effort. Soldiers were succumbing to otherwise non-fatal wounds primarily because timely blood transfusions could not be performed.
Concerted efforts to develop a method for storing and transferring blood to meet wartime demands led to several discoveries. Initially, clotting was inhibited by adding citrate to the donated blood.[3] Second, it was observed that adding glucose to the solution enabled red blood cells to maintain viability for several weeks when stored under refrigerated conditions.[4][5] Captain Oswald Hope Robertson of the US Army Medical Corps was the first to "bank" blood in a manner similar to today's practices. In November 1917, before the Battle of Cambrai, Captain Robertson gathered group O blood, combined it with glucose, and stored it. This marked a crucial transition from the earlier method of "direct" to "indirect" blood donation, notably separating donors from their recipients, both geographically and temporally. This advancement significantly enhanced the practicality and utility of blood donation and transfusion.[6]
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
The 2 primary uses for blood obtained through donation include transfusions to treat anemia and acute blood loss. Blood donation, referred to as "therapeutic phlebotomy" in contemporary medical terminology or "blood-letting" historically, also treats a small subset of medical conditions. Despite being misused for thousands of years as a supposed cure for various diseases, phlebotomy is now primarily indicated as a therapeutic intervention for the treatment of hereditary hemochromatosis and other disorders related to hemoglobin synthesis or myeloproliferative disorders such as polycythemia and porphyria.[7]
Autologous donation is another indication of blood donation when preparing for surgery. Autologous donation was widely used in the past, from the 1980s to 1990s, due to the increased fear of transfusion-transmitted infections, such as HIV and hepatitis C (HCV).[8][9] Healthcare professionals may direct patients who will likely require an intraoperative or postoperative red blood cell transfusion to donate blood at least 72 hours before the surgery. The patient must maintain an adequate hemoglobin level, with the donated units being exclusively reserved for autologous use. Unfortunately, patients with suspected bacteremia or conditions associated with increased risk are not eligible for autologous donation.
Enhanced safety measures in blood donation, handling, screening, and transfusion practices have significantly minimized the occurrence of transfusion-related HIV and HCV infections. Consequently, preoperative autologous donation has declined in popularity. The most compelling reason to persist with autologous blood donation is for patients with common alloantibodies, where finding a suitable compatible blood supply from the general population poses challenges. Additional potential benefits include preserving the allogeneic blood supply, reducing the risk of alloimmunization, reducing or eliminating the risk of transmitting allogeneic infections, and promoting transfusion acceptance in some instances.
Donating and storing umbilical cord blood provides an excellent source for hematopoeitic stem cell transplantation. Cord blood has a significantly higher percentage of hematopoietic stem cells than non-cord blood. Cord blood is collected during the delivery of a newborn and processed and stored in blood banks specifically designed for cord blood, ensuring its availability for future use.[10]
Contraindications
The contraindications associated with blood donation center around donor ineligibility. These criteria are updated on the American Association of Blood Banks (AABB) recommendation and can be accessed online for the most updated information. For any questions or concerns regarding current blood donation regulations and practices, it is advisable to consult the AABB and the Food and Drug Administration (FDA).
As per the recent AABB guidelines, an individual should meet specific eligibility criteria to donate blood. Potential donors must be at least 16 years or older (although this age may be older in some states), maintain a body weight of at least 110 pounds or 50 kg, and should not have unregulated hypertension, diabetes, or anemia. In addition, the individual should be in good health and feel well during blood donation. A donor with a blood pressure above 180/100 mmHg or below 90/50 mmHg is excluded from blood donation.
Low hemoglobin is the leading cause of donor deferral, resulting in deferral of blood donors in as many as 1 out of 10 attempted blood donations. Although the causes of low hemoglobin may vary, one of the most common causes is insufficient dietary iron intake. In the United States, the minimum allowable hemoglobin level for blood donation is 13.0 g/dL for men and 12.5 g/dL for women.[11] A significant amount of iron is lost each time a person donates blood.
