Introduction
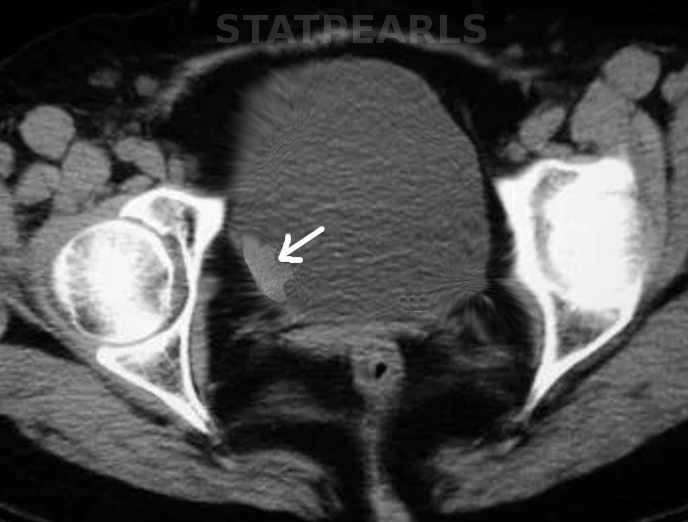
Bladder cancer, a prevalent malignancy affecting the urinary system, arises from the tissues of the bladder, a hollow organ responsible for storing urine (see Image. Bladder Cancer, Magnetic Resonance Image). Urothelial carcinoma is the most frequent type of bladder cancer, constituting over 90% of cases in industrialized nations. This type of cancer is notably common among older adults, with risk factors including smoking, chemical exposure, and chronic bladder inflammation. Presenting symptoms such as gross or microscopic hematuria, urinary frequency, and pelvic pain lead to its detection. Early diagnosis and treatment are crucial for improving outcomes, as bladder cancer can range from noninvasive forms that are confined to the inner layers of the bladder to invasive types that penetrate deeper and can spread to other parts of the body.
Treatment primarily involves transurethral resection and intravesical chemotherapy instillations but may also include laser ablation, Bacillus Calmette-Guerin bladder treatments, radiation therapy, chemotherapy, or surgical removal of part or all of the bladder.
This activity reviews the intricacies of bladder cancer, from its etiology to its therapeutic strategies. The latest advancements and comprehensive knowledge in diagnosing, treating, and managing this prevalent malignancy are discussed. Participants gain enhanced diagnostic acumen, learn evidence-based treatment protocols, and are updated on emerging research, fostering a deeper understanding of bladder cancer's evolving landscape and ultimately improving clinical practice. The role of the interprofessional team in improving care for patients with this common malignant condition.
Collaborating with an interprofessional team enhances patient outcomes by integrating diverse expertise, ensuring comprehensive treatment plans, and providing holistic care that addresses all aspects of the patient's health. This improves the overall management and prognosis of bladder cancer.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Urothelial carcinoma develops via two distinct pathways. One relates primarily to papillary neoplasms, and the other to flat or sessile lesions.
Non-muscle-invasive bladder cancers (NMIBC) are typically low-grade papillary tumors that usually arise from simple hyperplasia and/or minimal dysplasia. This category also includes carcinoma in situ (CIS), a high-grade but superficial cancer.
NMIBCs are characterized by the following:
- Activating mutations of fibroblast growth factor receptor 3
- Inactivating mutations of STAG2
- Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
- The loss of heterozygosity of chromosome 9
- Telomerase reverse transcriptase
Low-grade papillary NMIBC can progress to a muscle-invading malignancy in about 10% of cases due to cyclin-dependent kinase inhibitor 2A loss.[1]
Muscle-invasive bladder cancer (MIBC) arises from flat dysplasia or carcinoma in situ and is characterized by the following:
- The lesions show TP53 mutations and loss of heterozygosity of chromosome 9.
- Invasive carcinoma can then acquire metastatic potential by gaining RB1 and PTEN loss, together with other alterations.
- Copy number alterations and genetic instability correlate with tumor progression and a poorer prognosis.
- Overall, NMIBC usually shows diploid or near-diploid karyotypes as compared to muscle invading.
- MIBC is usually aneuploid, with numerous chromosomal alterations.[2][3]
Results from recent studies have demonstrated 4 subtypes of NMIBC based on abnormal ribonucleic acid (RNA) expression analyses.[4] These abnormal prognostic molecular subtypes involve early cell cycle abnormalities, chromosomal instability, stem cell-like disorders, and immune depletion problems.[4] Of these, chromosomal instability, which involves p53 and deoxyribonucleic acid damage repair genes, as well as apolipoprotein messenger RNA-editing enzyme, catalytic polypeptide, appears to be the most significant, as it has the highest recurrence and progression rates.[4]
Risk Factors
There are multiple known risk factors for bladder cancer. Important risk factors include smoking, schistosomiasis infection, and occupational exposure to certain chemicals.[2][5][6] Smoking is the most important known risk factor for bladder cancer as the average incidence in smokers is triple (2- to 6-fold) that of nonsmokers; the risk depends on smoking duration and intensity, with 10-pack-years generally considered the high-risk threshold.[7] At least 50% of bladder cancers will be found in current or former smokers.[8][9][10] Cigarette smokers also tend to develop more aggressive tumors and have a worse prognosis.[11] Smoking cessation lowers the bladder cancer risk, which eventually approaches that of nonsmokers.[8][10] Within the first 4 years after smoking cessation, the bladder cancer risk drops by 40% and is reduced by 60% after 25 years.[12]
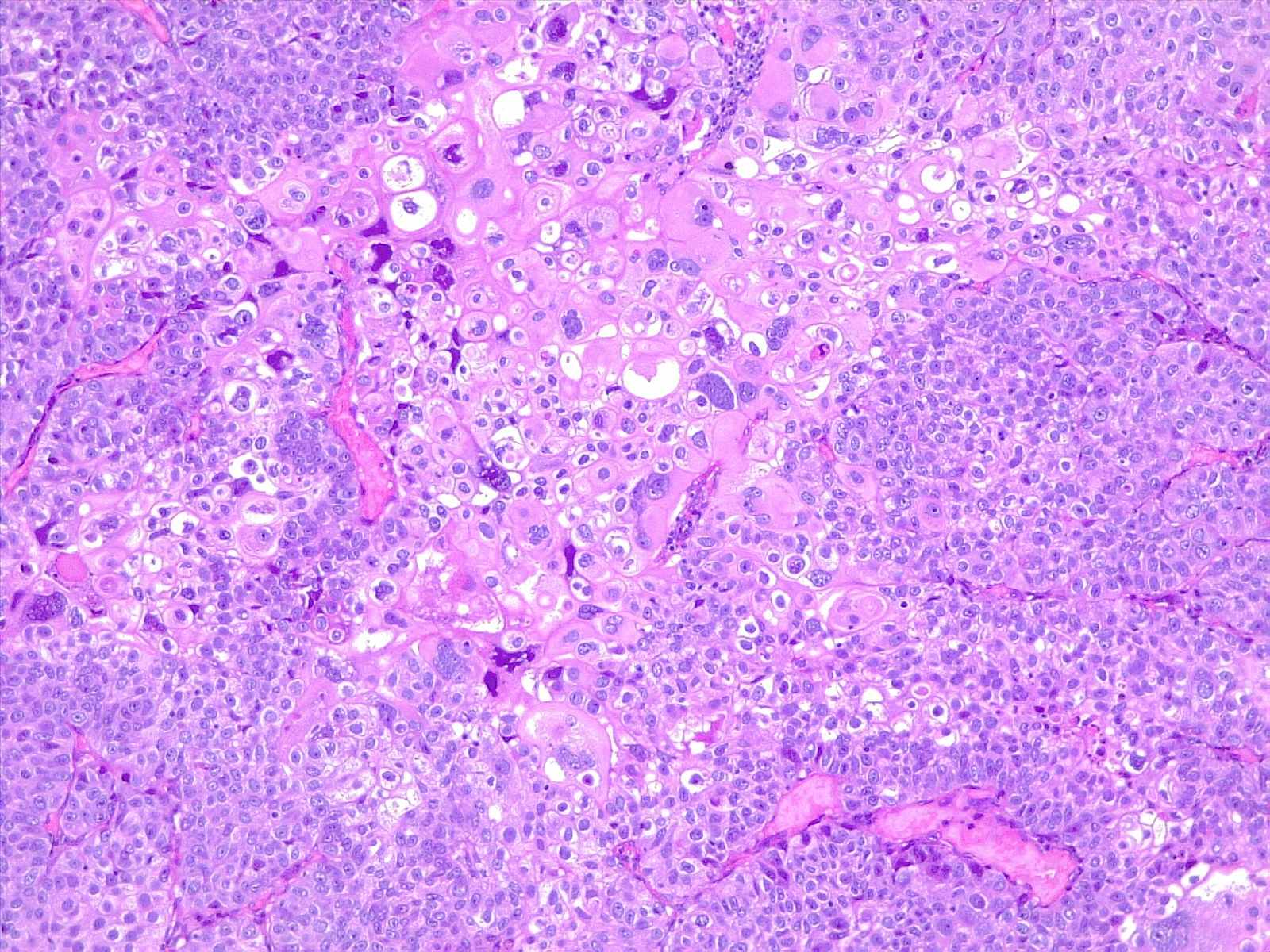
In developing countries, schistosomiasis infection is an important cause of bladder cancer. Schistosoma haematobium ova becomes embedded in the bladder wall, leading to irritation, chronic inflammation, squamous metaplasia, and dysplasia, ultimately progressing to squamous cell carcinoma of the urinary bladder (see Image. Poorly Differentiated Urothelial Carcinoma with Metaplastic Squamous Appearance). Worldwide, about 600 million people are at risk in endemic areas of Africa, Asia, the Caribbean, and South America.[13]
Genetic and/or environmental factors also affect the development of bladder cancer but only account for about 7% of all such cases.[14][15][16][17][18] Chemicals associated with bladder cancer include arylamine and aniline dyes, formaldehyde, phenacetin, cyclophosphamide, and arsenic in the drinking water.[19][20][21][22]
Occupational exposure to paint, rubber, petroleum products, agricultural chemicals, and dyes correlates with an increased risk of bladder cancer. Occupations that typically have the most exposure to such chemicals include the following:
- Agricultural crop production workers
- Barbers
- Bartenders and waiters
- Beauticians
- Chemical plant employees
- Dental office workers
- Dry cleaners
- Housecleaners
- Metalworkers (welding)
- Oil refinery workers
- Painters
- Paper production workers
- Pesticide production or use by agricultural workers
- Rope, string, and twine production workers [23]
Epidemiology
In the United States (US), bladder cancer is the fourth most common cancer in men, eighth in women, and fifth overall, with a man-to-woman ratio of 4:1.[24] While women have bladder cancer less often than men, they typically present with more advanced disease and have a poorer prognosis.[25][26][27][28][29] According to the National Cancer Institute, there were 82,290 new cases and 16,710 deaths from bladder cancer in the United States in 2023. Bladder cancer represents 4.2% of all new cancers detected and 2.7% of all cancer deaths, with an overall 5-year relative survival rate of 77.9%. The incidence of new cases and mortality rates are slowly declining in the US by about 1% a year.
Bladder cancer is twice as common in White populations (the ethnic group with the highest incidence) compared to those in the Black population, and the risk increases with age, particularly in those older than 70. However, disease-specific survival is lower for Black individuals.[30][31] In the US, almost all bladder cancer is urothelial, but worldwide, most (75% of cases) are squamous cell carcinomas, which correlates closely with the incidence of endemic schistosomiasis.[30][32][33]
Worldwide, bladder cancer is the seventh most common malignancy overall. The reported incidence globally is 9.5/100,000 population/year for men and 2.4/100,000 for women.[13] In 2020, 213,000 individuals died from bladder cancer worldwide.[34] The incidence of bladder cancer is twice as high in developing nations compared to highly industrialized countries. According to the World Health Institute (WHO), Greece has the highest overall risk of bladder cancer, followed by the Netherlands, Italy, Denmark, Belgium, and Spain. The WHO reports the highest mortality rate in the world from bladder cancer is in Egypt, followed by Tunisia, Libya, Poland, and Mali.
Pathophysiology
The most critical characteristics related to bladder cancer's aggressiveness and prognosis are its degree of invasiveness (penetration) into the bladder wall and its tumor cellular grade. Therefore, bladder cancers are classified into MIBC and NMIBC based on tumor invasiveness and low and high grade based on cellular characteristics.
Non-muscle-invasive bladder cancer is urothelial carcinoma that is confined to the mucosa and submucosa as it does not penetrate through the lamina propria into the underlying muscle layer.[35][36][37] This type tends to be less aggressive and is usually treated with localized therapy such as transurethral resection.[1][36] NMIBC is the most commonly found type of bladder cancer and accounts for about 75% of the total cases. One exception is CIS, which is an aggressive and high-grade but relatively rare (1% to 3%) and superficial form of bladder cancer.[38] While technically non-muscle-invasive, it is typically discussed and treated differently.[38]
Muscle-invasive bladder cancer penetrates the lamina propria and enters the superficial or deep muscle layers of the bladder.[30][35][37] It may also extend to other tissues surrounding the bladder or elsewhere. MIBC accounts for about 25% of all bladder malignancies.[39] This type of cancer is far more likely to metastasize than NMIBC and is treated much more aggressively, often with radical surgery and chemotherapy.[30]
Histopathology
The urinary bladder wall is comprised of 4 layers: mucosa, submucosa, muscularis, and serosa. The mucosa has a normal urothelium layer consisting of a 3– to 6-cell thick layer of stratified, uniform, nonsquamous (transitional cell) epithelium (urothelium). Large umbrella cells form on top, with an additional superficial glycosaminoglycans layer, which acts as a protective barrier to irritating urinary chemicals. Basal cells are found below, and intermediate cells are in between.
Bladder cancer originates from the basal cell layer (CIS, muscle-invasive urothelial cancer, and squamous cell carcinoma) or intermediate cells (noninvasive urothelial cancer).[40] The urothelium extends from the renal papilla to the urethra.[41] This is quite impermeable and chemically inert due to the protective glycosaminoglycans layer on the superficial mucosal surface, tight cellular junctions between the umbrella cells, and uroplakin proteins in the membrane of the umbrella cells, which block any mucosal penetration. Urothelial carcinoma stains positively for GATA-3, CK7, CK20, p63, thrombomodulin, and uroplakins II and III.
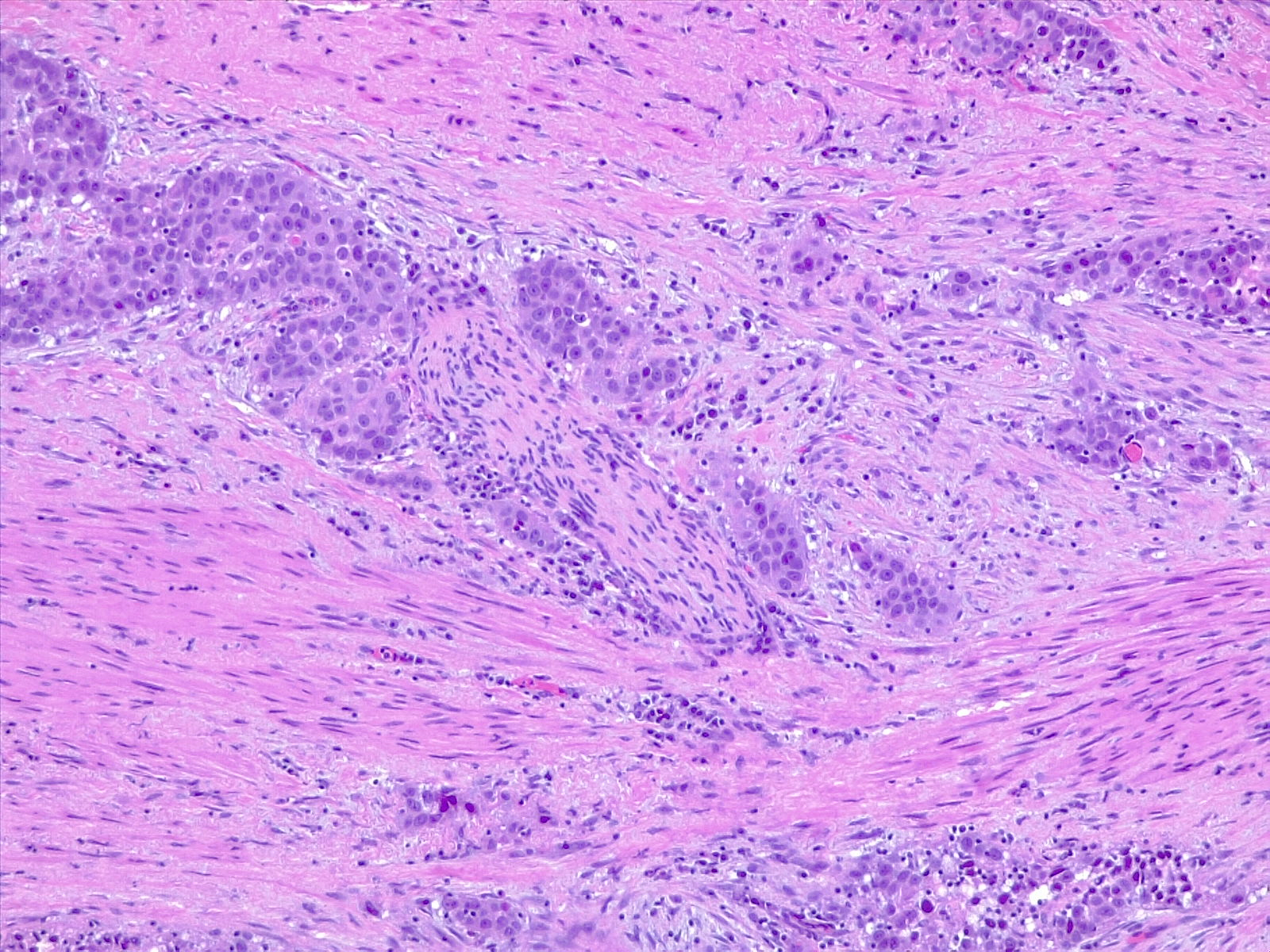
Bladder cancer is classified by stage (muscle-invading or not) and grade (low grade or high grade). Based on its morphology and pathway, it may also be subdivided into papillary (papilloma, low malignant potential, and papillary carcinoma) and flat (urothelial CIS and invasive) categories (see Image. Urothelial Carcinoma in Situ in the Setting of Cystitis Cystica and Glandularis).
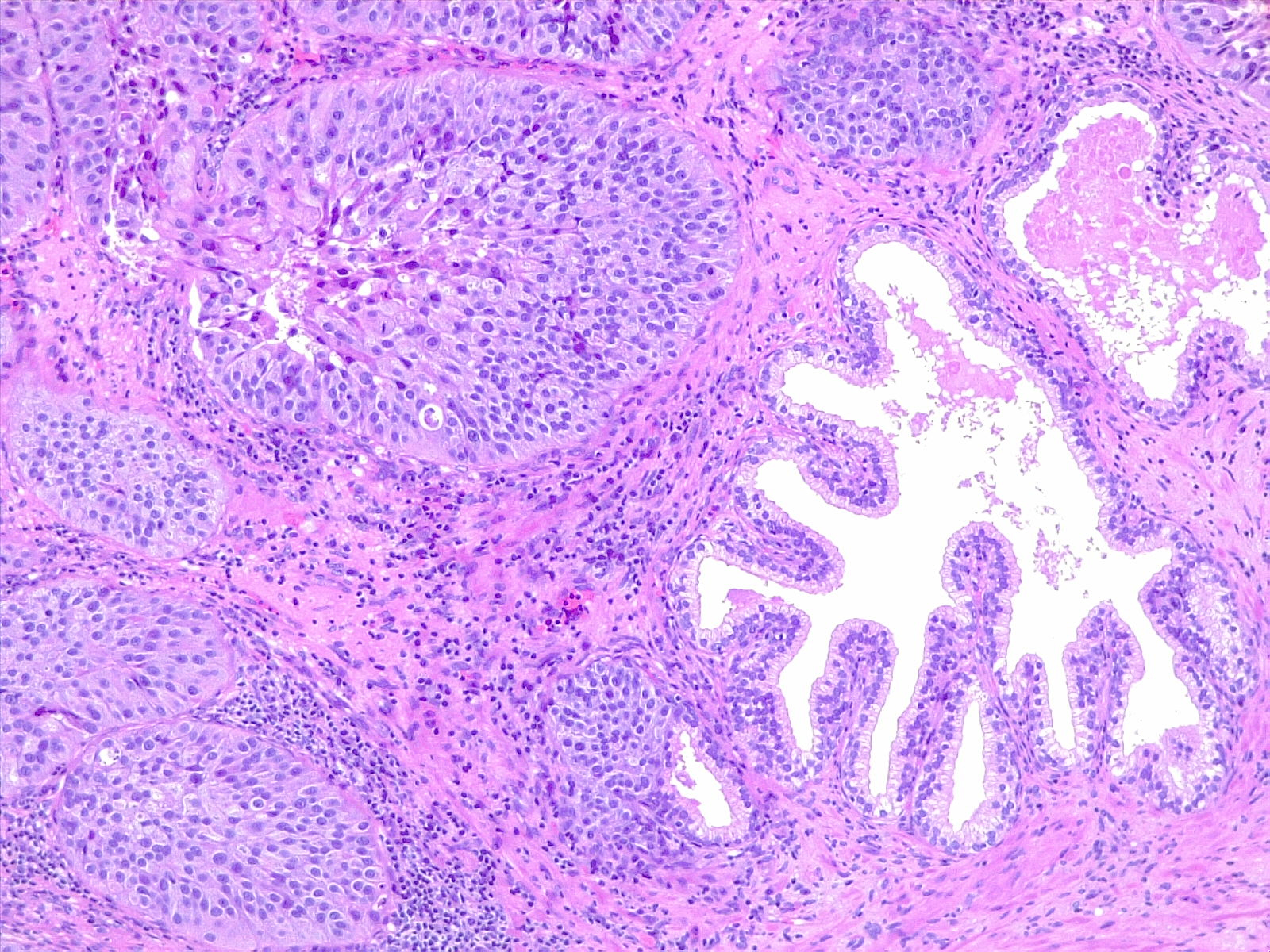
The WHO classifies urothelial carcinoma based on histopathology as low-grade or high-grade, depending on the degree of nuclear anaplasia and architectural abnormalities. The histology of infiltrating urothelial cancer is variable (see Image. Urothelial Carcinoma, Prostatic Infiltration and Image. Urothelial Carcinoma, Perineural Invasion). Most T1 (non-muscle-invading) cancers are papillary, low, or high grade, whereas T2-T4 (muscle-invading) carcinomas tend to be non-papillary and high grade.
Low-grade urothelial bladder tumors typically appear as thin papillary fronds with frequent branching.[42] Variation in nuclear polarity is common with enlarged irregular nuclei and frequent nucleoli.[42] Mitoses may be present, and most tumor cells are aneuploid.[42]
High-grade urothelial cancers show a more disordered arrangement with cytological atypia than low-grade neoplasms.[42] Papilla are often fused with more cytological and architectural abnormalities than in low-grade tumors.[42] Nuclei are pleomorphic, have prominent nucleoli, and have altered polarity with frequent mitoses present.[42] CIS is often seen in adjacent mucosal areas, and aneuploidy is common.[42]
Carcinoma in situ (CIS) of the urinary bladder describes a flat, superficial, high-grade urothelial malignancy with severe cytological atypia and nuclear anaplasia.[43] Other characteristics include loss of polarity, cellular crowding, and pleomorphism with markedly enlarged hyperchromatic nuclei and large nucleoli.[43] Loss of cellular adhesion results in denuded mucosal areas, common from shed cells, resulting in positive urinary cytological findings.[44] Urothelial CIS typically stains negatively with CD44 but strongly positively with CK20 and p53. CIS of the bladder has a 60% rate of disease recurrence and/or progression. CIS is relatively rare as a primary malignancy at no more than 3% of all bladder cancers, but it is frequently seen with invasive urothelial cancers and is considered a poor prognostic sign when detected.[43][45]
Divergent differentiation is the term used to describe urothelial carcinoma together with non-urothelial histology.[45] This is found in 15% to 25% of all invasive bladder cancers.[45] Possible associated morphologies with such "divergent differentiation" may occur single or in combination and include squamous, glandular, small-cell, micropapillary urothelial cancer, nested, microcytic, microtubular, sarcomatoid, high-grade neuroendocrine, and even trophoblastic lines.[46][47][48] All of these subtypes and variations are considered high-grade.[13][48] The percentage of such divergent differentiation should be reported when identified as it has prognostic significance.[46][47][49]
Pathology plays a crucial role in managing bladder cancer. Most patients (75%) present with NMIBC at initial diagnosis, which correlates with better overall survival and prognosis. The most critical factor in the pathological assessment of urothelial carcinoma is identifying the extent of invasion to set proper staging, followed by the tumor grade. Bladder cancers can be unifocal or multifocal. Most cases present as multifocal, which may be multiple and independent or arise from a common origin.
The pathology report should include the following:
- Depth of tumor penetration or invasion into the bladder wall (Microinvasion is defined as a depth of invasion ≤2 mm.)
- Histological type and grade
- Location of the tumor: where in the bladder it was found
- Number of separate specimens or tumors
- Presence or absence of CIS
- Presence or absence of detrusor muscle in the specimen
- Presence and percentage of any divergent differentiation
- Size of the specimen
In complex or difficult cases, an additional review by an experienced genitourinary pathologist is recommended.[46][47]
Squamous cell malignancies constitute the majority of non-urothelial bladder cancer cases worldwide, although over 90% of bladder tumors in the US and industrialized countries are urothelial carcinomas.[50]
In the US, squamous cell carcinoma of the bladder is related to chronic inflammation from foreign bodies, catheters, human papillomavirus (HPV) infections, cyclophosphamide exposure resulting in hemorrhagic cystitis, or schistosomiasis in travelers or immigrants.[51][52] Squamous cell carcinoma is the second most common cancer type found in the bladder at about 5%.[53] Squamous cell carcinoma is followed by adenocarcinoma at 2% and neuroendocrine malignancies.[51]
Up to approximately 40% of high-grade or invasive bladder cancers will have some squamous cell characteristics (polygonal cell shape, abundant light eosinophilic cytoplasm, distinct cell borders, and peritumoral lymphocytic aggregates).[45] The diagnosis of primary squamous cell carcinoma is reserved for keratin-forming squamous neoplasms that lack any typical urothelial components.[45] Squamous cell carcinoma of the bladder stains positive for cytokeratin CK 5/6, desmoglein 3, p63, CD44, and p40.
Treatment is generally surgical, possibly with preoperative radiation therapy. Patients with disease not amenable to surgical resection may receive chemoradiation. Metastatic disease is uncommon but may be best treated with carboplatin, gemcitabine, and paclitaxel.[54] Enrollment of patients with advanced disease in a clinical trial is strongly encouraged.
Adenocarcinoma of the bladder is far more likely to metastasize than squamous cell cancer; this cancer is typically classified as urachal or non-urachal. Urachal adenocarcinoma develops from the urachal remnant and is typically located in the dome of the bladder, often extending into the prevesical space. These malignancies are mucin-producing (90%) tumors that typically present as locally advanced with hematuria and a palpable lower abdominal mass.[55][56][57] Treatment is surgical, with a substantial number of patients able to receive partial cystectomies. Five-year overall survival has been reported at about 50%.[58]
Non-urachal adenocarcinoma of the bladder tends to be associated with schistosomiasis or bladder exstrophy.[59][60] Cystoscopic appearance may be papillary or flat; these tumors are usually muscle-invasive neoplasms at presentation.[61] Extrinsic adenocarcinomas need to be excluded. Treatment is primarily surgical, but partial cystectomies are generally avoided due to poor outcomes.[62][63][64][65] The prognosis for non-urachal adenocarcinoma of the bladder is usually bleak, with a 5-year overall survival of 35%.[66]
History and Physical
Bladder cancer is primarily a tumor in adults who are 60 and older. Hematuria, either gross or microscopic, is the most common initial symptom in patients with bladder cancer. Over a third of patients with gross hematuria and slightly over 10% of those with microscopic hematuria will ultimately be diagnosed with bladder cancer. Other less common symptoms include painful micturition, increased urinary frequency, a pelvic mass, and constitutional symptoms such as fatigue and weight loss.
A complete history and physical examination are recommended for all patients presenting with unexplained hematuria to identify risk factors for urothelial malignancy as well as other sources of bleeding such as medical kidney disease, renal neoplasms, urolithiasis, gynecological disorders, benign prostatic hyperplasia, and other nonmalignant genitourinary disorders.[67] In women, a pelvic examination, as well as a careful inspection of the external genitalia, introitus, and periurethral area, should be performed. The evaluation is the same for patients taking anticoagulants.[68][69][70]
If a nonmalignant cause of the hematuria is discovered, repeat urinalyses should be performed following treatment and resolution of the disorder. If the hematuria remains, a risk-based evaluation should be performed.[67] For example, patients with microscopic hematuria due to a urinary tract infection should have a follow-up microscopic urinalysis after treatment to verify that the hematuria has also resolved.[67]
There are no screening tests for the early detection of bladder cancer other than the finding of unexplained, persistent, gross, or microscopic hematuria. Gross hematuria may correlate somewhat with a more advanced disease stage.[46] Smoking history is a strong predisposing factor. A history of 10 pack-years or more is sufficient to increase the patient's bladder cancer risk significantly. Medical renal disease as a source of hematuria should be suspected in patients with proteinuria, dysmorphic red blood cells, cellular casts on microscopic urinalysis, or kidney failure.[67]
High-Risk Factors for Bladder Cancer
- A chronic indwelling catheter or foreign body
- Chemotherapy (cyclophosphamide, ifosfamide)
- Concurrent irritative voiding symptoms
- History of pelvic radiation
- Increasing age
- Male sex
- Positive family history of bladder cancer, Lynch syndrome, Peutz-Jeghers syndrome, or Cowden syndrome
- Smoking history of 10 or more pack-years
- Toxic chemical exposure
- Aromatic amines and hydrocarbons
- Arsenic
- Benzene products
- Formaldehyde
- Nitrosamine
- Petrochemicals
- Rubber [67]
Schistosoma hematobium is a risk factor for squamous cell carcinoma in those parts of the world where the parasite is endemic.
Evaluation
Screening patients for bladder cancer is not recommended. Patients who are found to have hematuria on routine urinalysis should be considered for an evaluation of possible bladder cancer. Modalities used in diagnosing bladder cancer include imaging (eg, ultrasound, computed tomography, and magnetic resonance imaging), cystoscopy, cytology, transurethral resection, and biopsy. (see Image. Bladder Cancer) Intravenous pyelography is no longer used.
American Urological Association Patient Risk-Stratification [67]
Low-Risk Patients
Microhematuria and must have all of the following:
- Women younger than 50, Men younger than 40
- Smoking: Never smoked or <10 pack-years
- Urinalysis shows ≤10 red blood cells/high power field (RBC/HPF) with no prior hematuria
- No high-risk factors
Patients with microscopic hematuria at low risk for urothelial cancer should proceed with an ultrasound and cystoscopy or have a repeat urinalysis in 6 months.
Intermediate-Risk Patients
Microhematuria and any of the following:
- Women 50 to 59 years; men, 40 to 59 years
- Smoking: 10 to 30 pack-years
- Urinalysis shows 11 to 25 RBC/HPF
- One or more high-risk factors
- Previously categorized as low risk without further evaluation
Patients with microscopic hematuria at moderate risk for urothelial cancer should proceed with ultrasound and cystoscopy.
High-Risk Patients
The patient does not qualify as low or intermediate risk OR microhematuria and any of the following:
- Gross hematuria
- Age 60 or older (women and men)
- Smoking >30 pack-years
- Urinalysis shows >25 RBC/HPF
Patients considered high risk or who fail to qualify as low or intermediate risk should be evaluated with a computed tomography (CT) urogram (ie, CT scan of the abdomen and pelvis without and with IV contrast) and a cystoscopy.
Imaging
Imaging is a critical diagnostic component for evaluating bladder cancer. Ultrasound may be used in patients considered low-risk (eg, microscopic hematuria, younger age, no significant smoking history, and no high-risk factors).[71] However, it is not as thorough or comprehensive as a CT urogram, and it will miss small tumors, cystic lesions, and CIS.[72]
A CT urogram is the gold-standard imaging modality for the upper urinary tract.[73] This imaging should be performed in all patients who are high-risk and those who do not otherwise qualify as low or intermediate risk.[67] A CT urogram is also indicated if a bladder tumor is confirmed on cystoscopy and the CT study has not already been performed. The incidence of finding a simultaneous urothelial malignancy at the same time as bladder cancer is quite low at 1.8%, except for trigonal neoplasms, where the reported incidence is 7.5%.[74]
A CT urogram is optimally performed in 3 phases as follows:
- The non-contrast phase optimizes the identification of calcifications and urinary calculi.
- An early arteriovenous phase study optimizes visualization of renal masses and neoplasms.
- Delayed imaging (about 15 minutes after contrast injection) opacifies the entire urinary collecting system and is optimized for identifying filling defects in the bladder and upper urinary tracts (possible urothelial tumors). Such upper urinary tract filling defects should be further evaluated with ureteroscopy.
CT urography has a reported diagnostic accuracy of 91.5%, sensitivity of 86.3%, and specificity of 92.4%.[73] Its positive predictive value was 63.6%, with a negative predictive value of 97.8%.[73] A magnetic resonance imaging (MRI) urogram should be performed if intravenous contrast cannot be administered due to inadequate renal function or an allergy. If this cannot be done, a non-contrast CT scan with bilateral retrograde pyelograms is recommended.[71] Filling defects in either the ureter or renal pelvis discovered on imaging should be further evaluated by ureteroscopy with biopsies, laser therapy, and/or selective cytology or washings for cytological examination.
MRI is superior to CT scanning for the staging of muscle-invasive disease, with a sensitivity and specificity reported at >90%.[75] MRI can also detect a significant tumor recurrence sooner by identifying early bladder wall neovascularization.[76][77] Newer modifications and improvements such as the Vesical Imaging-Reporting and Data System (VI-RADS), Synthetic Magnetic Resonance Imaging (SynMRI), multi-sequence MRI radiomics, and biparametric MRI techniques may be able to predict muscle invasion and histopathological tumor grade even better.[78][79][80] In general, pelvic lymph nodes >8 mm and abdominal nodes >10 mm in diameter should be considered pathological and consistent with metastasis.[81]
The 18F-fluoro-deoxy-glucose positron emission tomography (FDG-PET) is a test for metastatic urothelial malignant disease and may be helpful for staging before a radical cystectomy in selected patients, preferably in a clinical trial, but it should not be used for anatomical details or diagnostic imaging of the urinary tract and is not beneficial in the initial diagnosis of bladder cancer as its exact role is still being determined.[82][83][84][85][86][87][88] FDG-PET is highly specific for metastatic bladder cancer and provides superior sensitivity compared to CT scans.[86][89][90]
Cystoscopy
Cystoscopy is considered the gold standard for the initial management of bladder cancer, with a reported 98% sensitivity.[67][91] However, this high sensitivity rate has been questioned, suggesting that there may be a role for repeat cystoscopies, photodynamic enhancement techniques, and/or urinary biomarkers, particularly in high-risk or equivocal cases.[92] Diagnostic cystoscopy is usually performed with minimal anesthetic in the office or clinic, but it may also be performed in the operating room simultaneously with a biopsy or transurethral resection.
Non-muscle-invasive bladder cancer typically appears as 1 or more papillary lesions. Cystoscopically, they are frond-like neoplasms with multiple, tiny vascularized projections that tend to bleed easily. The hematuria they produce ultimately results in their early discovery in most cases. They are the most common (75%) forms of bladder cancer, are typically noninvasive, and tend to be low-grade histologically.[1][36] However, if undetected and untreated, they can progress and penetrate the lamina propria, becoming dangerous and muscle-invading, with a risk of metastasizing.
Muscle-invasive bladder cancer may appear as a solid tumor that is flat, sessile, or nodular. These tumors may also have a papillary appearance. They tend to be more dangerous and aggressive than NMIBC as they are more likely to be high-grade.
Cystoscopy has been reported to demonstrate a sensitivity of 92% and specificity of 88% for MIBC, along with a positive predictive value of 72% to 78% and a negative predictive value of 92% to 96%, making it a reasonably reliable method of identifying MIBC.[39][93] However, this does not negate the need for an accurate histological diagnosis and staging of bladder cancers with appropriate biopsies and transurethral cancer resections.
Using this evaluation protocol (CT urogram and cystoscopy), the etiology of hematuria in approximately 57% of patients with asymptomatic microscopic hematuria and 92% of those with gross hematuria will be identified. A malignancy will be found in about 3% to 5% of patients presenting with microscopic hematuria and 23% with gross hematuria.
Carcinoma in situ classically appears as a flat, irregular patch of erythematous mucosa with a velvety or granular surface, which looks very similar to routine inflammation, making cystoscopic diagnosis extremely difficult. Most of the patients with CIS (80%) will present with irritative voiding symptoms, which doubles the risk of finding CIS (from 5% to 10%) in patients with microscopic hematuria.[94][95] However, several other urological disorders can cause these same symptoms, such as urolithiasis, urinary tract infections, and benign prostatic hyperplasia.
Due to its similarity to inflammation, CIS can be easily overlooked on standard cystoscopy. For this reason, any inflammatory bladder lesions in patients with urothelial carcinomas should be considered for biopsy and/or cautery at the time of transurethral bladder tumor resection (TURBT).
Carcinoma in situ (CIS) may be clinically classified as primary, secondary, concurrent, or recurrent as follows:
- Primary: CIS with no prior or concurrent disease
- Secondary: CIS diagnosed during surveillance of patients previously diagnosed with bladder cancer but not CIS
- Concurrent: CIS identified simultaneously with any other bladder cancer
- Recurrent: CIS identified in a patient with a previous diagnosis of CIS
Bladder biopsies should be performed on all papillary and solid bladder neoplasms, as well as any suspicious erythematous lesions. If a TURBT is being performed, all erythematous areas should be considered suspicious and biopsied, along with possible fulguration. Cauterization or fulguration should be done to the biopsy bed and the superficial mucosa around the biopsy or resection site for about a 1 cm radius due to field change disease.
All erythematous and suspicious mucosal lesions should also be biopsied. Random biopsies for mapping should be performed if the cytology is positive or the tumor appears solid or sessile rather than papillary. Biopsies may be performed with cold cup biopsy forceps (flexible or preferably rigid) or transurethral resection. Electrosurgical resection is not preferred for biopsies as it causes cautery artifacts. Every effort should be made to obtain a deep sample, including muscle tissue, for staging, but it is absolutely essential to avoid a bladder perforation, which could spread malignant cells outside the bladder.
Keeping the bladder relatively empty and using continuous flow instrumentation will keep the bladder wall relatively thick and immobile, minimizing the risk of accidental perforation. Tumor and biopsy locations should be carefully recorded as this is associated with progression and prognosis.[13] Bladder cancers at the trigone, bladder neck, or prostatic urethra have an increased risk of nodal involvement and decreased survival.[96][97][98][99]
Cytology, Fluorescence In Situ Hybridization, and Urinary Biomarkers
Cytology, fluorescence in-situ hybridization (FISH), and other urinary biomarkers are not currently recommended by the American Urological Association (AUA) or European Association of Urology (EAU) guidelines for the routine initial evaluation of patients with hematuria due to their high cost and relatively poor specificity.[36][47][67] While they can be useful for monitoring progression in patients with known disease and borderline or equivocal situations, they are not considered an adequate substitute for cystoscopy.[36] Urinary biomarkers may also be useful in patients with persistent microscopic hematuria after a negative workup, particularly if they also have high-risk factors for CIS and/or irritative voiding symptoms.[67] Urine-based biomarkers can potentially reduce unnecessary referrals and cystoscopic procedures, but none has yet reached a level of clinical reliability to replace cystoscopy.[100][101][102]
Urine cytology has been recommended for high-risk patients with gross hematuria. Some healthcare professionals will also order it routinely for high-risk microhematuria patients, particularly those with a history of 10 or more pack-years of smoking. Cytology may also be useful in detecting and following CIS, which may be easily missed during cystoscopy.[67][103] Cytology is quite reliable when positive, but it lacks sensitivity and will miss many malignancies, especially low-grade neoplasms. It is also highly dependent on the cytopathologist's skill and experience.[104]
Cytology is considered a valuable tool for the follow-up of patients with high-grade bladder cancer and, especially, CIS of the bladder.[30][46][105] A large review of 36 separate studies with over 14,000 patients showed the sensitivity of urinary cytology to be only 44% with a high specificity of 96%.[106] The first morning voided urine is unsuitable for cytological evaluations due to cytolysis.[47] Cytology diagnostic reports should indicate the specimen's adequacy and the presence of high-grade urothelial carcinoma, atypical cells, or "suspicious" findings for high-grade cancer.[13]
Bladder washing for cytology is typically obtained during cystoscopy using normal saline. With the bladder emptied by a catheter, 50 mL to 100 mL of normal saline is instilled and then immediately extracted using a catheter tip syringe. This procedure is repeated 3 to 6 times. Bladder washing provides larger numbers of well-preserved urothelial cells than standard voided urine cytology and detects more urothelial cancers.[107][108] Washing can be performed immediately after TURBT to identify any invisible high-grade tumor and is often preferred in the follow-up of high-risk cancer cases, particularly in patients with CIS.[109][110] Cytology and bladder washing may be performed together, with the voided cytology collected first. Labeling the specimens correctly to avoid any potential confusion for the cytopathologist is important.
Fluorescence in-situ hybridization (FISH) is a relatively sensitive genetic test marker for bladder cancer that detects aneuploidy of chromosomes 3, 7, and 17 and the loss of the 9p21 locus.[111] Results are reported as either positive or negative, which makes interpretation easy. If the FISH test is positive, an increased frequency of surveillance is recommended, and further evaluation of the ureters and upper tracts bilaterally should be considered.[36]
- About 27% of patients with NMIBC on surveillance with negative cystoscopies will have a positive FISH test, and two-thirds of these will develop recurrent bladder cancers within the next 2 to 3 years.[112]
- A systematic review and meta-analysis reported the overall sensitivity and specificity of FISH testing as 68% and 64%, respectively.[113]
- The FISH test is most useful for monitoring bladder cancer, especially after bacillus Calmette-Guerin (BCG) therapy, and in the initial diagnosis of high-risk
- A positive FISH test following BCG therapy is suggestive of an impending bladder cancer recurrence, particularly within the following 6 months.[114][115][116][117] Close follow-up of such patients is therefore recommended.[114]
- The FISH test has a significant rate of false negatives for bladder cancer and is expensive, which limits its clinical utility.
Various other biomarkers for bladder cancer are available. They are primarily used to monitor the response to BCG therapy and where cystoscopy or cytology findings are equivocal or atypical, particularly in high-risk patients. Overall sensitivity ranges from 58% to 82%, and specificity from 78% to 88%.[36]
Genetics-based urine testing, using urinary mRNA, is a promising urinary biomarker for recurrent bladder cancer.[118][119][120] Preliminary results from a 2-year trial of 313 bladder cancer patients suggest that surveillance cystoscopies can be safely reduced by 54% using a new genetics-based urine test. The new urine test identifies 5 separate genes and identified bladder cancer recurrences earlier than cystoscopy.[121] While preliminary, this data appears quite promising, but it will require publication of the final trial results, independent confirmation, and additional testing before it can become clinically accepted and available.
Similarly, molecular markers for MIBC are still in the investigational stage and are not appropriate for use outside of a clinical trial.[13]
Enhanced cystoscopic diagnostic techniques use either drug-induced cystoscopic tumor cellular fluorescence or special image-processing techniques to help identify pathological urothelial tissue during cystoscopy.[122][123][124] These are very sensitive techniques for detecting CIS of the bladder and otherwise invisible superficial urothelial malignancies, reducing recurrences through better identification and elimination of affected urothelium, but their precise role is not yet clearly defined.[123][125][126] Overall, enhanced cystoscopic diagnostic techniques are significantly underutilized.[127]
The AUA and EAU guidelines now recommend photodynamic cystoscopic enhancement, where available, at the time of initial therapy for NMIBC, at the first 3-month surveillance cystoscopy and thereafter for the first 2 years, prior to intravesical chemotherapy, and in cases where there is a new finding of positive cytology or abnormal/equivocal cystoscopic findings.[36][47]
- Photodynamic cystoscopic diagnostic enhancement, also called "blue light" cystoscopy, uses 5-aminolevulinic acid (5-ALA) or hexylaminolevulinate (HAL) to help identify and visualize malignant tissue changes in the bladder mucosa.[124] This greatly enhances the diagnosis and localization of CIS of the bladder.[128][129][130][131] These medications cause the preferential accumulation of photoreactive porphyrins in malignant bladder mucosal urothelial cells. These become visible under blue cystoscopic light as the porphyrins fluoresce red, while normal tissue appears blue.[124]
- 5-ALA or HAL is instilled into the bladder 2 hours before the cystoscopy.[131]
- During the cystoscopy, blue light (380 to 440 nm) is used, and any malignant areas will fluoresce red.[132][133][134][135] These highlighted areas can then be selectively biopsied, cauterized, or resected.
- In 1 study, 5-year cancer-specific survival was improved over white light cystoscopy from 74% (with white light) to 91% using HAL and blue light.[136]
- False positives from photodynamic-enhanced diagnostic cystoscopy may be seen when 5-ALA or HAL is taken up by mucosal cells due to localized inflammation, recent transurethral surgery, or intravesical therapy.[137][138][139]
- Photodynamic cystoscopic enhancement is now available and FDA-approved for flexible cystoscopy in the office or clinic.
- Bladder urine can cause a green discoloration, and visualization in recorded images may be "foggy" due to hardware or irrigation fluid issues. Advanced image processing techniques can correct these.[140]
- Narrow-band imaging is a technique to enhance the cystoscopic visualization of malignant bladder urothelium through enhanced digital image processing. Narrow-band imaging does not require fluorescent drugs but instead uses special image processing of bandwidths 415 and 540 nm (blue and green). This improves the identification and visualization of abnormal urothelial tissue but requires special equipment.[141][142][143] Overall results are similar to photodynamic cystoscopic enhancement with fluorescing drugs.
Treatment / Management
The treatment strategy for urothelial cancer depends on whether there is histological evidence of muscle invasion (eg, therefore, it is either NMIBC or MIBC). This initial staging is determined after an initial biopsy and/or TURBT.
Transurethral resection of a bladder tumor is both diagnostic and therapeutic. Smaller tumors may be removed with cold cup biopsy forceps, which is preferred due to the preservation of the histology without any cautery artifact. Transurethral resection is reserved for larger tumors. Attaining hemostasis is important as active bleeding will interfere with endoscopic visualization. However, unnecessary cautery should be avoided to minimize coagulation artifacts in the tissue specimens.
A complete cystoscopic inspection of the bladder and urethra should be performed, with all lesions, masses, and erythematous patches carefully noted. While the patient is under full anesthesia, a bimanual examination should be performed to determine if there is any indication of a palpable mass or any evidence of possible tumor fixation to the pelvic sidewall or other structures.[144][145] A standard TURBT should contain at least 3 separate specimens: the main tumor, a deep biopsy from the tumor base if any muscle invasion might be present, and a random biopsy sample from "normal" bladder mucosa.[1][13][36][47] In addition, any abnormal mucosal areas should be biopsied with their location carefully documented and sent to pathology separately.[1][13][36](B3)
Possible involvement of the prostatic urethra has been reported in up to one-third of men with bladder cancer.[146][147][148][149][150] This risk is increased for patients who have a tumor located at the bladder neck, in the trigone, or if the patient has multifocal disease or CIS.[151][152][153]
TURBT tips include the following:
- The bladder should not be overstretched or overfilled, as this will attenuate the bladder wall thickness; should be filled enough so all major bladder folds are flattened to avoid overly deep unintended trauma and possible perforation.