According to the AABB guidelines, eligible donors can donate blood once every 8 weeks or 56 days. However, if donors choose to donate 2 units of blood simultaneously, they must refrain from donating red blood cells again for 16 weeks. The 56-day interval may not provide sufficient time to replenish iron stores lost during blood donation, potentially contributing to low-hemoglobin deferrals in repeat blood donors. Repeat donors may benefit from iron supplementation to rebuild iron stores more quickly. The amount of iron depletion from a single donation varies among individuals and between males and females. The ability to replenish iron stores also differs between individuals. Infrequent donors, especially men and post-menopausal women, who are deferred due to low hemoglobin may have an underlying medical condition that causes anemia. Thus, it is recommended that these individuals should follow up with their primary care provider for evaluation.[12]
Multiple medications can pose a risk of teratogenic effects for potential recipients who become pregnant. Notably, using these medications does not necessarily result in a permanent exclusion from blood donation. Examples of such medications include thalidomide, finasteride, and retinoids.[13][14] Etretinate is teratogenic and is absorbed into adipose tissue, posing a continuous risk of the compound leaking from adipose tissue into the serum. Due to this ongoing threat, individuals who take or have taken this medication are strongly discouraged from being blood donors.[15]
In Japan, individuals who have taken etretinate must refrain from using it for at least 2 years before becoming eligible to donate blood. However, the restriction is permanent in the United States, the United Kingdom, and Quebec.[14] For thalidomide and finasteride, the waiting period is 1 month. Antiplatelet and nonsteroidal anti-inflammatory medications impose restrictions on platelet apheresis donors for a duration ranging from 2 to 30 days, depending on the specific medication. The American Red Cross maintains an updated list of immunizations and medications, including their associated deferral periods.
Historically, men who have sex with men (MSM) have been deferred from donating blood. As of May 2023, MSM are deferred from blood donation if they have had a new sexual partner or more than one sexual partner and engaged in anal sex within the past 3 months. Additional criteria for a 3-month deferral include a history of syphilis or gonorrhea, engaging in sex in exchange for money or drugs, acquiring new tattoos or piercings, being on antiretroviral therapy for preexposure prophylaxis (unless it is the injectable form, in which case it is a 2-year deferral), and exposure to blood through transfusion, needlestick, or intravenous recreational drugs. In addition, individuals with solid organ cancers are deferred from blood donation until they have been symptom-free and considered cured for a period of 1 to 5 years.
According to the AABB, additional contraindications to donating blood are listed below.
- Individuals with a positive test for HIV
- Individuals who have ever received antiretroviral therapy for HIV
- Individuals with a history of hematologic cancer
- Individuals who have had hepatitis since their eleventh birthday
- Individuals who have had babesiosis or Chagas disease
- Individuals who have taken etretinate for psoriasis
- Individuals with risk factors for variant Creutzfeldt-Jakob disease (CJD) or who have a blood relative with the disease
- Individuals with risk factors for CJD, including:
- Those who spent 3 months or more in the United Kingdom from 1980 to 1996
- Those who received a blood transfusion in the United Kingdom or France from 1980 to the present
- Those who spent 5 years in Europe from 1980 to the present
- Those who received a cadaveric dura mater transplant
In addition, the AABB provides a list of current infectious disease testing conducted on donated blood, as provided below.
- Hepatitis B surface antigen (HBsAg)
- Hepatitis B core antibody (anti-HBc)
- HCV antibody (anti-HCV)
- HIV-1 and HIV-2 antibody (anti-HIV-1 and anti-HIV-2)
- Human T-lymphotropic virus (HTLV) types I and II antibodies (anti-HTLV-I and anti-HTLV-II)
- Serologic test for syphilis
- Nucleic acid amplification testing for HIV-1 RNA, HCV RNA, and West Nile virus RNA
- Nucleic acid amplification testing for HBV DNA
- Antibody test for Trypanosoma cruzi—the agent of Chagas disease
Donors with the infections mentioned above are deferred from blood donation. Furthermore, infectious agents may exist at levels that are too low to detect or for which there is no approved test, potentially leading to false-negative screening tests. Moreover, some infectious agents are not tested due to cost, availability, and prevalence. The list of at-risk pathogens tested for in the blood supply continues to expand over time, contributing to increased costs per donation event and restricting the number of eligible donors.
Pathogen reduction or inactivation is a relatively recent advancement in blood donation and the field of blood banking or transfusion. Pathogen reduction involves the proactive elimination of potential pathogens from donated blood. Currently, the most widely used method for minimizing transfusion-associated infections involves testing blood for specific pathogens and subsequently removing the units or deferring the donors found positive for those particular agents. Pathogen reduction modalities proactively target all nucleic acids to prevent transfusion-transmitted infections. However, the universal adoption of pathogen inactivation in blood banks has not been achieved. The same donor eligibility and deferral criteria are enforced in institutions where it has been adopted.