- Continuous-flow instrumentation is recommended to minimize the motion of the bladder wall during resection. Adjust the flow rate to the minimum necessary to maintain a constant level of filling.
- The resection motion should generally be straight, towards the operator, and without actively angling the scope laterally.
- One helpful technique is to place the end of the resectoscope loop under the far edge of the tumor and gently lift it away from the bladder wall before electrifying the cutting loop to perform the resection. This will help minimize unintentional bladder wall damage.
- If there are multiple or large tumors, start resecting at the edge, where there will be complete visualization.
- The goal is to complete the resection in 1 area or 1 tumor at a time until it is flush with the rest of the bladder wall. At that point, consider using a cold cup biopsy for tissue samples and try to avoid an inadvertent perforation.
- Do not resect in a bladder diverticulum, as these areas do not have a muscle layer, and perforation may easily occur; careful fulguration may be used instead. A partial cystectomy may be appropriate in some situations.
- Regardless of the technique, a deeper biopsy, including muscle tissue, is recommended for staging. This deeper biopsy is usually sent as a separate specimen for identification.
- TURBT will fail to identify muscle invasion in about 30% of cases, usually due to inadequate tissue sampling or problems with postsurgical processing, handling, or analysis.[39][154][155]
- A separate biopsy of the edge of the tumor should also be sent for a separate pathological examination if muscle invasion is suspected.
- There is evidence that laser ablation and bipolar resection are superior to monopolar transurethral resection for primary NMIBC. (Laser ablation does not yield any tissue for pathology.)
- Larger tumors may be resected in stages, starting with the endoluminal portion. The tumor should be resected until it is flush with the neighboring bladder wall. Deeper resection should be performed cautiously, and the specimen should be sent separately to help pathology with staging.
- Superficial cauterization should be done to the bladder mucosa for about a 1 cm margin around the base of any resected tumor.
- If available, enhanced cystoscopic techniques ("blue light" cystoscopy or narrow-band imaging) should be performed during the TURBT to enhance disease detection and allow for optimal therapy of all involved bladder mucosa.
- A biopsy should be taken from any abnormal urothelium at the time of the TURBT.
- Random mapping biopsies should be performed even from normal-appearing mucosa if the cytology is positive or the neoplasm is non-papillary.
- Any erythematous or suspicious areas should be biopsied and superficial cauterized, especially if highlighted by "blue light" fluorescence or narrow-band imaging.
- At the end of the procedure, the bladder should be thoroughly examined for any signs of a possible perforation, remaining tumor, or tumor fragments. Use the extra time to continue irrigation to facilitate the removal of as many floating malignant cells as possible.
An en-bloc resection may also be done endoscopically and is preferred when technically feasible. This technique removes the entire tumor, along with surrounding stromal tissue and some underlying detrusor muscle, as a single specimen. A systemic meta-analysis has shown that en-bloc resection facilitates the pathological examination, decreases the incidence of inadvertent perforation, reduces the incidence of finding residual tumor on repeat resections, and lowers the risk of perforation.[156][157](A1)
The obturator reflex is a sudden, violent spasm of the adductor thigh muscles from unintended obturator nerve stimulation during TURBT. This stimulation causes the patient to experience a sudden and severe jerk of the hips and thighs without warning. This unexpected violent motion can easily result in an inadvertent bladder perforation during TURBT.[158]
The obturator nerve passes close to the inferolateral wall of the bladder and bladder neck. During transurethral bladder surgery, electrosurgical resection or cautery (both monopolar and bipolar) can cause unintended stimulation of the nearby obturator nerve, resulting in an unintended reflex.[158] Minimizing electrosurgical current levels and avoiding bladder overdistension will also help reduce the risk of an inadvertent obturator reflex.[159] Using bipolar resection is thought to help as well, but studies have failed to show a consistent benefit.[159][160]
Effective elimination of the obturator reflex can be done by neuromuscular blockade during general anesthesia or using an obturator nerve block.[158][161] Spinal anesthesia alone will not block the obturator reflex.[159] Performing an obturator nerve block when using spinal anesthesia for a TURBT will reduce the incidence of obturator reflex reactions and bladder perforations.[162][163](A1)
Intravesical instillation of a chemotherapy agent (usually mitomycin C [40 mg/20 mL sterile water], gemcitabine [2000 mg/50 mL normal saline], or epirubicin [50 mg/50 mL normal saline]) immediately after a TURBT (within 24 hours) has been shown to reduce the incidence of urothelial cancer recurrence and progression significantly.[164][165][166][167][168][169][170][171] TURBT surgery releases tumor cells, which can be implanted elsewhere in the bladder.[174][175][176] This process can be impeded by a single intravesical chemotherapy bladder instillation within 24 hours of the resection.(A1)
Intravesical instillation should not be performed if there is a known or suspected bladder perforation, active bleeding, continuous irrigation, or if the resection is particularly extensive.[36] Gemcitabine is typically the preferred agent as it is equally effective but has been associated with fewer adverse events than other agents.[36]
Techniques for enhancing the effectiveness and pharmacodynamics of this intravesical chemotherapy include the following:
- The pH should be lowered. This can be accomplished by oral sodium bicarbonate starting the night before surgery, preoperatively, and intravenously during the TURBT procedure.
- The duration of the intravesical therapy should be at least 1 hour. If possible, a longer dwell time of 2 hours is suggested.
- Urine production during the instillation period should be minimized. Intraoperative and postoperative IV fluids should be minimized to limit urine production for the first 2 hours after surgery and avoid dilution of the intravesical chemotherapeutic agent.
- A high concentration of the intravesical chemotherapeutic agent should be used.[172][173][174] (A1)
A single intravesical chemotherapy instillation after TURBT has been shown to reduce the recurrence rate from 10% to 15% when compared to TURBT alone in a number of studies, including 3 separate meta-analyses.[175][176][177][178][179][180][181] Results from a single trial of combination intravescial chemotherapy with mitomycin C and epirubicin showed a relative risk reduction of 31%.[182] While promising, this result requires further testing for confirmation.[180](A1)
The rationale for this treatment is that during TURBT surgery, numerous malignant cells are released into the bladder and may implant on the bladder wall. They become quickly covered by extracellular matrix but are initially susceptible to intravesical agents.[183] Preparing a bladder diagram of tumor locations is also recommended.[165](B2)
Operative reports of TURBTs should optimally contain all of the elements as follows:
- Describe the number, location, and estimated size of the bladder tumors (single largest diameter and aggregate size).
- Describe the American Joint Commission on Cancer clinical stage (cTa, cT1, cTis, cT2, cT3, cT4).
- Describe tumor morphology (nodular, solid, papillary, flat, sessile) .
- Indicate if this was a primary or recurrent tumor.
- Indicate if there was any evidence or suspicion of CIS.
- Indicate if any immediate postoperative intravesical chemotherapy was used, the drug used, the indwelling duration, and the dose.
- Indicate if the resection was visually complete and if a deeper biopsy was performed.
- Report any random biopsies taken and from where.
- Report findings from the bimanual examination.
- Report if there was any evidence of perforation or if no such perforation was performed.
- Report the results of a complete cystoscopic evaluation of the bladder and urethra.
- Report whether any muscle was visually resected.[184][185] (B2)
Repeat or "second look" TURBT is often recommended within 6 weeks of the original resection, as indicated below. Care should be taken to avoid bladder perforations, but underlying muscle tissue needs to be present in the specimen. Repeat or "second look" TURBTs have been shown to increase recurrence-free survival, improve outcomes from BCG therapy, and provide important prognostic information if performed within 6 weeks of the original surgery.[186][187][188][189][190][191](A1)
Indications for a "second look" TURBT include the following:
- High-grade superficial papillary (high-grade Ta) or any invasive carcinoma into the submucosa or lamina propria (any T1), even if bladder muscle was found in the original specimen
- Highly multifocal tumor
- Incomplete initial resection of the bladder tumor and/or visible tumor left behind
- Large bladder tumor (defined as >3 cm) [36]
Such second-look TURBTs have found residual cancer in half of all Ta cancers, resulting in upstaging in 15% of cases. These procedures also found persistent tumor in about half of the T1 lesions (48%), and 30% were upstaged to include muscle invasion.[36] (Cancer risk staging for low-, intermediate-, and high-risk NMIBC malignancies can be found below in the "Postoperative and Rehabilitation Care" section)
Non-muscle-invasive bladder cancer is managed primarily with transurethral (endoscopic) resection, which includes all Ta and T1 lesions. This procedure may be followed by risk-based intravesical therapy, like Bacillus Calmette-Guérin (BCG). CIS is usually treated with BCG as first-line therapy.
Low-risk bladder NMIBC malignancies will do well with a single intravesical chemotherapy instillation and routine surveillance cystoscopy.[36] (See surveillance protocol below in "Postoperative and Rehabilitation Care.") Additional intravesical chemotherapy installations have not shown any additional benefit beyond the first, single, initial treatment for low-risk malignancies.[192][193][194][195] Low-risk, noninvasive malignancies rarely metastasize, so a cystectomy is rarely indicated. Intravesical chemotherapy or BCG can be considered in selected cases.(A1)
Intermediate-risk NMIBC urothelial cancers should be considered for a 6-week induction course of Bacillus Calmette–Guérin (BCG) immunotherapy or intravesical chemotherapy with mitomycin C, doxorubicin, or epirubicin.[36] All meta-analyses have shown reduced recurrences using such therapies, with BCG and mitomycin showing the best overall results.[36] Given the difficulty in obtaining BCG due to shortages, its increased adverse effects, and complications, intravesical mitomycin is usually preferred for intermediate-risk disease.[36]
Mitomycin C intravesical treatment efficacy can be enhanced by the following:
- No oral fluids for 8 hours prior to the instillation
- Urinary alkalinization therapy (1.3 g sodium bicarbonate by mouth taken the evening prior to, the following, and one-half hour before the instillation
- Complete evacuation of the bladder just prior to the instillation. (Checked by a bladder scanner or a quick urethral catheterization)
- High concentration of mitomycin C (40 mg in 20 mL sterile water)[172] (A1)
Intermediate-risk patients who have a complete response to an initial induction course of intravesical chemotherapy may be considered for maintenance BCG therapy for 1 year.[36]
High-risk NMIBC malignancies, including CIS, are best treated after TURBT, with a 6-week course of BCG induction (1 dose weekly for 6 weeks), which has been shown to provide superior results to all other immuno- or chemotherapies.[196][197][198][199] Transurethral resection and/or fulguration is of limited value in CIS of the bladder due to the extent of the disease, so BCG is considered first-line therapy.[38][200] Treatment of squamous cell carcinoma of the bladder is primarily surgical, as radiation, chemotherapy, and combined chemoradiation therapy do not appear to provide an overall survival benefit.[53](A1)
Bacillus Calmette–Guérin is a weakened form of Mycobacterium bovis (cowpox). Initially designed as a vaccine for tuberculosis, it is now also used as an immunotherapy for high-grade urothelial cancer.[117] Compared to chemotherapy, BCG has been shown to decrease disease recurrences and reduce progression (up to 37% compared to no BCG therapy) of high-grade urothelial carcinoma. The mechanism of action of BCG against tumor cells is incompletely understood. Originally, it was thought that the immune response to BCG was cross-reacting with bladder tumor cell antigens, but this simplistic mechanism has been largely disproven. Now it appears that BCG has a direct toxic effect on high-grade bladder cancer cells and a local immune-stimulating effect, which allows easier identification of BCG-affected tumor cells by the body's natural anti-tumor cellular defenses (macrophages, natural killer cells, neutrophils, and T lymphocytes.)[201][202](B3)
BCG initially attaches to malignant urothelial cells due to a unique cellular fibronectin protein interaction with the bacteria, after which the BCG becomes internalized. Once inside the tumor cell, the BCG causes an increase in intracellular cytokine production (granulocyte-macrophage colony-simulating factor, interferon-γ, interleukins 1, 2, 6, and 8, and tumor necrosis factor).[38][203] Nitric oxide production may also be increased, which further slows tumor growth.[204][205](A1)
For optimal efficacy from BCG bladder instillations, the following conditions are required:
- The patient is not immunocompromised.
- There is a relatively small tumor burden.
- Direct contact of the BCG with the tumor is possible (superficial disease).
- The dose used is sufficient to generate an immunological reaction.
- There is sufficient BCG available to complete the course of therapy.
BCG is particularly useful in CIS, where it is relatively easy to meet the above criteria as the tumor is completely superficial. In such cases, complete eradication of CIS is possible in 70% or more of these patients.[206] Standard treatment is 6 weekly installations, followed by 3 weekly installations at 3 months and 6 months, then every 6 months afterward for maintenance therapy, which should be continued for 3 years.[207][208][209] Recently, an alternate protocol utilizing 12 weekly induction BCG installations with no maintenance therapy has shown good efficacy; it utilizes fewer treatment vials per patient, making this treatment modality more efficient when BCG is in short supply.[210](A1)
There does not appear to be any significant difference between different BCG strains or dosage amounts, except that adjustments should be made as needed to maintain efficacy and tolerability.[211] Reduced dosages (ie, one-half, one-third) still appear to maintain efficacy, but there is no evidence that combination therapy with other chemotherapy agents improves the results.[36] If recurrent or persistent disease is found after completing the initial 6-week course of BCG, a second induction course may be given.[36]
Recurrent or persistent disease and/or positive cytologies after full intravesical BCG therapy suggests undetected disease. The upper urinary tracts should evaluated with contrast-enhanced imaging along with upper tract cytologies, and a prostatic urethral biopsy should be considered before administering further intravesical therapy.[36]
- Recurrent high-grade Ta disease should be managed by TURBT and intravesical therapy. A radical cystectomy should not be considered until such therapies have been tried and failed.[36]
- For recurrent high-grade T1 disease after an initial 6-week course of BCG, a radical cystectomy should be considered in eligible patients.[36]
- Recurrent high-grade urothelial cancer (CIS or NMIBC) within 6 months of starting BCG therapy constitutes a BCG failure, and additional immunotherapy should not be given.[36] The patient is then a candidate for radical cystectomy or alternative intravesical chemotherapy.[36]
- Recurrent high-grade urothelial cancer (CIS or NMIBC) within 1 year of completion of appropriate and adequate BCG therapy is an indication for a radical cystectomy. If the patient does not want such surgery or is otherwise unfit, alternative intravesical chemotherapy protocols may be used, but a clinical trial is recommended if possible.[36]
Expected symptoms from BCG therapy include dysuria, urinary frequency, hematuria, urgency, general malaise, and fever.[212] Such symptoms typically develop within 48 hours of BCG instillation and are often dose-related.[213][214] The dose of BCG may be reduced in patients who do not tolerate it well. Often, the morbidity and severity of the adverse events can be minimized by reducing the BCG dosage. Still, about 8% of patients will not be able to tolerate BCG treatment due to adverse effects.[212](B3)
BCG-osis, also known as disseminated BCG, occurs in less than 1% of patients receiving BCG therapy. This condition may present with mild flu-like symptoms or as a major systemic septic infection, ultimately proving fatal in some cases.[213][215] BCG-osis may occur years after the last BCG instillation, so clinicians should maintain a level of suspicion even long after BCG therapy has ceased.(B3)
Severe cases are quite rare at only a fraction of 1% and are thought to be due to disseminated BCG-related sepsis and/or hypersensitivity to Mycobacterium bovis.[216] These cases may present with mental status changes, chills, high-grade fevers, granulomatous prostatitis, arthritis, pneumonitis, granulomatous epididymo-orchitis, respiratory failure, hypotension, liver failure, jaundice, hepatitis, and coagulopathies.[217] Negative blood cultures do not exclude the diagnosis, as BCG-osis is still found in about 40% of such cases.[217] Treatment involves antituberculosis medications (ethambutol, isoniazid, and rifampicin) and oral steroids.[213] Antituberculosis treatment is typically continued for at least 3 to 6 months.(B3)
Therapy for BCG failures
Radical cystectomy should be the primary option for consideration in patients who are surgical candidates with tumors refractory to conservative management such as BCG therapy.[36]
Nonsurgical alternatives include the following:
- Pembrolizumab, an alternative IV immunotherapy, may be considered for patients with recurrent CIS that returns within a year of completing BCG therapy.[36]
- Valrubicin has been US Food and Drug Administration (FDA)-approved for intravesical therapy in CIS patients who fail BCG therapy and cannot or will not undergo a cystectomy. However, the complete response rate is only 18%.[218]
- Nadofaragene is another FDA-approved intravesical therapy for patients with CIS who are not responding to BCG therapy.[219] Early data from phase III trials indicates an initial complete response of just over 50% at 3 months, and about 45% of these patients maintained their response for 1 year.[219]
- Nogapendekin alfa inbakicept plus BCG was recently FDA-approved as intravesical therapy for treating high-risk BCG-unresponsive CIS of the bladder. This approval was based on a study of 160 patients with BCG-unresponsive CIS in which the complete response rate was 71% with a median response duration of over 2 years and cancer-specific overall survival of almost 100%.[220] Cystectomy was avoided for over 90% of patients in this very high-risk group during the 2-year follow-up period.[220]
- Experimental treatments under investigation include intravesical gemcitabine, gemcitabine alternating with mitomycin or docetaxel, and intravesical paclitaxel.[36][221][222]
- Other investigational therapies include immune checkpoint inhibitors, oncolytic virus regimens, recombinant fusion proteins, immune modulation therapies, cytotoxic agents (cabazitaxel, cisplatinum, and gemcitabine,) and targeted small molecule kinase inhibitors.[36][223] (A1)
A systematic review of the currently available treatment modalities available for patients who fail BCG who are not candidates for cystectomy suggested the following protocol as follows:
- For CIS only, intravesical nadofaragene, CG0070 adenovirus, or mitomycin C hyperthermic intravesical chemotherapy
- For CIS with Ta or T1 disease, intravesical nogapendekin alfa inbakicept plus BCG, intravesical radiofrequency-induced chemohyperthermia, or electromotive mitomycin C administration
- For Ta or T1 disease, intravesical nogapendekin alfa inbakicept plus BCG, intravesical hyperthermic chemotherapy with epirubicin, or electromotive mitomycin C followed by mitomycin C chemohyperthermia
- If the above treatment fails, then combination chemotherapy with cabazitaxel, cisplatin, and gemcitabine is suggested.[219][220][224] (A1)
Due to BCG shortage issues, the National Comprehensive Cancer Network has recommended adjustments as follows:
- BCG should be prioritized for CIS and high-grade T1 cancers, particularly at 3 and 6 months after induction.
- Induction and maintenance dosages may be reduced (one-half or one-third) to allow for more treatments from a single vial.
- Alternative intravesical agents may be used (mitomycin C, gemcitabine, etc).
- Consider early radical cystectomy in patients at high risk for recurrence or progression.
Intravesical gemcitabine for an hour, followed by docetaxel for 2 hours, has been successfully used as an alternative to BCG therapy and for BCG failures.[221][225][226] It is associated with reduced adverse effects, fewer treatment discontinuations, better tolerability, and equal or greater efficacy (as defined by progression-free, cancer-specific, and overall survival) than BCG treatments.[221][225][226]
Long-term analysis has demonstrated that intravesical gemcitabine followed by docetaxel for high-risk NMIBC patients who failed BCG therapy provided a 5-year cancer-specific survival of 91%, and 75% of patients were able to avoid cystectomy.[222] These results indicate that intravesical gemcitabine combined with docetaxel therapy may be reasonably considered an alternative to BCG treatment for suitable high-risk bladder cancer patients.[225]
Chemohyperthermia (heated intravesical chemotherapy, HIVEC), electromotive drug administration (EMDA), and radiofrequency-induced thermo-chemotherapy (RITE) are promising technologies designed to improve bladder wall penetration of intravesical chemotherapeutic agents. Still, they are not yet recommended by guidelines due to insufficient clinical data and evidence.[227][228][229][230] However, they are not unreasonable to use where available during BCG shortages.[230](A1)
An abstract from the 2023 European Society of Medical Oncology (ESMO) by Necchi and associates described their early results with a unique investigational implantable drug delivery device (TAR-200) allowing for the sustained delivery of intravesical chemotherapy to the bladder for patients with high-risk BCG-unresponsive CIS. This intravesical device using gemcitabine has shown a complete response rate of 77%, of which over 90% have been maintained over a median of 48 weeks. It is now being tested in combination with cetrelimab, an investigational programmed cell death receptor-1 (PD-1) monoclonal antibody. Further studies are needed to determine and verify the duration and optimal usage of such therapies.
Cretostimogene is a unique investigational oncolytic immunotherapy that has produced durable complete response rates of more than 75% lasting over a year in patients with BCG-unresponsive CIS. These preliminary results are quite encouraging.
MUSCLE-INVASIVE BLADDER CANCER
MIBC accounts for approximately 25% of all primary bladder cancer cases.[13][30] Once a malignant bladder tumor invades the detrusor muscle, even superficially, there is a markedly increased rate of metastases and reduced life expectancy as the overall 5-year recurrence-free survival rate is only about 50%.[30][31][231]
Staging is done with CT or MRI urogram imaging plus a chest x-ray or CT.[30] Despite advances in imaging technology, upstaging after surgery due to unanticipated extravesical disease is found in as many as 40% of cases.[232][233][234] Preoperative imaging that demonstrates hydronephrosis has been found to be a strong predictor of extravesical disease and a more guarded prognosis.[233][234](A1)
Involvement of the prostatic urethra may be found in as many as one-third of all patients with MIBC.[146][148][149] The risk is highest with tumors located in the trigone or bladder neck as well as in patients with CIS, positive cytologies without visible lesions, and in multifocal disease.[151][152][153] A prostatic urethral biopsy involves a limited transurethral prostate resection from the bladder neck to the verumontanum between 5 and 7 o'clock.[13] Any abnormal appearing urethral tissue should also be biopsied.[13]
The initial management of MIBC is transurethral (endoscopic) resection, but it will require further therapy once it has been staged as a T2 or higher lesion. Higher-stage lesions are managed with local palliative therapy and as metastatic cancers (see "Medical Oncology" section)
Treating MIBC without metastases is generally neoadjuvant cisplatin-based neoadjuvant chemotherapy followed by radical cystectomy with bilateral pelvic lymphadenectomy.[13][235] In men, removal of the prostate and seminal vesicles is usually included, especially if urethral or prostatic involvement is demonstrated or suspected. In women, the uterus, cervix, ovaries, and anterior vaginal wall are usually removed.[235] Perioperative pharmacologic thromboembolic prophylaxis is recommended for patients undergoing radical cystectomy surgery.[30] There does not appear to be any difference in overall complications, progression-free survival, or overall survival between robotic and open surgical cystectomies.[236][237][238][239][240](A1)
Indications for a radical cystectomy include the following: [13][235]
- MIBC with no known nodes or metastases
- Very high-risk NMIBC (Lymphovascular invasion, variant or divergent histology, extensive lamina propria involvement, large (>5 cm) high-grade T1 tumors, persistent or unresectable malignant tissue after repeat TURBT, and prostatic urethral involvement)
- CIS not responding to BCG
- Extensive papillary tumors are not controllable with TURBT and intravesical therapy
Salvage cystectomy should be considered in the following: [13][30]
- Recurrence after bladder-sparing procedures
- Palliation for intractable pain, unmanageable hematuria, or extensive fistula formation
The limits of dissection for the lymphadenectomy are as follows:
- Distal: Node of Cloquet (The uppermost of the deep inguinal nodes, the node of Cloquet, is located under the inguinal ligament and is the lowest of the external iliac lymph nodes.)[241][242][243][244]
- Inferior: Internal iliac lymph nodes and the pelvic floor
- Lateral: Genitofemoral nerve
- Posterior: Sacrum
- Proximal: Up to the bifurcation of the iliac arteries is standard, but the dissection may be extended to the aortic bifurcation. Further extension up to the inferior mesenteric artery (superextended dissection) does not appear to offer any additional survival benefit and is not recommended. (A1)
An extended dissection may also include the presacral lymph nodes and the triangle of Marcille (medial edge of the psoas major, lateral border of the vertebra, and the iliolumbar ligament below. The obturator nerve passes through this area.)[245] Currently, most surgeries use the standard template, and the benefits of extended dissections are unclear.[246][247][248](A1)
Neoadjuvant therapy for MIBC has been proven beneficial as demonstrated in results of several large randomized clinical trials and systematic meta-analyses.[249][250][251][252][253][254][255] Neoadjuvant chemotherapy for bladder cancer typically consists of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC).[30] These medicines are usually given now in a dose-dense (ddMVAC) manner where the chemotherapy is combined with growth factors.[13][30] This technique is better tolerated, is less likely to be interrupted by adverse effects, requires a shorter course of therapy, and may provide a slight improvement in cancer control compared to standard MVAC treatment.[256][257] (A1)
The combination of gemcitabine and cisplatin appears to be an acceptable, safer, and more tolerable substitute for MVAC, but additional studies are needed for definitive confirmation.[258][259][260] Patients who responded well to neoadjuvant chemotherapy tend to have better outcomes and improved overall survival, especially if they are complete responders.[261][262](A1)
Adjuvant chemotherapy is given after surgery and reserved for patients with a high recurrence risk. This would include patients with extravesical disease and those with positive lymph nodes at the time of surgery who did not receive preoperative chemotherapy. Nivolumab, an immune checkpoint inhibitor, is also FDA-approved for adjuvant therapy in patients with urothelial bladder cancer at high risk for a recurrence who refused, could not tolerate, or were ineligible for prior chemotherapy.[263][264] Unlike neoadjuvant chemotherapy, there is no definitive evidence of any significant benefit to adjuvant chemotherapy. However, adjuvant radiation therapy may help with local control and disease-specific survival but has not demonstrated any overall survival benefit when used alone. Fiduciary markers can help target radiation therapy.(B3)
Urinary reconstruction after radical cystectomy is typically a choice between an ileal conduit, continent cutaneous reservoir, or an orthotopic neobladder. The advantages and disadvantages of each of these should be discussed with patients prior to surgery.[13][30] Patients and their families should be thoroughly taught how to manage their chosen urinary diversion drainage system.[30]
- The ileal conduit remains the most popular urinary diversion after a cystectomy, most likely due to its relative simplicity. A short section of the distal ileum is used to passively pass urine from the anastomosed ureters at the proximal end through a distal stoma on the abdominal skin surface. While technically easier to perform, its associated complications are frequently underappreciated. These include hyperchloremic, hypokalemic metabolic acidosis as well as long-term deterioration of the renal units over time, ureteral strictures, stenosis of the stomal opening, frequent urinary tract infections, stomal hernias, skin irritation and ulceration, and urostomy bag leakage problems. Leaving the terminal 15 cm of the distal ileum can minimize problems of impaired absorption of bile salts and fat-soluble vitamins, particularly vitamin B12.[30][265]
- Continent cutaneous (Indiana pouch, Miami, and similar) urinary diversions create a continence reservoir, usually from the right colon, and the ileocecal valve is used as a continence mechanism. The ileal segment may need to be tapered, and the ileocecal valve may require plication or reconstruction to maintain continence. The ileal stomas open onto the abdominal skin surface and are drained periodically by self-catheterization. Variations include using ileal segments to form the reservoir and the appendix as a ready-made continence mechanism and catheterizable access point. Continence rates are reported as high as 90%.[266][267] The Kock reservoir has been largely abandoned due to its technical complexity and unacceptably high complication/reoperation rate.
- Continent orthotopic neobladder diversions require a clear margin at the urethral margin. If this is positive on a frozen section, a neobladder is absolutely contraindicated. Neobladders are constructed from a tabularized intestinal segment, usually the ileum, which is folded back on itself to minimize any contractions. The ileum is usually selected because of its reduced complication rate and superior mobility, which means it can easily reach the urethra without tension. The Studor, Hartmann, and Y-pouch ileal orthotopic neobladder techniques are frequently utilized.[268][269][270] Daytime continence rates of 85% have been reported.[271]
All of these urinary reconstruction methods are associated with many possible complications, such as hyperchloremic, hypokalemic metabolic acidosis, as well as kidney failure, decreased bone density, chronic diarrhea, vitamin B12 deficiency, calcium oxalate nephrolithiasis (from enteric hyperoxaluria), and hyperammonemia. Overall, patient satisfaction and quality of life scores are similar between the various urinary reconstructive surgical options, although a few studies indicated marginally higher quality of life scores with neobladders.[272][273][274][275][276](A1)
Bladder preservation techniques may be considered in selected patients with MIBC who are not candidates for cystectomy or who refuse such treatment.[30]
- A partial cystectomy may be considered in carefully selected patients with a solitary tumor without CIS or a malignancy in a bladder diverticulum that TURBT cannot completely or safely remove. A lymph node dissection is recommended in patients undergoing a partial cystectomy. Patients with isolated tumors of the bladder dome would be the best candidates for this treatment option. Overall, 5-year survival is reported to be about 70%.[277]
- Maximal or radical transurethral resection has been recommended as a treatment for selected patients with MIBC who are not candidates for a radical cystectomy. Essentially, a repeat TURBT is performed until no residual tumor is left. Enhanced cystoscopic detection techniques are suggested to maximize the results. Mandatory regular upper tract imaging with surveillance cystoscopies is required due to the high rate of local recurrence. Understaging is common with this treatment modality. Five-year survival rates have been reported as only 30% in an older series.[278]
- Maximal transurethral resection of the bladder tumor with chemoradiation ("trimodal therapy") has been reported to achieve a 5-year survival of 60% to 76%. This therapy appears to be the best alternative to radical cystectomy in selected patients based on the limited available data.[279] This data makes it comparable to the 5-year overall survival (73%) in patients who underwent radical cystectomy surgery.[280] Good long-term studies are not available. The AUA and EAU guidelines, therefore, recommend trimodal and multimodal bladder-preserving therapy for selected patients unable or unwilling to undergo radical cystectomy and who clearly understand the risks and the mandatory need for careful follow-up.[13][30]
About 30% of patients who undergo bladder-preserving therapy will develop a recurrence of their muscle-invasive disease.[281] These patients should be offered radical cystectomy surgery.[30] They will experience a higher recurrence rate if treated with local therapy (TURBT, etc) and not treated with cystectomy.[282]
Differential Diagnosis
Accurate diagnosis is crucial for ensuring appropriate treatment and management. When evaluating a patient for bladder cancer, it is essential to consider a range of differential diagnoses due to the overlap of symptoms with other urological and systemic conditions. The differential diagnosis for bladder cancer includes the following:
- Amyloidosis
- Benign prostatic hyperplasia
- Bladder wall thickening from obstruction or infection
- Cystitis cystica and glandularis
- Diverticulitis
- Enterovesical fistula
- Gynecologic and other pelvic malignancies
- Hemangioma
- Hematuria unrelated to urothelial carcinoma
- Hemorrhagic cystitis
- Inverted papilloma
- Interstitial cystitis
- Leiomyoma
- Malakoplakia
- Median lobe of the prostate with intravesical extension
- Nephrogenic adenoma
- Nephrolithiasis
- Overactive bladder
- Paraganglioma
- Prostatitis
- Radiation cystitis
- Renal masses and neoplasms
- Urinary tract infection
Medical Oncology
Metastatic Urothelial Disease
The National Comprehensive Cancer Network has indicated that FDG-PET/CT scanning may be useful in identifying metastases in selected patients with muscle-invasive disease and may change treatment in patients with stage T3 or higher disease. The initial spread of urothelial bladder cancer is by direct extension into adjacent organs and/or the pelvic side wall, through the lymph nodes, or hematogenously. The most likely locations for distant metastases are bone, liver, and lung.
An extensive review of chemotherapy for metastatic bladder cancer is beyond the scope of this review. Metastatic urothelial cancer generally has a good response rate to chemotherapy (about 70%), but the overall prognosis is still poor as the duration of the benefit is short. MVAC given in a dose-dense manner has been the most studied, but cisplatin and gemcitabine offer similar results with greater safety and less morbidity.[258]
Paclitaxel binds to tubulin and halts intracellular microtubule production, which blocks cell mitosis.[283][284] This therapy has demonstrated a very good response rate, with 27% of patients exhibiting a complete response.[283][285] Immune checkpoint inhibitors have shown activity in metastatic urothelial malignancies. These drugs reduce natural T-cell inhibition from their programmed death protein-1 (PD-1) cell surface protein, essentially releasing the T-cell's full immune potential. Examples include pembrolizumab, atezolizumab, avelumab, and nivolumab, which have all been approved for use in bladder cancer patients. Avelumab and pembrolizumab have shown improved overall survival in clinical trials.[286][287][288][289]
Erdafitinib is a tyrosine kinase inhibitor, which acts as a fibroblast growth factor blocker that is indicated for metastatic urothelial cancer patients who progressed on cisplatin-based chemotherapy. Hyperphosphatemia is common, and up to 25% of patients will need to stop therapy due to significant retinal eye issues.[290][291][292][293] Enfortumab vedotin is an antibody-drug conjugate, where a monoclonal antibody is attached to an anticancer drug.[294][295] Pembrolizumab is an immune checkpoint (PD-1) inhibitor. Results from a large, multinational trial involving 185 centers in 25 different countries recently demonstrated that combination therapy with enfortumab vedotin and pembrolizumab (EF+P) in advanced urothelial cancer patients nearly doubled their progression-free and overall survival.[296][297]
The latest recommendation from the FDA and the European Society of Medical Oncology is to use a combination of enfortumab vedotin and pembrolizumab (EV+P) as first-line chemotherapy for advanced or metastatic urothelial (bladder) cancer.[286][295]
Staging
TNM system: The American Joint Committee on Cancer
Tumor T Stage
- Ta lesions: Ta tumors are papillary (exophytic) lesions that tend to recur
- Tis lesions: High-grade intraepithelial neoplasm without invasion into subepithelial connective tissue
- T1 lesions: T1 tumors invade the submucosa or lamina propria
- T2 lesions: Invasion into muscle is present in T2 lesions
- T2a: Invasion into superficial muscle
- T2b: Invasion into deep muscle
- T3 lesions: T3 tumors extend beyond muscle into the perivesical fat
- T3a: Microscopic invasion only
- T3b: Macroscopic extension or invasion, extravesical mass
- T4 lesions: Tumor invading surrounding structures
- T4a: Invasion of the prostate, vagina, uterus, or bowel
- T4b: Invasion of the abdominal or pelvic wall structures or other organs
Lymph Node N Stage
- NX: Lymph nodes cannot be assessed
- N0: No lymph node metastasis
- N1: Single regional lymph node metastasis within the true pelvis (perivesical, obturator, internal and external iliac, or sacral lymph node)
- N2: Multiple regional lymph node metastases within the true pelvis
- N3: Lymph node metastasis spread to the common iliac lymph nodes
Distant Metastasis M Stage
- M0: No distant metastasis
- M1: Distant metastasis
- M1a: Distant metastasis limited to lymph nodes beyond the common iliac arteries
- M1b: Non-lymph node distant metastases
Clinical Staging
- Stage I: T1, N0, M0
- Stage II: T2, N0, M0
- Stage III:
- Stage IIIA: T3a, T3b, or T4a; N0; M0 or T1 to T4a, N1, M0
- Stage IIIB: T1 to T4a, N2 or N3, M0
- Stage IV:
- Stage IVA: T4b, any N, M0 or any T, any N, M1a
- Stage IVB: any T, any N, M1b
Prognosis
The prognosis of urothelial bladder cancer depends on multiple factors. TNM stage is the single most important prognostic factor of urinary bladder carcinoma. The 5-year overall survival for pT1 is 75%, pT2 is 50%, and pT3 is 20%. Invasion of the muscularis propria determines whether the patient's staging is pT1 (NMIBC) or pT2 (MIBC). Histological variants of urothelial carcinomas tend to have a poorer prognosis than typical bladder cancer. These variants include urothelial carcinoma with rhabdoid features, urothelial micropapillary carcinoma, plasmacytoid carcinoma, sarcomatoid carcinoma, small cell carcinoma, and undifferentiated carcinoma. Other poor prognostic factors of urothelial carcinoma include high histological cancer grade, lymphovascular invasion, the presence of CIS, recurrence, large tumor size, sessile nature, incomplete tumor removal, positive margins, and multicentricity.[46]
The National Cancer Institute reports that the 5-year relative bladder cancer survival rates are as follows: [298][299][300][301]
- CIS: 97%. About 50% will become invasive within 5 years, and just over half will demonstrate tumor progression unless treated with BCG, in which case the risk of progression is less than 10%.
- Localized bladder cancer: 71%.
- Regional bladder cancer (spread to adjacent organs or nearby lymph nodes): 39%.
- Metastatic bladder cancer: 8%.
Complications
Complications of urothelial cancer of the bladder include symptoms related to the tumor and adverse effects of treatment. Complications related to the tumor include weight loss, fatigue, urinary tract infection, metastasis, and urinary obstruction leading to chronic kidney failure.[302] The adverse effects of surgical management include urinary tract infections, urinary leak, pouch stones, urinary tract obstruction, erectile dysfunction, and vaginal narrowing. Almost two-thirds of patients who receive a radical cystectomy will develop at least 1 postoperative problem within the first 3 months after surgery, and 13% will have a severe (grade 3 or more) complication.[303]
Complications of a neobladder include voiding dysfunction, ureteroenteric strictures, urinary infections, bladder stones, metabolic abnormalities, urinary retention, and rupture.[304] Of these, rupture or leakage of a neobladder is potentially the most dangerous and should be suspected in all patients after cystectomy with neobladders who develop abdominal pain.[304][305][306] A CT cystogram is diagnostic.[304] Standard treatment of a ruptured neobladder is laparotomy with surgical repair, but selected cases (diagnosed early, hemodynamically stable, no significant comorbidities, neobladders are well drained and empty well) may be treated conservatively.[304][307] Failure of the patient to clinically improve or persistence of leakage from the neobladder should be treated surgically.[308]
The list of potential complications is exceptionally long. In addition to all of the standard complications associated with general anesthesia and surgical procedures, the possibilities include the following:
- Bleeding and vascular: Anemia, coagulopathy, deep vein thrombosis, hematoma formation, phlebitis, prolonged hematuria, pulmonary embolism, and the need for transfusions
- Gastrointestinal: Anastomotic bowel leak, ascites, diarrhea, emesis, enterocutaneous fistulas, gastrointestinal hemorrhage, ileus, peptic ulcers, pseudomembranous colitis, and small bowel obstruction
- Genitourinary: Bladder dysfunction or perforation, bladder neck stricture, erectile dysfunction, fistula formation, hydronephrosis, parastomal hernias, renal failure, stomal issues, ureteral or urethral strictures, voiding issues including urinary retention and incontinence
- Infections: Abscesses, cellulitis, cholecystitis, diverticulitis, incisional and wound infections, peritonitis, pyelonephritis, sepsis, and unexplained fevers
- Surgical: Bladder perforation, cellulitis, failure to identify CIS, fistula formation, iatrogenic obturator nerve injury, incomplete tumor resection, incisional hernia, intestinal injury (unrecognized), parastomal hernia, rectal trauma, retained drain, seromas, stomal ischemia, vascular injury, and wound dehiscence/infections.[235]
Postoperative and Rehabilitation Care
Patients with a history of NMIBC who have a negative cystoscopy but a positive cytology should be considered suspicious for a recurrence or of hidden disease. Upper tract imaging, ureteroscopy, random bladder biopsies, prostatic urethral biopsies, and photodynamic enhanced cystoscopy (blue light or narrow band imaging) should be considered.[36]
AUA NMIBC Cancer Risk StratificationLow risk:
- Solitary Ta tumor ≤3 cm
Intermediate risk:
- Solitary Ta tumor >3 cm
- Low-grade Ta tumor, which has recurred within 1 year
- Low-grade Ta multifocal tumors (at least 2 separate locations)
- Low-grade T1 tumor
- High-grade Ta tumor ≤3 cm
High risk:
- High-grade Ta tumor that is recurrent, >3 cm, or multifocal
- High-grade T1 tumor
- Any CIS
- Any BCG failure in a patient with a history of high-grade tumor histology
- Prostatic urethral involvement with high-grade histology
- Lymphovascular invasion [309]
- Any variant histology (extensive squamous or glandular differentiation, micropapillary, neuroendocrine, nested, plasmacytoid, or sarcomatoid changes) [310]
Follow-up Cystoscopic Surveillance after TURBT Surgery for NMIBC - AUA Guidelines
The first surveillance cystoscopy should be 3 to 4 months after surgery. If negative, follow-up depends on the risk stratification.[36]
Low-risk cancers
- The next surveillance cystoscopy can be postponed for an additional 6 to 9 months and then performed annually for at least 5 years.
- Continued annual surveillance cystoscopies after 5 years without a recurrence is optional and should be decided on a shared decision-making basis with the patient.
- Cytologies and urinary biomarkers with negative cystoscopies are not recommended for surveillance in low-risk cancers.
- Routine surveillance imaging of the upper tracts is unnecessary.
- Overall, the 5-year recurrence rate is 30% to 40%.[311]
Intermediate-risk cancers
- Surveillance cystoscopy with cytology should be performed every 3 to 6 months for 2 years.
- After 2 years, surveillance cystoscopy with cytology should be repeated every 6 to 12 months for an additional 2 years, and then annually.
- Upper tract imaging should be considered every 1 to 2 years.
- The reported recurrence rate for intermediate-risk bladder cancers is 45%.[36][312]
High-risk cancers
- Surveillance cystoscopy with cytology should be performed every 3 to 4 months for 2 years.
- After 2 years, surveillance cystoscopy with cytology should be performed every 6 months for an additional 2 years, and then annually.
- Some practitioners will add a bladder washing for cytology at every cystoscopy in patients with a history of high-grade cancer or CIS.
- Upper tract imaging should be considered every 1 to 2 years.
- One-fifth (20%) of patients with high-grade NMIBC will develop metastases.
- Consider early cystectomy in patients with variant histology due to the high rate of tumor upstaging in such cases.
- Overall, the 5-year recurrence rate is 60% to 70%, with a 10% to 45% risk of progression to muscle-invasive or metastatic disease.[36][311]
Follow-up MIBC Post-Cystectomy: AUA Guidelines
- Imaging of the chest together with an MRI or CT of the abdomen and pelvis should be obtained at 6- to 12-month intervals for 2 to 3 years.
- After the first 2 to 3 years, this may be continued annually.
- If the urethra was not removed, it should be evaluated regularly for possible recurrences.
Follow-up after MIBC Bladder Preservation Therapy: AUA Guidelines
- Surveillance cystoscopy with cytology should be performed every 3 months for the first year.
- After the first year, surveillance cystoscopy with cytology should be done every 4 to 6 months for another year, then every 6 to 12 months.[318][319]
- Imaging of the chest together with an MRI or CT of the abdomen and pelvis should be obtained at 6-month intervals for 2 years.[30]
Deterrence and Patient Education
Deterrence and patient education are pivotal in managing and preventing bladder cancer. Educating patients about reducing risk factors, such as smoking cessation and minimizing exposure to industrial chemicals, can significantly reduce their overall risk. Help for smoking cessation may be found at the American Cancer Society and SmokeFree.gov. Healthcare professionals should emphasize the importance of early symptom recognition, such as noticing blood in the urine and seeking prompt medical attention. Patients should know that painless gross or microscopic hematuria, urinary tract infection symptoms unrelated to infections and unresponsive to antibiotics, or irritative bladder symptoms may be early symptoms of urothelial bladder cancer.
Regular follow-up and monitoring are crucial for those with a history of bladder cancer due to the high recurrence rate. Patients who have been diagnosed with NMIBC should be given a clear schedule for their surveillance cystoscopies and strongly encouraged to stop smoking if they haven't already. The National Comprehensive Cancer Network offers a free guideline for patients with bladder cancer at NCCN.org/patientguidelines. Additionally, discussing lifestyle modifications, including maintaining a healthy diet and staying hydrated, can support overall urinary tract health. Empowering patients with knowledge fosters proactive health behaviors, ultimately improving outcomes and quality of life.
Pearls and Other Issues
Essential clinical pearls for bladder cancer management can enhance clinical practice. These insights aim to provide practical tips and key considerations for diagnosing, treating, and monitoring this prevalent malignancy effectively and include the following:
- Screening patients who are at high risk for bladder cancer is not recommended, but a routine urinalysis would not be unreasonable, especially in patients who express concern.
- When there is doubt about the risk stratification for determining the workup, it is never wrong to go with a CT urogram and cystoscopy. The consequences of a missed bladder cancer far outweigh the negatives of an unnecessary but more comprehensive evaluation.
- Cytology is not recommended for the routine initial evaluation of patients with microscopic hematuria, but it may be used selectively.
- Photodynamic-enhanced cystoscopic diagnostic technology ("blue light") and narrow-band imaging are probably underutilized with TURBT and could substantially improve outcomes.
- Inflammatory areas on cystoscopic examination of the bladder in patients with known urothelial cancers should be considered suspicious for CIS and biopsied.
- Consider using FISH and similar urinary biomarkers for borderline or equivocal cases.
- Most bladder cancer cases are low-grade and superficial, with minimal health risks if treated promptly and monitored appropriately according to guidelines.
- A persistently positive FISH test after BCG induction therapy is a poor prognostic indicator.[36]
- When performing a TURBT, the bladder should be filled enough so there are no significant mucosal folds and the surface is relatively flat, but the walls should not be thinned or attenuated.
- High-volume centers (>20 cases annually) have demonstrated substantially reduced perioperative mortality rates for radical cystectomy surgery.[320]
- "Trimodal" therapy utilizing maximal TURBT with chemoradiation has shown equivalent survival to radical cystectomy in carefully selected patients.
- Intravesical nogapendekin alfa inbakicept plus BCG therapy is now approved for high-risk BCG-unresponsive patients with CIS with or without Ta or T1 disease.[220]
- Enfortumab vedotin and pembrolizumab (EV+P) have replaced cisplatin-based treatments as first-line chemotherapy for advanced or metastatic urothelial (bladder) cancer.[295][296][297]
Enhancing Healthcare Team Outcomes
Bladder cancer is a prevalent disease in the United States and around the world, with substantial morbidity and mortality that requires interprofessional medical and surgical management. Patients with high-risk factors such as a family history of urothelial cancer, prolonged chemical exposure, and smoking >10-pack-years should be routinely screened with periodic urinalyses to identify any unexplained microhematuria that might be an early warning sign of bladder cancer.
The interprofessional team includes primary care clinicians, urologists, radiologists, radiation oncologists, medical oncologists, intensive care unit specialists, hospitalists, anesthesiologists, pharmacologists, physical therapists, laboratory technicians, a cancer coordinator, specialty-trained nurses, such as stomal therapists and other healthcare professionals. Nurses assist in patient and family education, patient monitoring, and reporting significant clinical issues to the healthcare team. Nurses also arrange close follow-ups when needed.