Notably, pathogen reduction is intended to augment the prevention of transfusion-associated infection and is not used as a replacement for the testing and screening processes. Testing and deferrals could be significantly decreased if pathogen reduction is proven to be both safe and effective. The promise of pathogen inactivation lies in the potential for creating a safer blood supply and increasing the donor pool. However, further data and trials are necessary to assess the cost, safety, and efficacy.[1]
An individual's eligibility to donate blood can be influenced by the results of malaria serology tests and their connection to an endemic area.[16][17] Individuals with a history of malaria are deferred from blood donation for 3 years following diagnosis, whereas residents of malaria-endemic regions are usually ineligible for 3 to 4 years. If the malaria serology test results are negative, individuals who have traveled to malarial zones may temporarily be unable to donate blood for 3 to 4 months. However, a positive malaria serology results in a complete exclusion from blood donation in the United Kingdom and France. In some cases, the travel location is considered in predicting exposure and relegating prohibition. Efforts are ongoing to develop an algorithm to aid in this evaluation.[18]
Equipment
The following list of equipment is necessary for blood donation:
- Blood pressure cuffs
- Thermometers
- Scales
- Donor chairs and tables
- Blood collection monitors or mixers
- Blood bag tube sealers
- Blood transportation boxes
- Blood bank refrigerators
- Tourniquet
- Blood collection mixer and weigher
- Bandages
- Cotton balls or gauze
- Needles, 16G or 18G
- Drinks and snacks for donors
- Chlorhexidine gluconate (2%) and isopropyl alcohol solution (70%)
- Labels
- Pens
- Hemoglobin analyzer
- Apheresis machine (only necessary if doing apheresis)
Personnel
Most blood collection personnel involved in blood donation are certified phlebotomists. These personnel typically hold a high school diploma and undergo 1 to 2 months of both classroom and hands-on training before obtaining certification. In addition to phlebotomists, other collection personnel may also receive training as licensed vocational nurses, registered nurses, and emergency medical technicians.
Preparation
In preparation for blood donation, donors are advised to ensure they are well-rested, consume a full meal, and drink ample amounts of non-alcoholic, non-caffeinated beverages. Following donation, donors should refrain from heavy lifting, drinking alcohol, and smoking for several hours.
Technique or Treatment
The donor undergoes a screening questionnaire and a medical assessment, including an evaluation of their general well-being, vital signs, and a fingerstick hemoglobin test. Following the screening process, the donor will proceed to the donor's table or chair for the blood collection. The venipuncture site is prepared by scrubbing it with a 2% chlorhexidine gluconate and 70% isopropyl alcohol solution, and a sterile needle is inserted into the antecubital vein.
In some individuals who do not have an otherwise easily accessible antecubital vein, veins on the dorsum of the hand or other prominent veins may be used for blood collection. The donor is continuously monitored throughout the blood collection process. Blood is collected into a bag containing an anticoagulant and is continually mixed mechanically. Once 450 mL of blood is collected, the needle is removed, and pressure is applied to the area.
Apheresis is the process by which platelets or plasma are separated from whole blood, and the remaining blood is returned to the donor's body. This procedure requires the use of 2 catheters that are connected to an apheresis machine. Both arms will be utilized during the process. Apheresis typically takes around 1 hour, whereas a regular blood donation lasts approximately 10 to 15 minutes.