Effective interprofessional communication fosters a collaborative environment where information is shared, questions are encouraged, and concerns are addressed promptly. Care coordination is critical to ensuring seamless and efficient patient care and optimizing outcomes. The interprofessional team must work together to streamline the patient's journey, from diagnosis through treatment and follow-up. This coordination minimizes errors, reduces delays, and enhances patient safety, ultimately leading to improved outcomes and patient-centered care that prioritizes the well-being and satisfaction of those affected by bladder cancer and their families.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
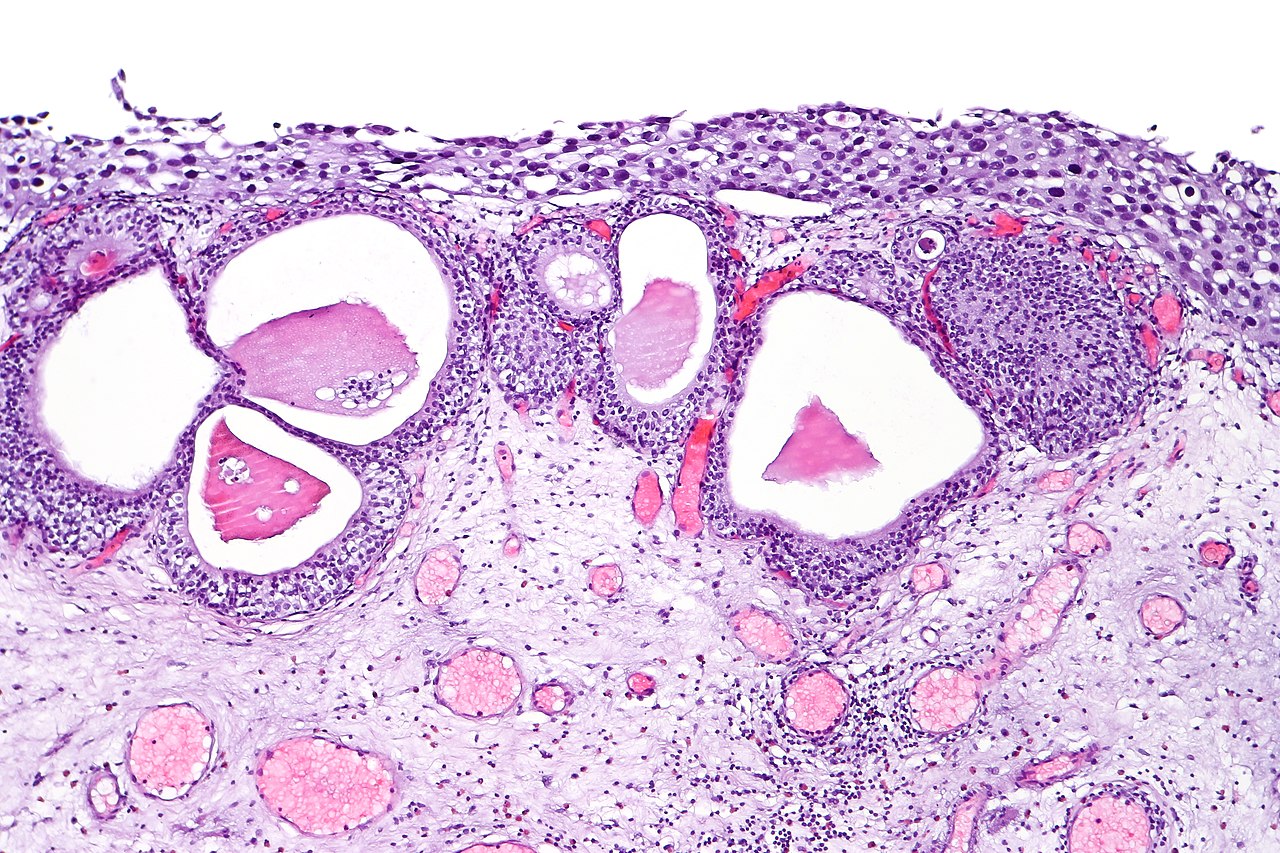
Urothelial Carcinoma In Situ. Urothelial carcinoma in situ in the setting of cystitis cystica glandularis.
CoRus13, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Matulewicz RS, Steinberg GD. Non-muscle-invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Reviews in urology. 2020:22(2):43-51 [PubMed PMID: 32760227]
Level 3 (low-level) evidenceHumphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. European urology. 2016 Jul:70(1):106-119. doi: 10.1016/j.eururo.2016.02.028. Epub 2016 Mar 17 [PubMed PMID: 26996659]
Linn JF, Sesterhenn I, Mostofi FK, Schoenberg M. The molecular characteristics of bladder cancer in young patients. The Journal of urology. 1998 May:159(5):1493-6 [PubMed PMID: 9554340]
Level 2 (mid-level) evidenceLindskrog SV, Prip F, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtröder K, Jensen JB, Strandgaard T, Nordentoft I, Christensen E, Sokac M, Birkbak NJ, Maretty L, Hermann GG, Petersen AC, Weyerer V, Grimm MO, Horstmann M, Sjödahl G, Höglund M, Steiniche T, Mogensen K, de Reyniès A, Nawroth R, Jordan B, Lin X, Dragicevic D, Ward DG, Goel A, Hurst CD, Raman JD, Warrick JI, Segersten U, Sikic D, van Kessel KEM, Maurer T, Meeks JJ, DeGraff DJ, Bryan RT, Knowles MA, Simic T, Hartmann A, Zwarthoff EC, Malmström PU, Malats N, Real FX, Dyrskjøt L. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nature communications. 2021 Apr 16:12(1):2301. doi: 10.1038/s41467-021-22465-w. Epub 2021 Apr 16 [PubMed PMID: 33863885]
Hinotsu S, Akaza H, Miki T, Fujimoto H, Shinohara N, Kikuchi E, Mizutani Y, Koga H, Okajima E, Okuyama A, Japanese Urological Association. Bladder cancer develops 6 years earlier in current smokers: analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. International journal of urology : official journal of the Japanese Urological Association. 2009 Jan:16(1):64-9. doi: 10.1111/j.1442-2042.2008.02194.x. Epub 2008 Nov 27 [PubMed PMID: 19054170]
Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: The epidemiology of bladder cancer. Nature clinical practice. Urology. 2006 Jun:3(6):327-40 [PubMed PMID: 16763645]
Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011 Aug 17:306(7):737-45. doi: 10.1001/jama.2011.1142. Epub [PubMed PMID: 21846855]
van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Quantified relations between exposure to tobacco smoking and bladder cancer risk: a meta-analysis of 89 observational studies. International journal of epidemiology. 2016 Jun:45(3):857-70. doi: 10.1093/ije/dyw044. Epub 2016 Apr 20 [PubMed PMID: 27097748]
Level 1 (high-level) evidenceMasaoka H, Matsuo K, Ito H, Wakai K, Nagata C, Nakayama T, Sadakane A, Tanaka K, Tamakoshi A, Sugawara Y, Mizoue T, Sawada N, Inoue M, Tsugane S, Sasazuki S, Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Cigarette smoking and bladder cancer risk: an evaluation based on a systematic review of epidemiologic evidence in the Japanese population. Japanese journal of clinical oncology. 2016 Mar:46(3):273-83. doi: 10.1093/jjco/hyv188. Epub 2016 Jan 17 [PubMed PMID: 26941372]
Level 1 (high-level) evidenceMasaoka H, Matsuo K, Oze I, Kimura T, Tamakoshi A, Sugawara Y, Tsuji I, Sawada N, Tsugane S, Ito H, Wada K, Nagata C, Kitamura T, Zha L, Sakata R, Ozasa K, Lin Y, Mizoue T, Tanaka K, Abe SK, Inoue M. Cigarette Smoking, Smoking Cessation, and Bladder Cancer Risk: A Pooled Analysis of 10 Cohort Studies in Japan. Journal of epidemiology. 2023 Nov 5:33(11):582-588. doi: 10.2188/jea.JE20220085. Epub 2023 Mar 31 [PubMed PMID: 36310059]
van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP. Significant Role of Lifetime Cigarette Smoking in Worsening Bladder Cancer and Upper Tract Urothelial Carcinoma Prognosis: A Meta-Analysis. The Journal of urology. 2016 Apr:195(4 Pt 1):872-9. doi: 10.1016/j.juro.2015.10.139. Epub 2015 Oct 31 [PubMed PMID: 26523878]
Level 1 (high-level) evidenceBrennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, Lopez-Abente G, Tzonou A, Chang-Claude J, Bolm-Audorff U, Jöckel KH, Donato F, Serra C, Wahrendorf J, Hours M, T'Mannetje A, Kogevinas M, Boffetta P. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. International journal of cancer. 2000 Apr 15:86(2):289-94 [PubMed PMID: 10738259]
Level 2 (mid-level) evidenceAlfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A, Martini A, Mertens LS, Meijer RP, Milowsky MI, Neuzillet Y, Panebianco V, Redlef J, Rink M, Rouanne M, Thalmann GN, Sæbjørnsen S, Veskimäe E, van der Heijden AG. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. European urology. 2024 Jan:85(1):17-31. doi: 10.1016/j.eururo.2023.08.016. Epub 2023 Oct 17 [PubMed PMID: 37858453]
Martin C, Leiser CL, O'Neil B, Gupta S, Lowrance WT, Kohlmann W, Greenberg S, Pathak P, Smith KR, Hanson HA. Familial Cancer Clustering in Urothelial Cancer: A Population-Based Case-Control Study. Journal of the National Cancer Institute. 2018 May 1:110(5):527-533. doi: 10.1093/jnci/djx237. Epub [PubMed PMID: 29228305]
Level 2 (mid-level) evidenceFigueroa JD, Ye Y, Siddiq A, Garcia-Closas M, Chatterjee N, Prokunina-Olsson L, Cortessis VK, Kooperberg C, Cussenot O, Benhamou S, Prescott J, Porru S, Dinney CP, Malats N, Baris D, Purdue M, Jacobs EJ, Albanes D, Wang Z, Deng X, Chung CC, Tang W, Bas Bueno-de-Mesquita H, Trichopoulos D, Ljungberg B, Clavel-Chapelon F, Weiderpass E, Krogh V, Dorronsoro M, Travis R, Tjønneland A, Brenan P, Chang-Claude J, Riboli E, Conti D, Gago-Dominguez M, Stern MC, Pike MC, Van Den Berg D, Yuan JM, Hohensee C, Rodabough R, Cancel-Tassin G, Roupret M, Comperat E, Chen C, De Vivo I, Giovannucci E, Hunter DJ, Kraft P, Lindstrom S, Carta A, Pavanello S, Arici C, Mastrangelo G, Kamat AM, Lerner SP, Barton Grossman H, Lin J, Gu J, Pu X, Hutchinson A, Burdette L, Wheeler W, Kogevinas M, Tardón A, Serra C, Carrato A, García-Closas R, Lloreta J, Schwenn M, Karagas MR, Johnson A, Schned A, Armenti KR, Hosain GM, Andriole G Jr, Grubb R 3rd, Black A, Ryan Diver W, Gapstur SM, Weinstein SJ, Virtamo J, Haiman CA, Landi MT, Caporaso N, Fraumeni JF Jr, Vineis P, Wu X, Silverman DT, Chanock S, Rothman N. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Human molecular genetics. 2014 Mar 1:23(5):1387-98. doi: 10.1093/hmg/ddt519. Epub 2013 Oct 24 [PubMed PMID: 24163127]
Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, Real FX, Van Den Berg D, Matullo G, Baris D, Thun M, Kiemeney LA, Vineis P, De Vivo I, Albanes D, Purdue MP, Rafnar T, Hildebrandt MA, Kiltie AE, Cussenot O, Golka K, Kumar R, Taylor JA, Mayordomo JI, Jacobs KB, Kogevinas M, Hutchinson A, Wang Z, Fu YP, Prokunina-Olsson L, Burdett L, Yeager M, Wheeler W, Tardón A, Serra C, Carrato A, García-Closas R, Lloreta J, Johnson A, Schwenn M, Karagas MR, Schned A, Andriole G Jr, Grubb R 3rd, Black A, Jacobs EJ, Diver WR, Gapstur SM, Weinstein SJ, Virtamo J, Cortessis VK, Gago-Dominguez M, Pike MC, Stern MC, Yuan JM, Hunter DJ, McGrath M, Dinney CP, Czerniak B, Chen M, Yang H, Vermeulen SH, Aben KK, Witjes JA, Makkinje RR, Sulem P, Besenbacher S, Stefansson K, Riboli E, Brennan P, Panico S, Navarro C, Allen NE, Bueno-de-Mesquita HB, Trichopoulos D, Caporaso N, Landi MT, Canzian F, Ljungberg B, Tjonneland A, Clavel-Chapelon F, Bishop DT, Teo MT, Knowles MA, Guarrera S, Polidoro S, Ricceri F, Sacerdote C, Allione A, Cancel-Tassin G, Selinski S, Hengstler JG, Dietrich H, Fletcher T, Rudnai P, Gurzau E, Koppova K, Bolick SC, Godfrey A, Xu Z, Sanz-Velez JI, D García-Prats M, Sanchez M, Valdivia G, Porru S, Benhamou S, Hoover RN, Fraumeni JF Jr, Silverman DT, Chanock SJ. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nature genetics. 2010 Nov:42(11):978-84. doi: 10.1038/ng.687. Epub 2010 Oct 24 [PubMed PMID: 20972438]
Kiemeney LA, Thorlacius S, Sulem P, Geller F, Aben KK, Stacey SN, Gudmundsson J, Jakobsdottir M, Bergthorsson JT, Sigurdsson A, Blondal T, Witjes JA, Vermeulen SH, Hulsbergen-van de Kaa CA, Swinkels DW, Ploeg M, Cornel EB, Vergunst H, Thorgeirsson TE, Gudbjartsson D, Gudjonsson SA, Thorleifsson G, Kristinsson KT, Mouy M, Snorradottir S, Placidi D, Campagna M, Arici C, Koppova K, Gurzau E, Rudnai P, Kellen E, Polidoro S, Guarrera S, Sacerdote C, Sanchez M, Saez B, Valdivia G, Ryk C, de Verdier P, Lindblom A, Golka K, Bishop DT, Knowles MA, Nikulasson S, Petursdottir V, Jonsson E, Geirsson G, Kristjansson B, Mayordomo JI, Steineck G, Porru S, Buntinx F, Zeegers MP, Fletcher T, Kumar R, Matullo G, Vineis P, Kiltie AE, Gulcher JR, Thorsteinsdottir U, Kong A, Rafnar T, Stefansson K. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nature genetics. 2008 Nov:40(11):1307-12. doi: 10.1038/ng.229. Epub 2008 Sep 14 [PubMed PMID: 18794855]
Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP. Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. European journal of epidemiology. 2016 Sep:31(9):811-51. doi: 10.1007/s10654-016-0138-6. Epub 2016 Mar 21 [PubMed PMID: 27000312]
Level 1 (high-level) evidenceCumberbatch MG, Rota M, Catto JW, La Vecchia C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. European urology. 2016 Sep:70(3):458-66. doi: 10.1016/j.eururo.2015.06.042. Epub 2015 Jul 3 [PubMed PMID: 26149669]
Level 1 (high-level) evidenceZeegers MP, Swaen GM, Kant I, Goldbohm RA, van den Brandt PA. Occupational risk factors for male bladder cancer: results from a population based case cohort study in the Netherlands. Occupational and environmental medicine. 2001 Sep:58(9):590-6 [PubMed PMID: 11511746]
Level 2 (mid-level) evidenceAmes BN, Kammen HO, Yamasaki E. Hair dyes are mutagenic: identification of a variety of mutagenic ingredients. Proceedings of the National Academy of Sciences of the United States of America. 1975 Jun:72(6):2423-7 [PubMed PMID: 1094469]
Gaertner RR, Trpeski L, Johnson KC, Canadian Cancer Registries Epidemiology Research Group. A case-control study of occupational risk factors for bladder cancer in Canada. Cancer causes & control : CCC. 2004 Dec:15(10):1007-19 [PubMed PMID: 15801485]
Level 2 (mid-level) evidenceCassidy A, Wang W, Wu X, Lin J. Risk of urinary bladder cancer: a case-control analysis of industry and occupation. BMC cancer. 2009 Dec 15:9():443. doi: 10.1186/1471-2407-9-443. Epub 2009 Dec 15 [PubMed PMID: 20003537]
Level 2 (mid-level) evidencePloeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World journal of urology. 2009 Jun:27(3):289-93. doi: 10.1007/s00345-009-0383-3. Epub 2009 Feb 15 [PubMed PMID: 19219610]
Liu S, Yang T, Na R, Hu M, Zhang L, Fu Y, Jiang H, Ding Q. The impact of female gender on bladder cancer-specific death risk after radical cystectomy: a meta-analysis of 27,912 patients. International urology and nephrology. 2015 Jun:47(6):951-8. doi: 10.1007/s11255-015-0980-6. Epub 2015 Apr 19 [PubMed PMID: 25894962]
Level 1 (high-level) evidenceUhlig A, Seif Amir Hosseini A, Simon J, Lotz J, Trojan L, Schmid M, Uhlig J. Gender Specific Differences in Disease-Free, Cancer Specific and Overall Survival after Radical Cystectomy for Bladder Cancer: A Systematic Review and Meta-Analysis. The Journal of urology. 2018 Jul:200(1):48-60. doi: 10.1016/j.juro.2017.11.150. Epub 2018 Mar 1 [PubMed PMID: 29477716]
Level 1 (high-level) evidenceToren P, Wilkins A, Patel K, Burley A, Gris T, Kockelbergh R, Lodhi T, Choudhury A, Bryan RT. The sex gap in bladder cancer survival - a missing link in bladder cancer care? Nature reviews. Urology. 2024 Mar:21(3):181-192. doi: 10.1038/s41585-023-00806-2. Epub 2023 Aug 21 [PubMed PMID: 37604983]
Patafio FM, Robert Siemens D, Wei X, Booth CM. Is there a gender effect in bladder cancer? A population-based study of practice and outcomes. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2015 Jul-Aug:9(7-8):269-74. doi: 10.5489/cuaj.2927. Epub [PubMed PMID: 26316913]
Cohn JA, Vekhter B, Lyttle C, Steinberg GD, Large MC. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: a nationwide claims-based investigation. Cancer. 2014 Feb 15:120(4):555-61. doi: 10.1002/cncr.28416. Epub 2013 Nov 13 [PubMed PMID: 24496869]
Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, Lotan Y, Meeks JJ, Michalski JM, Morgan TM, Quale DZ, Rosenberg JE, Zietman AL, Holzbeierlein JM. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. The Journal of urology. 2017 Sep:198(3):552-559. doi: 10.1016/j.juro.2017.04.086. Epub 2017 Apr 26 [PubMed PMID: 28456635]
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y. Epidemiology and risk factors of urothelial bladder cancer. European urology. 2013 Feb:63(2):234-41. doi: 10.1016/j.eururo.2012.07.033. Epub 2012 Jul 25 [PubMed PMID: 22877502]
Stephenson LA, Kolka MA. Acetylcholinesterase inhibitor, pyridostigmine bromide, reduces skin blood flow in humans. The American journal of physiology. 1990 Apr:258(4 Pt 2):R951-7 [PubMed PMID: 2184685]
Abern MR, Dude AM, Tsivian M, Coogan CL. The characteristics of bladder cancer after radiotherapy for prostate cancer. Urologic oncology. 2013 Nov:31(8):1628-34. doi: 10.1016/j.urolonc.2012.04.006. Epub 2012 May 9 [PubMed PMID: 22575239]
Level 2 (mid-level) evidenceZhang Y, Rumgay H, Li M, Yu H, Pan H, Ni J. The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. Journal of global health. 2023 Sep 15:13():04109. doi: 10.7189/jogh.13.04109. Epub 2023 Sep 15 [PubMed PMID: 37712386]
Chen S, Zhu S, Cui X, Xu W, Kong C, Zhang Z, Qian W. Identifying non-muscle-invasive and muscle-invasive bladder cancer based on blood serum surface-enhanced Raman spectroscopy. Biomedical optics express. 2019 Jul 1:10(7):3533-3544. doi: 10.1364/BOE.10.003533. Epub 2019 Jun 24 [PubMed PMID: 31467792]
Level 2 (mid-level) evidenceHolzbeierlein JM, Bixler BR, Buckley DI, Chang SS, Holmes R, James AC, Kirkby E, McKiernan JM, Schuckman AK. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. The Journal of urology. 2024 Apr:211(4):533-538. doi: 10.1097/JU.0000000000003846. Epub 2024 Jan 24 [PubMed PMID: 38265030]
Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, Weider J. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005 Dec:66(6 Suppl 1):4-34 [PubMed PMID: 16399414]
Tang DH, Chang SS. Management of carcinoma in situ of the bladder: best practice and recent developments. Therapeutic advances in urology. 2015 Dec:7(6):351-64. doi: 10.1177/1756287215599694. Epub [PubMed PMID: 26622320]
Level 3 (low-level) evidencevan Straten CGJI, Bruins MH, Dijkstra S, Cornel EB, Kortleve MDH, de Vocht TF, Kiemeney LALM, van der Heijden AG. The accuracy of cystoscopy in predicting muscle invasion in newly diagnosed bladder cancer patients. World journal of urology. 2023 Jul:41(7):1829-1835. doi: 10.1007/s00345-023-04428-6. Epub 2023 May 17 [PubMed PMID: 37195314]
Harland SJ, Kynaston H, Grigor K, Wallace DM, Beacock C, Kockelbergh R, Clawson S, Barlow T, Parmar MK, Griffiths GO, National Cancer Research Institute Bladder Clinical Studies Group. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. The Journal of urology. 2007 Sep:178(3 Pt 1):807-13; discussion 813 [PubMed PMID: 17631326]
Level 1 (high-level) evidenceLewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. American journal of physiology. Renal physiology. 2000 Jun:278(6):F867-74 [PubMed PMID: 10836974]
Cheng L, MacLennan GT, Lopez-Beltran A. Histologic grading of urothelial carcinoma: a reappraisal. Human pathology. 2012 Dec:43(12):2097-108. doi: 10.1016/j.humpath.2012.01.008. Epub 2012 Apr 26 [PubMed PMID: 22542126]
Nese N, Gupta R, Bui MH, Amin MB. Carcinoma in situ of the urinary bladder: review of clinicopathologic characteristics with an emphasis on aspects related to molecular diagnostic techniques and prognosis. Journal of the National Comprehensive Cancer Network : JNCCN. 2009 Jan:7(1):48-57 [PubMed PMID: 19176205]
Elliott GB, Moloney PJ, Anderson GH. "Denuding cystitis" and in situ urothelial carcinoma. Archives of pathology. 1973 Aug:96(2):91-4 [PubMed PMID: 4718234]
Gandhi J, Chen JF, Al-Ahmadie H. Urothelial Carcinoma: Divergent Differentiation and Morphologic Subtypes. Surgical pathology clinics. 2022 Dec:15(4):641-659. doi: 10.1016/j.path.2022.07.003. Epub 2022 Oct 13 [PubMed PMID: 36344181]
Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, van Rhijn BWG, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. European urology. 2017 Mar:71(3):447-461. doi: 10.1016/j.eururo.2016.05.041. Epub 2016 Jun 17 [PubMed PMID: 27324428]
Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). European urology. 2022 Jan:81(1):75-94. doi: 10.1016/j.eururo.2021.08.010. Epub 2021 Sep 10 [PubMed PMID: 34511303]
Compérat E, Amin MB, Berney DM, Cree I, Menon S, Moch H, Netto GJ, Rao V, Raspollini MR, Rubin MA, Srigley JR, Tan PH, Tickoo SK, Turajlic S, Tsuzuki T. What's new in WHO fifth edition - urinary tract. Histopathology. 2022 Oct:81(4):439-446. doi: 10.1111/his.14764. Epub 2022 Aug 16 [PubMed PMID: 35942645]
Willis DL, Fernandez MI, Dickstein RJ, Parikh S, Shah JB, Pisters LL, Guo CC, Henderson S, Czerniak BA, Grossman HB, Dinney CP, Kamat AM. Clinical outcomes of cT1 micropapillary bladder cancer. The Journal of urology. 2015 Apr:193(4):1129-34. doi: 10.1016/j.juro.2014.09.092. Epub 2014 Sep 22 [PubMed PMID: 25254936]
Level 2 (mid-level) evidenceSaginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of Bladder Cancer. Medical sciences (Basel, Switzerland). 2020 Mar 13:8(1):. doi: 10.3390/medsci8010015. Epub 2020 Mar 13 [PubMed PMID: 32183076]
Park S, Reuter VE, Hansel DE. Non-urothelial carcinomas of the bladder. Histopathology. 2019 Jan:74(1):97-111. doi: 10.1111/his.13719. Epub [PubMed PMID: 30565306]
Dahm P, Gschwend JE. Malignant non-urothelial neoplasms of the urinary bladder: a review. European urology. 2003 Dec:44(6):672-81 [PubMed PMID: 14644119]
Larkins MC, Pasli M, Bhatt A, Burke A. Squamous cell carcinoma of the bladder: Demographics and outcomes associated with surgery and radiotherapy. Journal of surgical oncology. 2024 Mar:129(3):649-658. doi: 10.1002/jso.27525. Epub 2023 Nov 20 [PubMed PMID: 37985369]
Hussain M, Vaishampayan U, Du W, Redman B, Smith DC. Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001 May 1:19(9):2527-33 [PubMed PMID: 11331332]
Johnson DE, Hodge GB, Abdul-Karim FW, Ayala AG. Urachal carcinoma. Urology. 1985 Sep:26(3):218-21 [PubMed PMID: 4035835]
Kakizoe T, Matsumoto K, Andoh M, Nishio Y, Kishi K. Adenocarcinoma of urachus. Report of 7 cases and review of literature. Urology. 1983 Apr:21(4):360-6 [PubMed PMID: 6836822]