Complications
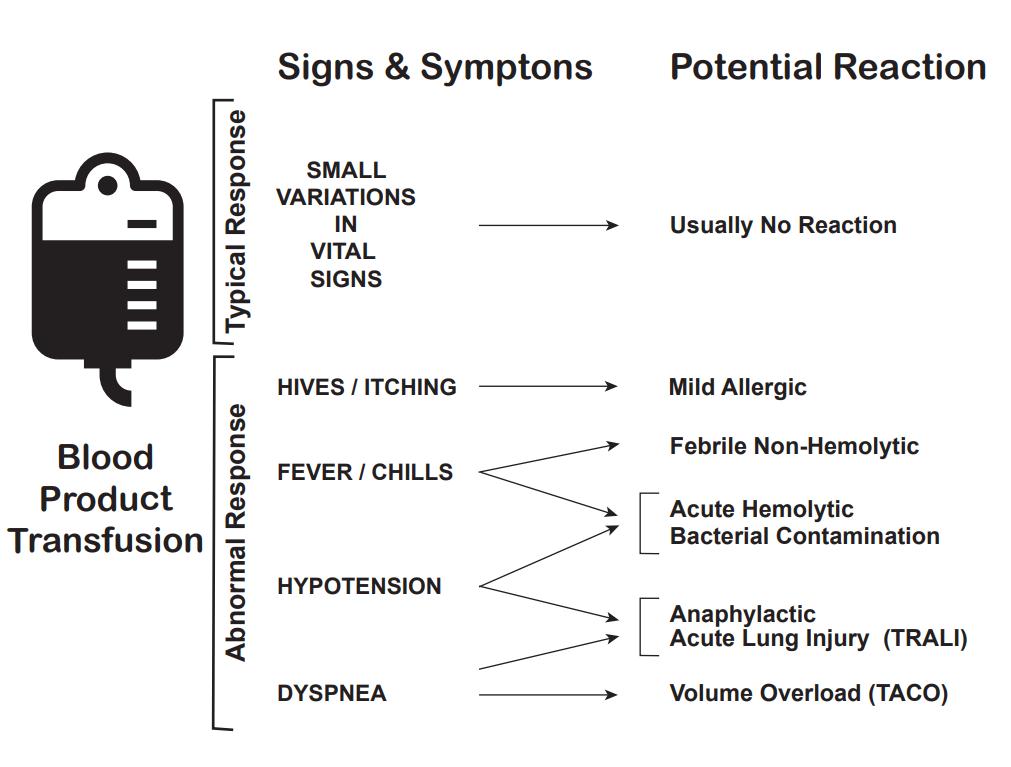
Certified phlebotomists are mandated to conduct blood donation procedures. These professionals undergo thorough training in screening potential blood donors, using sterile collection methods, and overseeing the blood donation process. Although adverse effects from blood donation are rare, the most frequent complication is the occurrence of a local traumatic hematoma upon needle removal. Applying pressure and using ice or cold compresses in the affected area can aid in preventing the formation or progression of hematomas. While these hematomas are typically small and do not pose significant issues, there is a rare possibility that they may advance to cause damage to neighboring structures and tissues. Therefore, it is crucial to monitor the hematoma closely for the potential development of local damage.[7]
The second most common adverse effect of blood donation is syncope, which is often vasovagal or related to hypovolemia. During syncopal episodes associated with blood donation, there is typically a decrease in systolic blood pressure and pulse. The blood donor may experience weakness, sweating, dizziness, or pallor symptoms. The donation is temporarily halted or discontinued, and supportive measures are initiated when syncope occurs. Post-donation syncope may also occur, and the risk can be reduced by having the donor sit in a reclined position for a few minutes. When feasible, donors should gradually transition from a reclined position to an upright one and then move to an area where they can consume food and beverages. Encouraging increased fluid intake and advising against consuming alcoholic or caffeinated beverages over the following few hours can aid in replenishing the lost circulating volume.[7][19][20]
One study showed that first-time donor status, young age, and female gender were associated with an increased risk of adverse events. Although transient syncopal events may not be dangerous, injuries sustained from falls due to syncope may result. Thus, it is crucial to mandate rest and sitting during both the blood donation process and the post-donation period to prevent such injuries. Donors who experience injuries during donation may be less inclined to return for subsequent donations.[21][20]
Although nausea and vomiting are reported infrequently with blood donation, if they occur, the donation is temporarily paused, and supportive measures are initiated.[7][22] Other risks include anemia and iron deficiency without anemia. When collecting platelets or plasma via apheresis, red blood cells are not removed, and risks include thrombocytopenia, lymphopenia, and citrate toxicity.
Additional risks associated with autologous donation include procedural-induced anemia, loss of donated units due to unforeseen circumstances, and a potential delay in necessary procedures. Ironically, this delay may lead to an increased requirement for allogeneic transfusion in these patients.[9] Despite these considerations, blood donation is generally considered a safe practice, with many individuals perceiving it as an altruistic act. With minimal cost to themselves, donors can significantly contribute to others' healthcare and save lives.
Clinical Significance
Blood donation and transfusion procedures are crucial for modern medicine, as millions of patients rely on these life-saving procedures yearly. However, maintaining a sufficient blood supply remains an ongoing challenge primarily due to the limited shelf life of blood and its derived products. In the United States alone, over 24 million units of whole blood and blood-derived products are transfused annually.[23]
The future of our blood supply depends solely on volunteer donors. Currently, several factors are influencing the availability of donors, including increased restrictions on potential donors due to concerns about infectious agents, the implementation of more effective screening methods, the discovery of new pathogens, and the evolving eligibility criteria for donors. Collectively, these factors contribute to limitations in the donor pool.[24] Recent efforts have strongly emphasized patient blood management and the appropriate utilization of blood resources. These initiatives have proven valuable in safeguarding the sustainability and effectiveness of our blood supply.[25]
Enhancing Healthcare Team Outcomes
To address the increasing medical demands of our population, we need to boost the blood supply and donor numbers. Hospitals and other establishments continue to create campaigns and motivators to increase the donor pool. These marketing strategies often center around themes of altruism, addressing genuine medical needs, and appealing to a sense of duty and service.[22][26][24] Collaboration among healthcare professionals, hospitals, and blood banks is essential to sustain the required blood donations. Ensuring the protection of blood donors from injury during the donation process is not only inherently important but also crucial for preventing donor dropout and encouraging retention and recruitment.[21][20]
The aging population in the United States is steadily growing, and European studies indicate that this trend is diminishing the donor pool.[27] Simultaneously, in-hospital blood needs have significantly increased over the past 3 decades. The collaboration of an interprofessional healthcare team is crucial for educating and initiating blood donation campaigns to expand the pool of donors. Due to the changing demographics, healthcare professionals such as doctors, pharmacists, nurses, phlebotomists, laboratory technicians, and other allied healthcare workers play crucial roles in enhancing blood donations.[27]
Media
(Click Image to Enlarge)
References
Drew VJ, Barro L, Seghatchian J, Burnouf T. Towards pathogen inactivation of red blood cells and whole blood targeting viral DNA/RNA: design, technologies, and future prospects for developing countries. Blood transfusion = Trasfusione del sangue. 2017 Oct:15(6):512-521. doi: 10.2450/2017.0344-16. Epub 2017 Apr 13 [PubMed PMID: 28488960]
Lee RI. A SIMPLE AND RAPID METHOD FOR THE SELECTION OF SUITABLE DONORS FOR TRANSFUSION BY THE DETERMINATION OF BLOOD GROUPS. British medical journal. 1917 Nov 24:2(2969):684-5 [PubMed PMID: 20768818]
LEWISOHN R. The citrate method of blood transfusion in retrospect. Acta haematologica. 1958 Jul-Oct:20(1-4):215-7 [PubMed PMID: 13582565]
Wang JC. A Call to Arms: Wartime Blood Donor Recruitment. Transfusion medicine reviews. 2018 Jan:32(1):52-57. doi: 10.1016/j.tmrv.2017.06.004. Epub 2017 Jul 6 [PubMed PMID: 28778577]
Rous P, Turner JR. THE PRESERVATION OF LIVING RED BLOOD CELLS IN VITRO : II. THE TRANSFUSION OF KEPT CELLS. The Journal of experimental medicine. 1916 Feb 1:23(2):239-48 [PubMed PMID: 19867982]
Robertson OH. TRANSFUSION WITH PRESERVED RED BLOOD CELLS. British medical journal. 1918 Jun 22:1(2999):691-5 [PubMed PMID: 20769077]
Cook LS. Therapeutic phlebotomy: a review of diagnoses and treatment considerations. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2010 Mar-Apr:33(2):81-8. doi: 10.1097/NAN.0b013e3181d00010. Epub [PubMed PMID: 20228645]
Brecher ME, Goodnough LT. The rise and fall of preoperative autologous blood donation. Transfusion. 2001 Dec:41(12):1459-62 [PubMed PMID: 11778055]
Vassallo R, Goldman M, Germain M, Lozano M, BEST Collaborative. Preoperative Autologous Blood Donation: Waning Indications in an Era of Improved Blood Safety. Transfusion medicine reviews. 2015 Oct:29(4):268-75. doi: 10.1016/j.tmrv.2015.04.001. Epub 2015 May 6 [PubMed PMID: 26006319]