Level 3 (low-level) evidenceSheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. The Journal of urology. 1984 Jan:131(1):1-8 [PubMed PMID: 6361280]
Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, Zincke H. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006 Aug 15:107(4):712-20 [PubMed PMID: 16826585]
CULP DA. THE HISTOLOGY OF THE EXSTROPHIED BLADDER. The Journal of urology. 1964 May:91():538-48 [PubMed PMID: 14154540]
Roy S, Parwani AV. Adenocarcinoma of the urinary bladder. Archives of pathology & laboratory medicine. 2011 Dec:135(12):1601-5. doi: 10.5858/arpa.2009-0713-RS. Epub [PubMed PMID: 22129192]
Hughes MJ, Fisher C, Sohaib SA. Imaging features of primary nonurachal adenocarcinoma of the bladder. AJR. American journal of roentgenology. 2004 Nov:183(5):1397-401 [PubMed PMID: 15505310]
Xiaoxu L, Jianhong L, Jinfeng W, Klotz LH. Bladder adenocarcinoma: 31 reported cases. The Canadian journal of urology. 2001 Oct:8(5):1380-3 [PubMed PMID: 11718635]
Level 3 (low-level) evidenceThomas DG, Ward AM, Williams JL. A study of 52 cases of adenocarcinoma of the bladder. British journal of urology. 1971 Feb:43(1):4-15 [PubMed PMID: 4926456]
Level 3 (low-level) evidenceAbenoza P, Manivel C, Fraley EE. Primary adenocarcinoma of urinary bladder. Clinicopathologic study of 16 cases. Urology. 1987 Jan:29(1):9-14 [PubMed PMID: 2432717]
Level 3 (low-level) evidenceAnderström C, Johansson SL, von Schultz L. Primary adenocarcinoma of the urinary bladder. A clinicopathologic and prognostic study. Cancer. 1983 Oct 1:52(7):1273-80 [PubMed PMID: 6883290]
Wright JL, Porter MP, Li CI, Lange PH, Lin DW. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer. 2006 Aug 15:107(4):721-8 [PubMed PMID: 16826584]
Barocas DA, Boorjian SA, Alvarez RD, Downs TM, Gross CP, Hamilton BD, Kobashi KC, Lipman RR, Lotan Y, Ng CK, Nielsen ME, Peterson AC, Raman JD, Smith-Bindman R, Souter LH. Microhematuria: AUA/SUFU Guideline. The Journal of urology. 2020 Oct:204(4):778-786. doi: 10.1097/JU.0000000000001297. Epub 2020 Jul 23 [PubMed PMID: 32698717]
Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. The Journal of urology. 2000 Feb:163(2):524-7 [PubMed PMID: 10647670]
Jeong CW, Lee S, Byun SS, Lee DH, Lee SE. No increase in risk of microscopic hematuria with aspirin use by asymptomatic healthy people. JAMA internal medicine. 2013 Jun 24:173(12):1145-6. doi: 10.1001/jamainternmed.2013.567. Epub [PubMed PMID: 23609065]
Level 2 (mid-level) evidenceCulclasure TF, Bray VJ, Hasbargen JA. The significance of hematuria in the anticoagulated patient. Archives of internal medicine. 1994 Mar 28:154(6):649-52 [PubMed PMID: 8129498]
Tan WS, Sarpong R, Khetrapal P, Rodney S, Mostafid H, Cresswell J, Hicks J, Rane A, Henderson A, Watson D, Cherian J, Williams N, Brew-Graves C, Feber A, Kelly JD, DETECT I Trial Collaborators. Can Renal and Bladder Ultrasound Replace Computerized Tomography Urogram in Patients Investigated for Microscopic Hematuria? The Journal of urology. 2018 Nov:200(5):973-980. doi: 10.1016/j.juro.2018.04.065. Epub 2018 Apr 24 [PubMed PMID: 29702097]
Gharibvand MM, Kazemi M, Motamedfar A, Sametzadeh M, Sahraeizadeh A. The role of ultrasound in diagnosis and evaluation of bladder tumors. Journal of family medicine and primary care. 2017 Oct-Dec:6(4):840-843. doi: 10.4103/jfmpc.jfmpc_186_17. Epub [PubMed PMID: 29564274]
Trinh TW, Glazer DI, Sadow CA, Sahni VA, Geller NL, Silverman SG. Bladder cancer diagnosis with CT urography: test characteristics and reasons for false-positive and false-negative results. Abdominal radiology (New York). 2018 Mar:43(3):663-671. doi: 10.1007/s00261-017-1249-6. Epub [PubMed PMID: 28677000]
Palou J, Rodríguez-Rubio F, Huguet J, Segarra J, Ribal MJ, Alcaraz A, Villavicencio H. Multivariate analysis of clinical parameters of synchronous primary superficial bladder cancer and upper urinary tract tumor. The Journal of urology. 2005 Sep:174(3):859-61; discussion 861 [PubMed PMID: 16093970]
Huang L, Kong Q, Liu Z, Wang J, Kang Z, Zhu Y. The Diagnostic Value of MR Imaging in Differentiating T Staging of Bladder Cancer: A Meta-Analysis. Radiology. 2018 Feb:286(2):502-511. doi: 10.1148/radiol.2017171028. Epub 2017 Dec 4 [PubMed PMID: 29206594]
Level 1 (high-level) evidenceRajesh A, Sokhi HK, Fung R, Mulcahy KA, Bankart MJ. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clinical radiology. 2011 Dec:66(12):1140-5. doi: 10.1016/j.crad.2011.05.019. Epub 2011 Sep 14 [PubMed PMID: 21924408]
Mallampati GK, Siegelman ES. MR imaging of the bladder. Magnetic resonance imaging clinics of North America. 2004 Aug:12(3):545-55, vii [PubMed PMID: 15271370]
Zhang X, Guo J, Yun Y, Shan D, Yang D, Xu C, Chen X. Differentiation of Muscular Invasion in Bladder Cancer: Additional Value of Synthetic Magnetic Resonance Imaging. Academic radiology. 2024 Mar 27:():. pii: S1076-6332(24)00153-3. doi: 10.1016/j.acra.2024.03.011. Epub 2024 Mar 27 [PubMed PMID: 38548534]
Li L, Zhang J, Zhe X, Tang M, Zhang L, Lei X, Zhang X. Prediction of histopathologic grades of bladder cancer with radiomics based on MRI: Comparison with traditional MRI. Urologic oncology. 2024 Jun:42(6):176.e9-176.e20. doi: 10.1016/j.urolonc.2024.02.008. Epub 2024 Mar 30 [PubMed PMID: 38556403]
Yang G, Bai J, Hao M, Zhang L, Fan Z, Wang X. Enhancing recurrence risk prediction for bladder cancer using multi-sequence MRI radiomics. Insights into imaging. 2024 Mar 25:15(1):88. doi: 10.1186/s13244-024-01662-3. Epub 2024 Mar 25 [PubMed PMID: 38526620]
Barentsz JO, Engelbrecht MR, Witjes JA, de la Rosette JJ, van der Graaf M. MR imaging of the male pelvis. European radiology. 1999:9(9):1722-36 [PubMed PMID: 10602944]
Ateş SG, Demirel BB, Başar H, Uçmak G. The Added-value of Staging (18)F-FDG PET/CT in the Prediction of Overall Survival in the Patients with Bladder Cancer. Molecular imaging and radionuclide therapy. 2024 Feb 22:33(1):11-18. doi: 10.4274/mirt.galenos.2023.65002. Epub [PubMed PMID: 38390706]
Longoni M, Scilipoti P, Re C, Rosiello G, Nocera L, Pellegrino F, Basile G, de Angelis M, Quarta L, Burgio G, Necchi A, Cigliola A, Chiti A, Picchio M, Salonia A, Briganti A, Montorsi F, Moschini M. Use of 18F-fluoro-2-deoxy-d-glucose (18F-FDG) PET/CT for lymph node assessment before radical cystectomy in bladder cancer patients. BJU international. 2024 Apr 15:():. doi: 10.1111/bju.16363. Epub 2024 Apr 15 [PubMed PMID: 38621771]
Jena R, Bhargava P, Tripathi S, Taywade S, Yadav T, Sandhu AS, Singh M, Navriya SC, Bhirud DP, Aggarwal A, Choudhary GR. 18F-fluoro-2-deoxy-2-d-glucose PET-CT (FDG PET-CT) in staging of high-risk renal and urothelial bladder cancers (COPPER-T) trial protocol. BJUI compass. 2023 Nov:4(6):662-667. doi: 10.1002/bco2.246. Epub 2023 May 10 [PubMed PMID: 37818027]
Bacchiani M, Salamone V, Massaro E, Sandulli A, Mariottini R, Cadenar A, Di Maida F, Pradere B, Mertens LS, Longoni M, Krajewski W, Del Giudice F, D'Andrea D, Laukhtina E, Shariat SF, Minervini A, Moschini M, Mari A, On Behalf Of European Association Of Urology-Young Academic Urologists Eau-Yau Urothelial Carcinoma Working Group. Assessing the Performance of 18F-FDG PET/CT in Bladder Cancer: A Narrative Review of Current Evidence. Cancers. 2023 May 28:15(11):. doi: 10.3390/cancers15112951. Epub 2023 May 28 [PubMed PMID: 37296913]
Level 3 (low-level) evidenceMarandino L, Capozza A, Bandini M, Raggi D, Farè E, Pederzoli F, Gallina A, Capitanio U, Bianchi M, Gandaglia G, Fossati N, Colecchia M, Giannatempo P, Serafini G, Padovano B, Salonia A, Briganti A, Montorsi F, Alessi A, Necchi A. [18F]Fluoro-Deoxy-Glucose positron emission tomography to evaluate lymph node involvement in patients with muscle-invasive bladder cancer receiving neoadjuvant pembrolizumab. Urologic oncology. 2021 Apr:39(4):235.e15-235.e21. doi: 10.1016/j.urolonc.2020.09.035. Epub 2020 Oct 16 [PubMed PMID: 33071107]
Vind-Kezunovic S, Bouchelouche K, Ipsen P, Høyer S, Bell C, Bjerggaard Jensen J. Detection of Lymph Node Metastasis in Patients with Bladder Cancer using Maximum Standardised Uptake Value and (18)F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Results from a High-volume Centre Including Long-term Follow-up. European urology focus. 2019 Jan:5(1):90-96. doi: 10.1016/j.euf.2017.06.005. Epub 2017 Jun 23 [PubMed PMID: 28753817]
Mertens LS, Meijer RP, Alfred Witjes J. Positron Emission Tomography/Computed Tomography for Staging of Bladder Cancer: A Continuing Clinical Controversy. European urology. 2023 Feb:83(2):95-96. doi: 10.1016/j.eururo.2022.09.017. Epub 2022 Oct 4 [PubMed PMID: 36202686]
Level 3 (low-level) evidenceHa HK, Koo PJ, Kim SJ. Diagnostic Accuracy of F-18 FDG PET/CT for Preoperative Lymph Node Staging in Newly Diagnosed Bladder Cancer Patients: A Systematic Review and Meta-Analysis. Oncology. 2018:95(1):31-38. doi: 10.1159/000488200. Epub 2018 May 30 [PubMed PMID: 29847834]
Level 1 (high-level) evidenceEinerhand SMH, van Gennep EJ, Mertens LS, Hendricksen K, Donswijk ML, van der Poel HG, van Rhijn BWG. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in muscle-invasive bladder cancer. Current opinion in urology. 2020 Sep:30(5):654-664. doi: 10.1097/MOU.0000000000000798. Epub [PubMed PMID: 32701719]
Level 3 (low-level) evidenceBlick CG, Nazir SA, Mallett S, Turney BW, Onwu NN, Roberts IS, Crew JP, Cowan NC. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU international. 2012 Jul:110(1):84-94. doi: 10.1111/j.1464-410X.2011.10664.x. Epub 2011 Nov 28 [PubMed PMID: 22122739]
Guldhammer CS, Vásquez JL, Kristensen VM, Norus T, Nadler N, Jensen JB, Azawi N. Cystoscopy Accuracy in Detecting Bladder Tumors: A Prospective Video-Confirmed Study. Cancers. 2023 Dec 28:16(1):. doi: 10.3390/cancers16010160. Epub 2023 Dec 28 [PubMed PMID: 38201586]
During VA, Sole GM, Jha AK, Anderson JA, Bryan RT. Prediction of histological stage based on cystoscopic appearances of newly diagnosed bladder tumours. Annals of the Royal College of Surgeons of England. 2016 Nov:98(8):547-551 [PubMed PMID: 27502337]
Mohr DN, Offord KP, Owen RA, Melton LJ 3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA. 1986 Jul 11:256(2):224-9 [PubMed PMID: 3723707]
Zincke H, Utz DC, Farrow GM. Review of Mayo Clinic experience with carcinoma in situ. Urology. 1985 Oct:26(4 Suppl):39-46 [PubMed PMID: 3931327]
Dutta R, Abdelhalim A, Martin JW, Vernez SL, Faltas B, Lotan Y, Youssef RF. Effect of tumor location on survival in urinary bladder adenocarcinoma: A population-based analysis. Urologic oncology. 2016 Dec:34(12):531.e1-531.e6. doi: 10.1016/j.urolonc.2016.06.009. Epub 2016 Jul 15 [PubMed PMID: 27427223]
Svatek RS, Clinton TN, Wilson CA, Kamat AM, Grossman HB, Dinney CP, Shah JB. Intravesical tumor involvement of the trigone is associated with nodal metastasis in patients undergoing radical cystectomy. Urology. 2014 Nov:84(5):1147-51. doi: 10.1016/j.urology.2014.05.011. Epub 2014 Aug 28 [PubMed PMID: 25174656]
Donat SM, Genega EM, Herr HW, Reuter VE. Mechanisms of prostatic stromal invasion in patients with bladder cancer: clinical significance. The Journal of urology. 2001 Apr:165(4):1117-20 [PubMed PMID: 11257650]
Moschini M, Soria F, Susani M, Korn S, Briganti A, Roupret M, Seitz C, Gust K, Haitel A, Montorsi F, Wirth G, Robinson BD, Karakiewicz PI, Özsoy M, Rink M, Shariat SF. Impact of the Level of Urothelial Carcinoma Involvement of the Prostate on Survival after Radical Cystectomy. Bladder cancer (Amsterdam, Netherlands). 2017 Jul 27:3(3):161-169. doi: 10.3233/BLC-160086. Epub 2017 Jul 27 [PubMed PMID: 28824943]
Kravchuk AP, Wolff I, Gilfrich C, Wirtz RM, Soares P, Braun KP, Brookman-May SD, Kollitsch L, Hauner K, Burchardt M, Bründl J, Burger M, May M. Urine-Based Biomarker Test Uromonitor(®) in the Detection and Disease Monitoring of Non-Muscle-Invasive Bladder Cancer-A Systematic Review and Meta-Analysis of Diagnostic Test Performance. Cancers. 2024 Feb 11:16(4):. doi: 10.3390/cancers16040753. Epub 2024 Feb 11 [PubMed PMID: 38398144]
Level 1 (high-level) evidenceAzawi N, Vásquez JL, Dreyer T, Guldhammer CS, Saber Al-Juboori RM, Nielsen AM, Jensen JB. Surveillance of Low-Grade Non-Muscle Invasive Bladder Tumors Using Uromonitor: SOLUSION Trial. Cancers. 2023 Apr 17:15(8):. doi: 10.3390/cancers15082341. Epub 2023 Apr 17 [PubMed PMID: 37190269]
Laukhtina E, Shim SR, Mori K, D'Andrea D, Soria F, Rajwa P, Mostafaei H, Compérat E, Cimadamore A, Moschini M, Teoh JY, Enikeev D, Xylinas E, Lotan Y, Palou J, Gontero P, Babjuk M, Witjes JA, Kamat AM, Roupret M, Shariat SF, Pradere B, European Association of Urology–Young Academic Urologists (EAU-YAU): Urothelial Carcinoma Working Group. Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis. European urology oncology. 2021 Dec:4(6):927-942. doi: 10.1016/j.euo.2021.10.003. Epub 2021 Nov 6 [PubMed PMID: 34753702]
Level 1 (high-level) evidenceDaneshmand S, Patel S, Lotan Y, Pohar K, Trabulsi E, Woods M, Downs T, Huang W, Jones J, O'Donnell M, Bivalacqua T, DeCastro J, Steinberg G, Kamat A, Resnick M, Konety B, Schoenberg M, Jones JS, Flexible Blue Light Study Group Collaborators. Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study. The Journal of urology. 2018 May:199(5):1158-1165. doi: 10.1016/j.juro.2017.11.096. Epub 2017 Dec 2 [PubMed PMID: 29203268]
Level 2 (mid-level) evidenceRaitanen MP, Aine R, Rintala E, Kallio J, Rajala P, Juusela H, Tammela TL, FinnBladder Group. Differences between local and review urinary cytology in diagnosis of bladder cancer. An interobserver multicenter analysis. European urology. 2002 Mar:41(3):284-9 [PubMed PMID: 12180229]
Casey RG, Catto JW, Cheng L, Cookson MS, Herr H, Shariat S, Witjes JA, Black PC. Diagnosis and management of urothelial carcinoma in situ of the lower urinary tract: a systematic review. European urology. 2015 May:67(5):876-88. doi: 10.1016/j.eururo.2014.10.040. Epub 2014 Nov 14 [PubMed PMID: 25466937]
Level 1 (high-level) evidenceParker J, Spiess PE. Current and emerging bladder cancer urinary biomarkers. TheScientificWorldJournal. 2011 May 26:11():1103-12. doi: 10.1100/tsw.2011.104. Epub 2011 May 26 [PubMed PMID: 21623456]
Flezar MS. Urine and bladder washing cytology for detection of urothelial carcinoma: standard test with new possibilities. Radiology and oncology. 2010 Dec:44(4):207-14. doi: 10.2478/v10019-010-0042-8. Epub 2010 Sep 29 [PubMed PMID: 22933917]
Zein T, Wajsman Z, Englander LS, Gamarra M, Lopez C, Huben RP, Pontes JE. Evaluation of bladder washings and urine cytology in the diagnosis of bladder cancer and its correlation with selected biopsies of the bladder mucosa. The Journal of urology. 1984 Oct:132(4):670-1 [PubMed PMID: 6471209]
Kiliçarslan A, Süngü N, Balci S, Canda E, Altinova S, Güler G. The role of collecting bladder wash fluid before biopsy procedure to help the cytological diagnosis of residual tumor. Journal of cytology. 2015 Apr-Jun:32(2):85-9. doi: 10.4103/0970-9371.160549. Epub [PubMed PMID: 26229243]
Bieri U, Kranzbühler B, Wettstein MS, Fankhauser CD, Kaufmann BP, Seifert B, Bode PK, Poyet C, Lenggenhager D, Hermanns T. Limited Value of Bladder Wash Cytology During Follow-Up of Patients With Non-muscle Invasive Bladder Cancer. Cureus. 2023 Jun:15(6):e40283. doi: 10.7759/cureus.40283. Epub 2023 Jun 12 [PubMed PMID: 37448431]
Zhou L, Yang K, Li X, Ding Y, Mu D, Li H, Yan Y, Li J, Wang D, Li W, Cong Y, Gao J, Ma K, Xiao Y, Zhang S, Jiang H, Hu W, Wei Q, Jin X, Guan Z, Liu Q, Xu D, Gao X, Jiang Y, Gan W, Sun G, Wang Q, Liu Y, Hou J, Xie L, Song X, Jin F, Feng J, Cai M, Liang Z, Zhang J, Ye D, Qi L, Ma L, Shou J, Dai Y, Shao J, Tian Y, Hong S, Xu T, Kong C, Kang Z, Liu Y, Qu X, Shi B, Zheng S, Lin Y, Xia S, Wei D, Wu J, Fu W, Wang Z, Liang J. Application of fluorescence in situ hybridization in the detection of bladder transitional-cell carcinoma: A multi-center clinical study based on Chinese population. Asian journal of urology. 2019 Jan:6(1):114-121. doi: 10.1016/j.ajur.2018.06.001. Epub 2018 Jun 8 [PubMed PMID: 30775255]
Level 2 (mid-level) evidenceYoder BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC, Liou LS, Brainard JA, Biscotti CV, Jones JS, Tubbs RR. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. American journal of clinical pathology. 2007 Feb:127(2):295-301 [PubMed PMID: 17210520]
Zheng W, Lin T, Chen Z, Cao D, Bao Y, Zhang P, Yang L, Wei Q. The Role of Fluorescence In Situ Hybridization in the Surveillance of Non-Muscle Invasive Bladder Cancer: An Updated Systematic Review and Meta-Analysis. Diagnostics (Basel, Switzerland). 2022 Aug 19:12(8):. doi: 10.3390/diagnostics12082005. Epub 2022 Aug 19 [PubMed PMID: 36010354]
Level 1 (high-level) evidenceKe C, Liu Z, Zhu J, Zeng X, Hu Z, Yang C. Fluorescence in situ hybridization (FISH) to predict the efficacy of Bacillus Calmette-Guérin perfusion in bladder cancer. Translational cancer research. 2022 Oct:11(10):3448-3457. doi: 10.21037/tcr-22-1367. Epub [PubMed PMID: 36388029]
Mengual L, Marín-Aguilera M, Ribal MJ, Burset M, Villavicencio H, Oliver A, Alcaraz A. Clinical utility of fluorescent in situ hybridization for the surveillance of bladder cancer patients treated with bacillus Calmette-Guérin therapy. European urology. 2007 Sep:52(3):752-9 [PubMed PMID: 17379395]
Lotan Y, Inman BA, Davis LG, Kassouf W, Messing E, Daneshmand S, Canter D, Marble HT, Joseph AM, Jewell S, Boorjian SA. Evaluation of the Fluorescence In Situ Hybridization Test to Predict Recurrence and/or Progression of Disease after bacillus Calmette-Guérin for Primary High Grade Nonmuscle Invasive Bladder Cancer: Results from a Prospective Multicenter Trial. The Journal of urology. 2019 Nov:202(5):920-926. doi: 10.1097/JU.0000000000000355. Epub 2019 Oct 9 [PubMed PMID: 31120373]
Level 1 (high-level) evidenceKamat AM, Dickstein RJ, Messetti F, Anderson R, Pretzsch SM, Gonzalez GN, Katz RL, Khanna A, Zaidi T, Wu X, Grossman HB, Dinney CP. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guérin therapy for bladder cancer: results of a prospective trial. The Journal of urology. 2012 Mar:187(3):862-7. doi: 10.1016/j.juro.2011.10.144. Epub 2012 Jan 15 [PubMed PMID: 22245325]
Sharma G, Sharma A, Krishna M, Devana SK, Singh SK. Xpert bladder cancer monitor in surveillance of bladder cancer: Systematic review and meta-analysis. Urologic oncology. 2022 Apr:40(4):163.e1-163.e9. doi: 10.1016/j.urolonc.2021.08.017. Epub 2021 Sep 14 [PubMed PMID: 34535354]
Level 1 (high-level) evidenceCancel-Tassin G, Roupret M, Pinar U, Gaffory C, Vanie F, Ondet V, Compérat E, Cussenot O. Assessment of Xpert Bladder Cancer Monitor test performance for the detection of recurrence during non-muscle invasive bladder cancer follow-up. World journal of urology. 2021 Sep:39(9):3329-3335. doi: 10.1007/s00345-021-03629-1. Epub 2021 Mar 26 [PubMed PMID: 33770241]
Smrkolj T, Cegovnik Primozic U, Fabjan T, Sterpin S, Osredkar J. The performance of the Xpert Bladder Cancer Monitor Test and voided urinary cytology in the follow-up of urinary bladder tumors. Radiology and oncology. 2020 Dec 29:55(2):196-202. doi: 10.2478/raon-2020-0072. Epub 2020 Dec 29 [PubMed PMID: 33764701]