Shearer WT, Lubin BH, Cairo MS, Notarangelo LD, SECTION ON HEMATOLOGY/ONCOLOGY, SECTION ON ALLERGY AND IMMUNOLOGY. Cord Blood Banking for Potential Future Transplantation. Pediatrics. 2017 Nov:140(5):. doi: 10.1542/peds.2017-2695. Epub [PubMed PMID: 29084832]
Food and Drug Administration, HHS. Requirements for blood and blood components intended for transfusion or for further manufacturing use. Final rule. Federal register. 2015 May 22:80(99):29841-906 [PubMed PMID: 26003966]
Mast AE. Low hemoglobin deferral in blood donors. Transfusion medicine reviews. 2014 Jan:28(1):18-22. doi: 10.1016/j.tmrv.2013.11.001. Epub 2013 Nov 12 [PubMed PMID: 24332843]
Al-Dabagh A, Feldman S. Restrictions on blood donations relevant to dermatology. Dermatology online journal. 2018 Apr 15:24(4):. pii: 13030/qt1s98s3v4. Epub 2018 Apr 15 [PubMed PMID: 29905997]
Becker CD, Stichtenoth DO, Wichmann MG, Schaefer C, Szinicz L. Blood Donors on Medication - an Approach to Minimize Drug Burden for Recipients of Blood Products and to Limit Deferral of Donors. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2009:36(2):107-113 [PubMed PMID: 20823991]
Jeong JH, Hyun GH, Park YJ, Kwon SW, Lee AY. Clinical Factors Affecting the Serum Retention of a Teratogenic Etretinate after the Acitretin Administration. Biomolecules & therapeutics. 2022 Nov 1:30(6):562-569. doi: 10.4062/biomolther.2022.069. Epub 2022 Jul 25 [PubMed PMID: 35871607]
Spencer B, Steele W, Custer B, Kleinman S, Cable R, Wilkinson S, Wright D. Risk for malaria in United States donors deferred for travel to malaria-endemic areas. Transfusion. 2009 Nov:49(11):2335-45. doi: 10.1111/j.1537-2995.2009.02290.x. Epub [PubMed PMID: 19903290]
Grande R, Petrini G, Silvani I, Simoneschi B, Marconi M, Torresani E. Immunological testing for malaria and blood donor deferral: the experience of the Ca' Granda Polyclinic Hospital in Milan. Blood transfusion = Trasfusione del sangue. 2011 Apr:9(2):162-6. doi: 10.2450/2011.0158-09. Epub 2011 Jan 17 [PubMed PMID: 21251462]
Acheampong DO, Aninagyei E. The Use of Screening Algorithm to Defer Blood Donors with Subclinical Malaria. Journal of tropical medicine. 2021:2021():9942721. doi: 10.1155/2021/9942721. Epub 2021 Aug 13 [PubMed PMID: 34426742]
Eder AF, Dy BA, Kennedy JM, Perez J, Demaris P, Procaccio A, Benjamin RJ. Improved safety for young whole blood donors with new selection criteria for total estimated blood volume. Transfusion. 2011 Jul:51(7):1522-31. doi: 10.1111/j.1537-2995.2011.03143.x. Epub 2011 Apr 29 [PubMed PMID: 21534981]
Eder AF. Improving safety for young blood donors. Transfusion medicine reviews. 2012 Jan:26(1):14-26. doi: 10.1016/j.tmrv.2011.07.008. Epub 2011 Aug 26 [PubMed PMID: 21872427]
Eder AF, Hillyer CD, Dy BA, Notari EP 4th, Benjamin RJ. Adverse reactions to allogeneic whole blood donation by 16- and 17-year-olds. JAMA. 2008 May 21:299(19):2279-86. doi: 10.1001/jama.299.19.2279. Epub [PubMed PMID: 18492969]
Bednall TC, Bove LL. Donating blood: a meta-analytic review of self-reported motivators and deterrents. Transfusion medicine reviews. 2011 Oct:25(4):317-34. doi: 10.1016/j.tmrv.2011.04.005. Epub 2011 Jun 8 [PubMed PMID: 21641767]
Level 1 (high-level) evidenceSullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007 Mar:47(3):385-94 [PubMed PMID: 17319817]
McCullough J. The nation's changing blood supply system. JAMA. 1993 May 5:269(17):2239-45 [PubMed PMID: 8474203]
Jenkins I, Doucet JJ, Clay B, Kopko P, Fipps D, Hemmen E, Paulson D. Transfusing Wisely: Clinical Decision Support Improves Blood Transfusion Practices. Joint Commission journal on quality and patient safety. 2017 Aug:43(8):389-395. doi: 10.1016/j.jcjq.2017.04.003. Epub 2017 Jun 27 [PubMed PMID: 28738984]
Level 2 (mid-level) evidenceGillespie TW, Hillyer CD. Blood donors and factors impacting the blood donation decision. Transfusion medicine reviews. 2002 Apr:16(2):115-30 [PubMed PMID: 11941574]
Greinacher A, Fendrich K, Brzenska R, Kiefel V, Hoffmann W. Implications of demographics on future blood supply: a population-based cross-sectional study. Transfusion. 2011 Apr:51(4):702-9. doi: 10.1111/j.1537-2995.2010.02882.x. Epub 2010 Sep 16 [PubMed PMID: 20849411]
Level 2 (mid-level) evidence