Valenberg FJPV, Hiar AM, Wallace E, Bridge JA, Mayne DJ, Beqaj S, Sexton WJ, Lotan Y, Weizer AZ, Jansz GK, Stenzl A, Danella JF, Cline KJ, Williams MB, Montgomery S, David RD, Harris R, Klein EW, Bradford TJ, Wolk FN, Westenfelder KR, Trainer AF, Richardson TA, Egerdie RB, Goldfarb B, Zadra JA, Lu X, Simon IM, Campbell SA, Bates MP, Higuchi RG, Witjes JA. Validation of an mRNA-based Urine Test for the Detection of Bladder Cancer in Patients with Haematuria. European urology oncology. 2021 Feb:4(1):93-101. doi: 10.1016/j.euo.2020.09.001. Epub 2020 Sep 28 [PubMed PMID: 33004290]
Level 1 (high-level) evidenceFan Z, Shi H, Luo J, Guo X, Wang B, Liu Y, Yu J. Diagnostic and therapeutic effects of fluorescence cystoscopy and narrow-band imaging in bladder cancer: a systematic review and network meta-analysis. International journal of surgery (London, England). 2023 Oct 1:109(10):3169-3177. doi: 10.1097/JS9.0000000000000592. Epub 2023 Oct 1 [PubMed PMID: 37526087]
Level 1 (high-level) evidenceRusso GI, Sholklapper TN, Cocci A, Broggi G, Caltabiano R, Smith AB, Lotan Y, Morgia G, Kamat AM, Witjes JA, Daneshmand S, Desai MM, Gill IS, Cacciamani GE. Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis. Cancers. 2021 Aug 30:13(17):. doi: 10.3390/cancers13174378. Epub 2021 Aug 30 [PubMed PMID: 34503188]
Level 1 (high-level) evidenceCahill EM, Chua K, Doppalapudi SK, Ghodoussipour S. The use of blue-light cystoscopy in the detection and surveillance of nonmuscle invasive bladder cancer. Current urology. 2022 Sep:16(3):121-126. doi: 10.1097/CU9.0000000000000142. Epub 2022 Aug 27 [PubMed PMID: 36204358]
Hagimoto H, Makita N, Mine Y, Kokubun H, Murata S, Abe Y, Kubota M, Tsutsumi N, Yamasaki T, Kawakita M. Comparison between 5-aminolevulinic acid photodynamic diagnosis and narrow-band imaging for bladder cancer detection. BMC urology. 2021 Dec 22:21(1):180. doi: 10.1186/s12894-021-00946-w. Epub 2021 Dec 22 [PubMed PMID: 34937543]
Fukuhara H, Yamamoto S, Lai HW, Karashima T, Kurabayashi A, Furihata M, Inoue K. Real-world experience with 5-aminolevulinic acid for photodynamic diagnosis of bladder cancer (2nd report): Reduced bladder recurrence after PDD-TURBT. Photodiagnosis and photodynamic therapy. 2022 Jun:38():102757. doi: 10.1016/j.pdpdt.2022.102757. Epub 2022 Feb 11 [PubMed PMID: 35151889]
Lewicki P, Arenas-Gallo C, Qiu Y, Venkat S, Basourakos SP, Scherr D, Shoag JE. Underutilization of Blue Light Cystoscopy for Bladder Cancer in the United States. European urology focus. 2022 Jul:8(4):968-971. doi: 10.1016/j.euf.2021.09.025. Epub 2021 Oct 26 [PubMed PMID: 34711530]
Level 2 (mid-level) evidenceDaniltchenko DI, Riedl CR, Sachs MD, Koenig F, Daha KL, Pflueger H, Loening SA, Schnorr D. Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. The Journal of urology. 2005 Dec:174(6):2129-33, discussion 2133 [PubMed PMID: 16280742]
Level 1 (high-level) evidenceWitjes JA, Douglass J. The role of hexaminolevulinate fluorescence cystoscopy in bladder cancer. Nature clinical practice. Urology. 2007 Oct:4(10):542-9 [PubMed PMID: 17921969]
Mowatt G, N'Dow J, Vale L, Nabi G, Boachie C, Cook JA, Fraser C, Griffiths TR, Aberdeen Technology Assessment Review (TAR) Group. Photodynamic diagnosis of bladder cancer compared with white light cystoscopy: Systematic review and meta-analysis. International journal of technology assessment in health care. 2011 Jan:27(1):3-10. doi: 10.1017/S0266462310001364. Epub 2011 Jan 25 [PubMed PMID: 21262078]
Level 1 (high-level) evidenceNakai Y, Inoue K, Tsuzuki T, Shimamoto T, Shuin T, Nagao K, Matsuyama H, Oyama M, Furuse H, Ozono S, Miyake M, Fujimoto K. Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study. International journal of urology : official journal of the Japanese Urological Association. 2018 Aug:25(8):723-729. doi: 10.1111/iju.13718. Epub 2018 Jul 12 [PubMed PMID: 29999205]
De Dominicis C, Liberti M, Perugia G, De Nunzio C, Sciobica F, Zuccalà A, Sarkozy A, Iori F. Role of 5-aminolevulinic acid in the diagnosis and treatment of superficial bladder cancer: improvement in diagnostic sensitivity. Urology. 2001 Jun:57(6):1059-62 [PubMed PMID: 11377304]
Fukuhara H, Hagiwara Y, Oba K, Inoue K. Real-world experience with 5-aminolevulinic acid for photodynamic diagnosis of bladder cancer (3rd report): Cost impact of transurethral resection of bladder tumor in Japan. Photodiagnosis and photodynamic therapy. 2023 Dec:44():103758. doi: 10.1016/j.pdpdt.2023.103758. Epub 2023 Aug 19 [PubMed PMID: 37604217]
Pohar KS, Patel S, Lotan Y, Trabulsi E, Woods M, Downs T, Huang WC, Jones J, Taylor J, O'Donnell M, Bivalacqua TJ, DeCastro J, Steinberg G, Kamat AM, Resnick MJ, Konety B, Schoenberg M, Jones JS, Daneshmand S, Flexible Blue Light Study Group Collaborators. Safety of repeat blue light cystoscopy with hexaminolevulinate (HAL) in the management of bladder cancer: Results from a phase III, comparative, multi-center study. Urologic oncology. 2022 Aug:40(8):382.e1-382.e6. doi: 10.1016/j.urolonc.2022.04.012. Epub 2022 Jun 22 [PubMed PMID: 35750559]
Level 2 (mid-level) evidenceDaneshmand S, Schuckman AK, Bochner BH, Cookson MS, Downs TM, Gomella LG, Grossman HB, Kamat AM, Konety BR, Lee CT, Pohar KS, Pruthi RS, Resnick MJ, Smith ND, Witjes JA, Schoenberg MP, Steinberg GD. Hexaminolevulinate blue-light cystoscopy in non-muscle-invasive bladder cancer: review of the clinical evidence and consensus statement on appropriate use in the USA. Nature reviews. Urology. 2014 Oct:11(10):589-96. doi: 10.1038/nrurol.2014.245. Epub 2014 Sep 23 [PubMed PMID: 25245244]
Level 3 (low-level) evidenceRenninger M, Fahmy O, Schubert T, Schmid MA, Hassan F, Stenzl A, Gakis G. The prognostic impact of hexaminolevulinate-based bladder tumor resection in patients with primary non-muscle invasive bladder cancer treated with radical cystectomy. World journal of urology. 2020 Feb:38(2):397-406. doi: 10.1007/s00345-019-02780-0. Epub 2019 Apr 27 [PubMed PMID: 31030231]
Pederzoli F, Murati Amador B, Samarska I, Lombardo KA, Kates M, Bivalacqua TJ, Matoso A. Diagnosis of urothelial carcinoma in situ using blue light cystoscopy and the utility of immunohistochemistry in blue light-positive lesions diagnosed as atypical. Human pathology. 2019 Aug:90():1-7. doi: 10.1016/j.humpath.2019.04.018. Epub 2019 May 6 [PubMed PMID: 31071342]
Ray ER, Chatterton K, Khan MS, Chandra A, Thomas K, Dasgupta P, O'Brien TS. Hexylaminolaevulinate fluorescence cystoscopy in patients previously treated with intravesical bacille Calmette-Guérin. BJU international. 2010 Mar:105(6):789-94. doi: 10.1111/j.1464-410X.2009.08839.x. Epub 2009 Oct 13 [PubMed PMID: 19832725]
Draga RO, Grimbergen MC, Kok ET, Jonges TN, van Swol CF, Bosch JL. Photodynamic diagnosis (5-aminolevulinic acid) of transitional cell carcinoma after bacillus Calmette-Guérin immunotherapy and mitomycin C intravesical therapy. European urology. 2010 Apr:57(4):655-60. doi: 10.1016/j.eururo.2009.09.037. Epub 2009 Oct 6 [PubMed PMID: 19819064]
Chang S, Bermoy ME, Chang SS, Scarpato KR, Luckenbaugh AN, Kolouri S, Bowden AK. Enhancing the image quality of blue light cystoscopy through green-hue correction and fogginess removal. Scientific reports. 2023 Dec 6:13(1):21484. doi: 10.1038/s41598-023-48882-z. Epub 2023 Dec 6 [PubMed PMID: 38057491]
Level 2 (mid-level) evidenceNaito S, Algaba F, Babjuk M, Bryan RT, Sun YH, Valiquette L, de la Rosette J, CROES Narrow Band Imaging Global Study Group. The Clinical Research Office of the Endourological Society (CROES) Multicentre Randomised Trial of Narrow Band Imaging-Assisted Transurethral Resection of Bladder Tumour (TURBT) Versus Conventional White Light Imaging-Assisted TURBT in Primary Non-Muscle-invasive Bladder Cancer Patients: Trial Protocol and 1-year Results. European urology. 2016 Sep:70(3):506-15. doi: 10.1016/j.eururo.2016.03.053. Epub 2016 Apr 23 [PubMed PMID: 27117749]
Level 1 (high-level) evidenceNaselli A, Introini C, Timossi L, Spina B, Fontana V, Pezzi R, Germinale F, Bertolotto F, Puppo P. A randomized prospective trial to assess the impact of transurethral resection in narrow band imaging modality on non-muscle-invasive bladder cancer recurrence. European urology. 2012 May:61(5):908-13. doi: 10.1016/j.eururo.2012.01.018. Epub 2012 Jan 20 [PubMed PMID: 22280855]
Level 1 (high-level) evidenceYe Z, Hu J, Song X, Li F, Zhao X, Chen S, Wang X, He D, Fan J, Ye D, Xing J, Pan T, Wang D. A comparison of NBI and WLI cystoscopy in detecting non-muscle-invasive bladder cancer: A prospective, randomized and multi-center study. Scientific reports. 2015 Jun 5:5():10905. doi: 10.1038/srep10905. Epub 2015 Jun 5 [PubMed PMID: 26046790]
Level 1 (high-level) evidenceFosså SD, Ous S, Berner A. Clinical significance of the "palpable mass" in patients with muscle-infiltrating bladder cancer undergoing cystectomy after pre-operative radiotherapy. British journal of urology. 1991 Jan:67(1):54-60 [PubMed PMID: 1993277]
Wijkström H, Norming U, Lagerkvist M, Nilsson B, Näslund I, Wiklund P. Evaluation of clinical staging before cystectomy in transitional cell bladder carcinoma: a long-term follow-up of 276 consecutive patients. British journal of urology. 1998 May:81(5):686-91 [PubMed PMID: 9634042]
Varinot J, Camparo P, Roupret M, Bitker MO, Capron F, Cussenot O, Witjes JA, Compérat E. Full analysis of the prostatic urethra at the time of radical cystoprostatectomy for bladder cancer: impact on final disease stage. Virchows Archiv : an international journal of pathology. 2009 Nov:455(5):449-53. doi: 10.1007/s00428-009-0849-0. Epub 2009 Oct 20 [PubMed PMID: 19841937]
Tabibi A, Simforoosh N, Parvin M, Abdi H, Javaherforooshzadeh A, Farrokhi F, Soltani MH. Predictive factors for prostatic involvement by transitional cell carcinoma of the bladder. Urology journal. 2011 Winter:8(1):43-7 [PubMed PMID: 21404202]
Mazzucchelli R, Barbisan F, Santinelli A, Scarpelli M, Galosi AB, Lopez-Beltran A, Cheng L, Kirkali Z, Montironi R. Prediction of prostatic involvement by urothelial carcinoma in radical cystoprostatectomy for bladder cancer. Urology. 2009 Aug:74(2):385-90. doi: 10.1016/j.urology.2009.03.010. Epub 2009 Jun 7 [PubMed PMID: 19501882]
Pettus JA, Al-Ahmadie H, Barocas DA, Koppie TM, Herr H, Donat SM, Dalbagni G, Reuter VE, Olgac S, Bochner BH. Risk assessment of prostatic pathology in patients undergoing radical cystoprostatectomy. European urology. 2008 Feb:53(2):370-5 [PubMed PMID: 17689003]
Kirkali Z, Canda AE. Superficial urothelial cancer in the prostatic urethra. TheScientificWorldJournal. 2006 Feb 28:6():2603-10 [PubMed PMID: 17619737]
Weiner AB, Desai AS, Meeks JJ. Tumor Location May Predict Adverse Pathology and Survival Following Definitive Treatment for Bladder Cancer: A National Cohort Study. European urology oncology. 2019 May:2(3):304-310. doi: 10.1016/j.euo.2018.08.018. Epub 2018 Sep 14 [PubMed PMID: 31200845]
Matzkin H, Soloway MS, Hardeman S. Transitional cell carcinoma of the prostate. The Journal of urology. 1991 Nov:146(5):1207-12 [PubMed PMID: 1942262]
Mungan MU, Canda AE, Tuzel E, Yorukoglu K, Kirkali Z. Risk factors for mucosal prostatic urethral involvement in superficial transitional cell carcinoma of the bladder. European urology. 2005 Nov:48(5):760-3 [PubMed PMID: 16005563]
Kulkarni GS, Hakenberg OW, Gschwend JE, Thalmann G, Kassouf W, Kamat A, Zlotta A. An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. European urology. 2010 Jan:57(1):60-70. doi: 10.1016/j.eururo.2009.08.024. Epub 2009 Sep 1 [PubMed PMID: 19740595]
Lonati C, Baumeister P, Ornaghi PI, Di Trapani E, De Cobelli O, Rink M, Karnes RJ, Poyet C, Simone G, Afferi L, Necchi A, Briganti A, Montorsi F, Krajewski W, Antonelli A, Cerruto MA, Zamboni S, Simeone C, Mordasini L, Mattei A, Moschini M, EAU-YAU Urothelial Cancer Working Party. Accuracy of Transurethral Resection of the Bladder in Detecting Variant Histology of Bladder Cancer Compared with Radical Cystectomy. European urology focus. 2022 Mar:8(2):457-464. doi: 10.1016/j.euf.2021.04.005. Epub 2021 Apr 16 [PubMed PMID: 33867307]
Yanagisawa T, Mori K, Motlagh RS, Kawada T, Mostafaei H, Quhal F, Laukhtina E, Rajwa P, Aydh A, König F, Pallauf M, Pradere B, D'Andrea D, Compérat E, Miki J, Kimura T, Egawa S, Shariat SF. En Bloc Resection for Bladder Tumors: An Updated Systematic Review and Meta-Analysis of Its Differential Effect on Safety, Recurrence and Histopathology. The Journal of urology. 2022 Apr:207(4):754-768. doi: 10.1097/JU.0000000000002444. Epub 2022 Jan 21 [PubMed PMID: 35060770]
Level 1 (high-level) evidenceCreta M, Celentano G, Califano G, La Rocca R, Longo N. En-bloc Laser Resection of Bladder Tumors: Where Are We Now? Journal of clinical medicine. 2022 Jun 16:11(12):. doi: 10.3390/jcm11123463. Epub 2022 Jun 16 [PubMed PMID: 35743533]
Augspurger RR, Donohue RE. Prevention of obturator nerve stimulation during transurethral surgery. The Journal of urology. 1980 Feb:123(2):170-2 [PubMed PMID: 7188780]
Panagoda PI, Vasdev N, Gowrie-Mohan S. Avoiding the Obturator Jerk during TURBT. Current urology. 2018 Oct:12(1):1-5. doi: 10.1159/000447223. Epub 2018 Jun 30 [PubMed PMID: 30374273]
Ozer K, Horsanali MO, Gorgel SN, Ozbek E. Bladder injury secondary to obturator reflex is more common with plasmakinetic transurethral resection than monopolar transurethral resection of bladder cancer. Central European journal of urology. 2015:68(3):284-8. doi: 10.5173/ceju.2015.565. Epub 2015 Sep 26 [PubMed PMID: 26568867]
Cesur M, Erdem AF, Alici HA, Yapanoglu T, Yuksek MS, Aksoy Y. The role of succinylcholine in the prevention of the obturator nerve reflex during transurethral resection of bladder tumors. Saudi medical journal. 2008 May:29(5):668-71 [PubMed PMID: 18454211]
Deng W, Zhang Q, Yao H. A Systematic Review and Meta-Analysis Comparing the Safety and Efficacy of Spinal Anesthesia and Spinal Anesthesia Combined with Obturator Nerve Block in Transurethral Resection of Bladder Tumors. Emergency medicine international. 2022:2022():8490462. doi: 10.1155/2022/8490462. Epub 2022 Jun 29 [PubMed PMID: 35811608]
Level 1 (high-level) evidenceErbay G, Akyol F, Karabakan M, Celebi B, Keskin E, Hirik E. Effect of obturator nerve block during transurethral resection of lateral bladder wall tumors on the presence of detrusor muscle in tumor specimens and recurrence of the disease. The Kaohsiung journal of medical sciences. 2017 Feb:33(2):86-90. doi: 10.1016/j.kjms.2016.11.006. Epub 2016 Dec 30 [PubMed PMID: 28137416]
Akand M, Muilwijk T, Raskin Y, De Vrieze M, Joniau S, Van Der Aa F. Quality Control Indicators for Transurethral Resection of Non-Muscle-Invasive Bladder Cancer. Clinical genitourinary cancer. 2019 Aug:17(4):e784-e792. doi: 10.1016/j.clgc.2019.04.014. Epub 2019 Apr 19 [PubMed PMID: 31097388]
Level 2 (mid-level) evidenceMariappan P, Johnston A, Padovani L, Clark E, Trail M, Hamid S, Hollins G, Simpson H, Thomas BG, Hasan R, Bhatt J, Ahmad I, Nandwani GM, Mitchell IDC, Hendry D, for members of the Scottish Bladder Cancer QPI Research Collaborative. Enhanced Quality and Effectiveness of Transurethral Resection of Bladder Tumour in Non-muscle-invasive Bladder Cancer: A Multicentre Real-world Experience from Scotland's Quality Performance Indicators Programme. European urology. 2020 Oct:78(4):520-530. doi: 10.1016/j.eururo.2020.06.051. Epub 2020 Jul 17 [PubMed PMID: 32690321]
Level 2 (mid-level) evidenceErsoy H, Yaytokgil M, Karakoyunlu AN, Topaloglu H, Sagnak L, Ozok HU. Single early instillation of mitomycin C and urinary alkalinization in low-risk non-muscle-invasive bladder cancer: a preliminary study. Drug design, development and therapy. 2013:7():1-6. doi: 10.2147/DDDT.S39541. Epub 2012 Dec 28 [PubMed PMID: 23300343]
Barghi MR, Rahmani MR, Hosseini Moghaddam SM, Jahanbin M. Immediate intravesical instillation of mitomycin C after transurethral resection of bladder tumor in patients with low-risk superficial transitional cell carcinoma of bladder. Urology journal. 2006 Fall:3(4):220-4 [PubMed PMID: 17559045]
Han MA, Maisch P, Jung JH, Hwang JE, Narayan V, Cleves A, Hwang EC, Dahm P. Intravesical gemcitabine for non-muscle invasive bladder cancer. The Cochrane database of systematic reviews. 2021 Jun 14:6(6):CD009294. doi: 10.1002/14651858.CD009294.pub3. Epub 2021 Jun 14 [PubMed PMID: 34125951]
Level 1 (high-level) evidenceManoharan M. Intravesical therapy for urothelial carcinoma of the bladder. Indian journal of urology : IJU : journal of the Urological Society of India. 2011 Apr:27(2):252-61. doi: 10.4103/0970-1591.82846. Epub [PubMed PMID: 21814318]
Ali-el-Dein B, Nabeeh A, el-Baz M, Shamaa S, Ashamallah A. Single-dose versus multiple instillations of epirubicin as prophylaxis for recurrence after transurethral resection of pTa and pT1 transitional-cell bladder tumours: a prospective, randomized controlled study. British journal of urology. 1997 May:79(5):731-5 [PubMed PMID: 9158511]
Level 1 (high-level) evidenceMitsumori K, Tsuchiya N, Habuchi T, Li Z, Akao T, Ohyama C, Sato K, Kato T. Early and large-dose intravesical instillation of epirubicin to prevent superficial bladder carcinoma recurrence after transurethral resection. BJU international. 2004 Aug:94(3):317-21 [PubMed PMID: 15291859]
Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, Pollifrone DL, Harbrecht JD, Chin JL, Lerner SP, Miles BJ, International Mitomycin C Consortium. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. Journal of the National Cancer Institute. 2001 Apr 18:93(8):597-604 [PubMed PMID: 11309436]
Level 1 (high-level) evidenceGiesbers AA, Van Helsdingen PJ, Kramer AE. Recurrence of superficial bladder carcinoma after intravesical instillation of mitomycin-C. Comparison of exposure times. British journal of urology. 1989 Feb:63(2):176-9 [PubMed PMID: 2495144]
Kuroda M, Niijima T, Kotake T, Akaza H, Hinotsu S, 6th Trial of the Japanese Urological Cancer Research Group. Effect of prophylactic treatment with intravesical epirubicin on recurrence of superficial bladder cancer--The 6th Trial of the Japanese Urological Cancer Research Group (JUCRG): a randomized trial of intravesical epirubicin at dose of 20mg/40ml, 30mg/40ml, 40mg/40ml. European urology. 2004 May:45(5):600-5 [PubMed PMID: 15082202]
Level 1 (high-level) evidenceTaylor CA, Tobert CM, Kahnoski RJ, Humphrey JE, Lane BR. The value of a comprehensive primary outcome - results of a negative randomized control trial in the non-muscle invasive bladder cancer population. The Canadian journal of urology. 2021 Aug:28(4):10756-10761 [PubMed PMID: 34378511]
Level 1 (high-level) evidenceSylvester RJ, Oosterlinck W, Holmang S, Sydes MR, Birtle A, Gudjonsson S, De Nunzio C, Okamura K, Kaasinen E, Solsona E, Ali-El-Dein B, Tatar CA, Inman BA, N'Dow J, Oddens JR, Babjuk M. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? European urology. 2016 Feb:69(2):231-44. doi: 10.1016/j.eururo.2015.05.050. Epub 2015 Jun 16 [PubMed PMID: 26091833]
Level 1 (high-level) evidenceSylvester RJ, Oosterlinck W. An immediate instillation after transurethral resection of bladder tumor in non-muscle-invasive bladder cancer: has the evidence changed? European urology. 2009 Jul:56(1):43-5. doi: 10.1016/j.eururo.2009.03.074. Epub 2009 Apr 3 [PubMed PMID: 19375216]
Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. The Journal of urology. 2004 Jun:171(6 Pt 1):2186-90, quiz 2435 [PubMed PMID: 15126782]
Level 1 (high-level) evidenceAbern MR, Owusu RA, Anderson MR, Rampersaud EN, Inman BA. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. Journal of the National Comprehensive Cancer Network : JNCCN. 2013 Apr 1:11(4):477-84 [PubMed PMID: 23584348]
Level 1 (high-level) evidencePerlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. European urology. 2013 Sep:64(3):421-30. doi: 10.1016/j.eururo.2013.06.009. Epub 2013 Jun 19 [PubMed PMID: 23830475]
Level 1 (high-level) evidenceKang M, Jeong CW, Kwak C, Kim HH, Ku JH. Single, immediate postoperative instillation of chemotherapy in non-muscle invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials using different drugs. Oncotarget. 2016 Jul 19:7(29):45479-45488. doi: 10.18632/oncotarget.9991. Epub [PubMed PMID: 27323781]
Level 1 (high-level) evidenceDaryanto B, Purnomo AF, Seputra KP, Budaya TN. Comparison Between Intravesical Chemotherapy Epirubicin and Mitomycin-C after TURB vs TURB Alone With Recurrence Rate of Non-Muscle Invasive Bladder Cancer: Meta-Analysis. Medical archives (Sarajevo, Bosnia and Herzegovina). 2022 Jun:76(3):198-201. doi: 10.5455/medarh.2022.76.198-201. Epub [PubMed PMID: 36200115]
Level 1 (high-level) evidencePode D, Alon Y, Horowitz AT, Vlodavsky I, Biran S. The mechanism of human bladder tumor implantation in an in vitro model. The Journal of urology. 1986 Aug:136(2):482-6 [PubMed PMID: 3525861]
Anderson C, Weber R, Patel D, Lowrance W, Mellis A, Cookson M, Lang M, Barocas D, Chang S, Newberger E, Montgomery JS, Weizer AZ, Lee CT, Kava BR, Jackson M, Meraney A, Sjoberg D, Bochner B, Dalbagni G, Donat M, Herr H. A 10-Item Checklist Improves Reporting of Critical Procedural Elements during Transurethral Resection of Bladder Tumor. The Journal of urology. 2016 Oct:196(4):1014-20. doi: 10.1016/j.juro.2016.03.151. Epub 2016 Apr 1 [PubMed PMID: 27044571]
Haddad J, Anderson C, Heinlen J, Stratton K, Mellis A, Herr H, Cookson M, Patel S. Improving the quality of operative reports for transurethral resection of bladder tumor surgery in resident education. The Canadian journal of urology. 2017 Oct:24(5):8976-8981 [PubMed PMID: 28971783]
Level 2 (mid-level) evidenceEroglu A, Ekin RG, Koc G, Divrik RT. The prognostic value of routine second transurethral resection in patients with newly diagnosed stage pT1 non-muscle-invasive bladder cancer: results from randomized 10-year extension trial. International journal of clinical oncology. 2020 Apr:25(4):698-704. doi: 10.1007/s10147-019-01581-0. Epub 2019 Nov 23 [PubMed PMID: 31760524]
Level 1 (high-level) evidenceGordon PC, Thomas F, Noon AP, Rosario DJ, Catto JWF. Long-term Outcomes from Re-resection for High-risk Non-muscle-invasive Bladder Cancer: A Potential to Rationalize Use. European urology focus. 2019 Jul:5(4):650-657. doi: 10.1016/j.euf.2017.10.004. Epub 2017 Oct 28 [PubMed PMID: 29089252]
Bishr M, Lattouf JB, Latour M, Saad F. Tumour stage on re-staging transurethral resection predicts recurrence and progression-free survival of patients with high-risk non-muscle invasive bladder cancer. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2014 May:8(5-6):E306-10. doi: 10.5489/cuaj.1514. Epub [PubMed PMID: 24940455]
Palou J, Pisano F, Sylvester R, Joniau S, Serretta V, Larré S, Di Stasi S, van Rhijn B, Witjes AJ, Grotenhuis A, Colombo R, Briganti A, Babjuk M, Soukup V, Malmstrom PU, Irani J, Malats N, Baniel J, Mano R, Cai T, Cha EK, Ardelt P, Varkarakis J, Bartoletti R, Dalbagni G, Shariat SF, Xylinas E, Karnes RJ, Gontero P. Recurrence, progression and cancer-specific mortality according to stage at re-TUR in T1G3 bladder cancer patients treated with BCG: not as bad as previously thought. World journal of urology. 2018 Oct:36(10):1621-1627. doi: 10.1007/s00345-018-2299-2. Epub 2018 May 2 [PubMed PMID: 29721611]
Gontero P, Sylvester R, Pisano F, Joniau S, Oderda M, Serretta V, Larré S, Di Stasi S, Van Rhijn B, Witjes AJ, Grotenhuis AJ, Colombo R, Briganti A, Babjuk M, Soukup V, Malmström PU, Irani J, Malats N, Baniel J, Mano R, Cai T, Cha EK, Ardelt P, Vakarakis J, Bartoletti R, Dalbagni G, Shariat SF, Xylinas E, Karnes RJ, Palou J. The impact of re-transurethral resection on clinical outcomes in a large multicentre cohort of patients with T1 high-grade/Grade 3 bladder cancer treated with bacille Calmette-Guérin. BJU international. 2016 Jul:118(1):44-52. doi: 10.1111/bju.13354. Epub 2015 Nov 6 [PubMed PMID: 26469362]
Level 2 (mid-level) evidenceBaltacı S, Bozlu M, Yıldırım A, Gökçe Mİ, Tinay İ, Aslan G, Can C, Türkeri L, Kuyumcuoğlu U, Mungan A. Significance of the interval between first and second transurethral resection on recurrence and progression rates in patients with high-risk non-muscle-invasive bladder cancer treated with maintenance intravesical Bacillus Calmette-Guérin. BJU international. 2015 Nov:116(5):721-6. doi: 10.1111/bju.13102. Epub 2015 May 11 [PubMed PMID: 25715815]
Tolley DA, Parmar MK, Grigor KM, Lallemand G, Benyon LL, Fellows J, Freedman LS, Grigor KM, Hall RR, Hargreave TB, Munson K, Newling DW, Richards B, Robinson MR, Rose MB, Smith PH, Williams JL, Whelan P. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: a further report with 7 years of follow up. The Journal of urology. 1996 Apr:155(4):1233-8 [PubMed PMID: 8632538]
Ali-el-Dein B, el-Baz M, Aly AN, Shamaa S, Ashamallah A. Intravesical epirubicin versus doxorubicin for superficial bladder tumors (stages pTa and pT1): a randomized prospective study. The Journal of urology. 1997 Jul:158(1):68-73; discussion 73-4 [PubMed PMID: 9186325]
Level 1 (high-level) evidenceLiu B, Wang Z, Chen B, Yu J, Zhang P, Ding Q, Zhang Y. Randomized study of single instillation of epirubicin for superficial bladder carcinoma: long-term clinical outcomes. Cancer investigation. 2006 Mar:24(2):160-3 [PubMed PMID: 16537185]
Level 1 (high-level) evidenceTürkeri L, Tanıdır Y, Çal Ç, Özen H, Şahin H, Turkish Urooncology Society. Comparison of the efficacy of single or double intravesical epirubicin instillation in the early postoperative period to prevent recurrences in non-muscle-invasive urothelial carcinoma of the bladder: prospective, randomized multicenter study. Urologia internationalis. 2010:85(3):261-5. doi: 10.1159/000300571. Epub 2010 Mar 20 [PubMed PMID: 20332605]
Level 1 (high-level) evidenceBöhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. The Journal of urology. 2003 Jan:169(1):90-5 [PubMed PMID: 12478111]
Level 1 (high-level) evidenceHan RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006 Jun:67(6):1216-23 [PubMed PMID: 16765182]
Level 1 (high-level) evidenceShelley MD, Wilt TJ, Court J, Coles B, Kynaston H, Mason MD. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU international. 2004 Mar:93(4):485-90 [PubMed PMID: 15008714]
Level 1 (high-level) evidenceShelley MD, Kynaston H, Court J, Wilt TJ, Coles B, Burgon K, Mason MD. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU international. 2001 Aug:88(3):209-16 [PubMed PMID: 11488731]
Level 1 (high-level) evidenceOkafor CN, Rewane A, Momodu II. Bacillus Calmette Guerin. StatPearls. 2024 Jan:(): [PubMed PMID: 30844212]
Kawai K, Miyazaki J, Joraku A, Nishiyama H, Akaza H. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: current understanding and perspectives on engineered BCG vaccine. Cancer science. 2013 Jan:104(1):22-7. doi: 10.1111/cas.12075. Epub 2013 Jan 3 [PubMed PMID: 23181987]
Level 3 (low-level) evidenceFuge O, Vasdev N, Allchorne P, Green JS. Immunotherapy for bladder cancer. Research and reports in urology. 2015:7():65-79. doi: 10.2147/RRU.S63447. Epub 2015 May 4 [PubMed PMID: 26000263]
Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. The Journal of urology. 2005 Jul:174(1):86-91; discussion 91-2 [PubMed PMID: 15947584]
Level 1 (high-level) evidenceShah G, Zhang G, Chen F, Cao Y, Kalyanaraman B, See WA. iNOS expression and NO production contribute to the direct effects of BCG on urothelial carcinoma cell biology. Urologic oncology. 2014 Jan:32(1):45.e1-9. doi: 10.1016/j.urolonc.2013.06.005. Epub 2013 Sep 17 [PubMed PMID: 24054867]
Morcos E, Jansson OT, Adolfsson J, Ehrén I, Wiklund NP. Bacillus Calmette-Guerin induces long-term local formation of nitric oxide in the bladder via the induction of nitric oxide synthase activity in urothelial cells. The Journal of urology. 2001 Feb:165(2):678-82 [PubMed PMID: 11176457]
Lamm DL, Morales A. A BCG success story: From prevention of tuberculosis to optimal bladder cancer treatment. Vaccine. 2021 Dec 8:39(50):7308-7318. doi: 10.1016/j.vaccine.2021.08.026. Epub 2021 Aug 18 [PubMed PMID: 34417051]
Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, Gontero P, Hoeltl W, Turkeri L, Marreaud S, Collette S, Oosterlinck W. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. European urology. 2013 Mar:63(3):462-72. doi: 10.1016/j.eururo.2012.10.039. Epub 2012 Nov 2 [PubMed PMID: 23141049]
Level 1 (high-level) evidenceLamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB, Beck TM, Leimert JT, Crawford ED. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. The Journal of urology. 2000 Apr:163(4):1124-9 [PubMed PMID: 10737480]
Level 1 (high-level) evidenceGrabe-Heyne K, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF. Intermediate and high-risk non-muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Frontiers in oncology. 2023:13():1170124. doi: 10.3389/fonc.2023.1170124. Epub 2023 Jun 2 [PubMed PMID: 37333804]
Level 3 (low-level) evidenceKatims AB, Tallman J, Vertosick E, Porwal S, Dalbagni G, Cha EK, Smith R, Benfante N, Herr HW. Response to 2 Induction Courses of Bacillus Calmette-Guèrin Therapy Among Patients With High-Risk Non-Muscle-Invasive Bladder Cancer: 5-year Follow-Up of a Phase 2 Clinical Trial. JAMA oncology. 2024 Apr 1:10(4):522-525. doi: 10.1001/jamaoncol.2023.6804. Epub [PubMed PMID: 38358761]
Level 1 (high-level) evidenceLlano A, Chan A, Kuk C, Kassouf W, Zlotta AR. Carcinoma In Situ (CIS): Is There a Difference in Efficacy between Various BCG Strains? A Comprehensive Review of the Literature. Cancers. 2024 Jan 5:16(2):. doi: 10.3390/cancers16020245. Epub 2024 Jan 5 [PubMed PMID: 38254736]
Liu Y, Lu J, Huang Y, Ma L. Clinical Spectrum of Complications Induced by Intravesical Immunotherapy of Bacillus Calmette-Guérin for Bladder Cancer. Journal of oncology. 2019:2019():6230409. doi: 10.1155/2019/6230409. Epub 2019 Mar 10 [PubMed PMID: 30984262]
Lukacs S, Tschobotko B, Szabo NA, Symes A. Systemic BCG-Osis as a Rare Side Effect of Intravesical BCG Treatment for Superficial Bladder Cancer. Case reports in urology. 2013:2013():821526. doi: 10.1155/2013/821526. Epub 2013 Jun 17 [PubMed PMID: 23844314]
Level 3 (low-level) evidencePérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, Lumbreras C, Tejido Á, San Juan R, Arrebola-Pajares A, Lizasoain M, Prieto S, Aguado JM. Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine. 2014 Oct:93(17):236-254. doi: 10.1097/MD.0000000000000119. Epub [PubMed PMID: 25398060]
Fernandes PF, Nunes P, Figueiredo A. Septic Shock After Intravesical Therapy With Bacillus Calmette-Guerin: A Case Report of a Rare Life-Threatening Complication. Cureus. 2023 Oct:15(10):e46563. doi: 10.7759/cureus.46563. Epub 2023 Oct 6 [PubMed PMID: 37933342]
Level 3 (low-level) evidenceLiaw F, Tan YY, Hendry D. Systemic BCG-osis following intravesical BCG instillation for bladder carcinoma. Clinical case reports. 2017 Oct:5(10):1569-1572. doi: 10.1002/ccr3.1129. Epub 2017 Aug 15 [PubMed PMID: 29026546]
Level 3 (low-level) evidenceOladiran O, Nwosu I, Oladunjoye A, Oladunjoye O. Disseminated BCG sepsis following intravesical therapy for Bladder Carcinoma: A case report and review of literature. Journal of community hospital internal medicine perspectives. 2020 May 21:10(2):168-170. doi: 10.1080/20009666.2020.1742475. Epub 2020 May 21 [PubMed PMID: 32850058]
Level 3 (low-level) evidenceDinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urologic oncology. 2013 Nov:31(8):1635-42. doi: 10.1016/j.urolonc.2012.04.010. Epub 2012 May 9 [PubMed PMID: 22575238]
Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, Bivalacqua TJ, Montgomery JS, Lerner SP, Busby JE, Poch M, Crispen PL, Steinberg GD, Schuckman AK, Downs TM, Svatek RS, Mashni J Jr, Lane BR, Guzzo TJ, Bratslavsky G, Karsh LI, Woods ME, Brown G, Canter D, Luchey A, Lotan Y, Krupski T, Inman BA, Williams MB, Cookson MS, Keegan KA, Andriole GL Jr, Sankin AI, Boyd A, O'Donnell MA, Sawutz D, Philipson R, Coll R, Narayan VM, Treasure FP, Yla-Herttuala S, Parker NR, Dinney CPN. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. The Lancet. Oncology. 2021 Jan:22(1):107-117. doi: 10.1016/S1470-2045(20)30540-4. Epub 2020 Nov 27 [PubMed PMID: 33253641]
Chamie K, Chang SS, Kramolowsky E, Gonzalgo ML, Agarwal PK, Bassett JC, Bjurlin M, Cher ML, Clark W, Cowan BE, David R, Goldfischer E, Guru K, Jalkut MW, Kaffenberger SD, Kaminetsky J, Katz AE, Koo AS, Sexton WJ, Tikhonenkov SN, Trabulsi EJ, Trainer AF, Spilman P, Huang M, Bhar P, Taha SA, Sender L, Reddy S, Soon-Shiong P. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM evidence. 2023 Jan:2(1):EVIDoa2200167. doi: 10.1056/EVIDoa2200167. Epub 2022 Nov 10 [PubMed PMID: 38320011]
McElree IM, Steinberg RL, Martin AC, Richards J, Mott SL, Gellhaus PT, Nepple KG, O'Donnell MA, Packiam VT. Sequential Intravesical Gemcitabine and Docetaxel for bacillus Calmette-Guérin-Naïve High-Risk Nonmuscle-Invasive Bladder Cancer. The Journal of urology. 2022 Sep:208(3):589-599. doi: 10.1097/JU.0000000000002740. Epub 2022 Jul 27 [PubMed PMID: 35892270]
Chevuru PT, McElree IM, Mott SL, Steinberg RL, O'Donnell MA, Packiam VT. Long-term follow-up of sequential intravesical gemcitabine and docetaxel salvage therapy for non-muscle invasive bladder cancer. Urologic oncology. 2023 Mar:41(3):148.e1-148.e7. doi: 10.1016/j.urolonc.2022.10.030. Epub 2022 Nov 28 [PubMed PMID: 36456454]
Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G. European urology. 2015 Aug:68(2):267-79. doi: 10.1016/j.eururo.2015.02.032. Epub 2015 Mar 29 [PubMed PMID: 25824720]
Level 1 (high-level) evidenceSyed S, Rahman M, Israr A, Anwar M, Khatroth S, Safi D, Kamran A. A systematic review on the available treatment modalities for Bacillus Calmette-Guérin-unresponsive carcinoma in situ and tumors in patients who are ineligible for or decline radical cystectomy. SAGE open medicine. 2023:11():20503121231160408. doi: 10.1177/20503121231160408. Epub 2023 Mar 16 [PubMed PMID: 36949824]
Level 1 (high-level) evidenceMcElree IM, Steinberg RL, Mott SL, O'Donnell MA, Packiam VT. Comparison of Sequential Intravesical Gemcitabine and Docetaxel vs Bacillus Calmette-Guérin for the Treatment of Patients With High-Risk Non-Muscle-Invasive Bladder Cancer. JAMA network open. 2023 Feb 1:6(2):e230849. doi: 10.1001/jamanetworkopen.2023.0849. Epub 2023 Feb 1 [PubMed PMID: 36853609]
McElree IM, Packiam VT, Steinberg RL, Mott SL, Gellhaus PT, Nepple KG, O'Donnell MA. Sequential Intravesical Valrubicin and Docetaxel for the Salvage Treatment of Non-Muscle-Invasive Bladder Cancer. The Journal of urology. 2022 Nov:208(5):969-977. doi: 10.1097/JU.0000000000002848. Epub 2022 Jul 5 [PubMed PMID: 35830552]
Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nature reviews. Urology. 2018 Nov:15(11):667-685. doi: 10.1038/s41585-018-0092-z. Epub [PubMed PMID: 30254383]
Jung JH, Gudeloglu A, Kiziloz H, Kuntz GM, Miller A, Konety BR, Dahm P. Intravesical electromotive drug administration for non-muscle invasive bladder cancer. The Cochrane database of systematic reviews. 2017 Sep 12:9(9):CD011864. doi: 10.1002/14651858.CD011864.pub2. Epub 2017 Sep 12 [PubMed PMID: 28898400]
Level 1 (high-level) evidenceMelgarejo Segura MT, Morales Martínez A, Yáñez Castillo Y, Arrabal Polo MÁ, Gómez Lechuga P, Pareja Vílchez M, Arrabal Martín M. Conductive hyperthermic chemotherapy versus electromotive drug administration of mitomycin C as intravesical adjuvant treatment of patients with intermediate or high-risk non-muscle invasive bladder cancer. Urologic oncology. 2023 Feb:41(2):109.e1-109.e8. doi: 10.1016/j.urolonc.2022.10.019. Epub 2022 Nov 12 [PubMed PMID: 36379812]
Guerrero-Ramos F, González-Padilla DA, González-Díaz A, de la Rosa-Kehrmann F, Rodríguez-Antolín A, Inman BA, Villacampa-Aubá F. Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial. World journal of urology. 2022 Apr:40(4):999-1004. doi: 10.1007/s00345-022-03928-1. Epub 2022 Jan 17 [PubMed PMID: 35037963]
Level 1 (high-level) evidenceSmith AB, Deal AM, Woods ME, Wallen EM, Pruthi RS, Chen RC, Milowsky MI, Nielsen ME. Muscle-invasive bladder cancer: evaluating treatment and survival in the National Cancer Data Base. BJU international. 2014 Nov:114(5):719-26. doi: 10.1111/bju.12601. Epub 2014 Apr 16 [PubMed PMID: 24325202]
Svatek RS, Shariat SF, Novara G, Skinner EC, Fradet Y, Bastian PJ, Kamat AM, Kassouf W, Karakiewicz PI, Fritsche HM, Izawa JI, Tilki D, Ficarra V, Volkmer BG, Isbarn H, Dinney CP. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU international. 2011 Mar:107(6):898-904. doi: 10.1111/j.1464-410X.2010.09628.x. Epub 2011 Jan 18 [PubMed PMID: 21244604]
Level 1 (high-level) evidenceTurker P, Bostrom PJ, Wroclawski ML, van Rhijn B, Kortekangas H, Kuk C, Mirtti T, Fleshner NE, Jewett MA, Finelli A, Kwast TV, Evans A, Sweet J, Laato M, Zlotta AR. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU international. 2012 Sep:110(6):804-11. doi: 10.1111/j.1464-410X.2012.10939.x. Epub 2012 Feb 9 [PubMed PMID: 22321341]
Ahmadi H, Mitra AP, Abdelsayed GA, Cai J, Djaladat H, Bruins HM, Daneshmand S. Principal component analysis based pre-cystectomy model to predict pathological stage in patients with clinical organ-confined bladder cancer. BJU international. 2013 Apr:111(4 Pt B):E167-72. doi: 10.1111/j.1464-410X.2012.11502.x. Epub 2012 Oct 4 [PubMed PMID: 23035696]
Aminoltejari K, Black PC. Radical cystectomy: a review of techniques, developments and controversies. Translational andrology and urology. 2020 Dec:9(6):3073-3081. doi: 10.21037/tau.2020.03.23. Epub [PubMed PMID: 33457280]
Venkatramani V, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, Weizer AZ, Konety BR, Tollefson M, Krupski TL, Smith ND, Shabsigh A, Barocas DA, Quek ML, Dash A, Kibel AS, Pruthi RS, Montgomery JS, Weight CJ, Sharp DS, Chang SS, Cookson MS, Gupta GN, Gorbonos A, Uchio EM, Skinner E, Soodana-Prakash N, Becerra MF, Swain S, Kendrick K, Smith JA Jr, Thompson IM, Parekh DJ. Predictors of Recurrence, and Progression-Free and Overall Survival following Open versus Robotic Radical Cystectomy: Analysis from the RAZOR Trial with a 3-Year Followup. The Journal of urology. 2020 Mar:203(3):522-529. doi: 10.1097/JU.0000000000000565. Epub 2019 Sep 24 [PubMed PMID: 31549935]
Venkatramani V, Reis IM, Gonzalgo ML, Castle EP, Woods ME, Svatek RS, Weizer AZ, Konety BR, Tollefson M, Krupski TL, Smith ND, Shabsigh A, Barocas DA, Quek ML, Dash A, Parekh DJ. Comparison of Robot-Assisted and Open Radical Cystectomy in Recovery of Patient-Reported and Performance-Related Measures of Independence: A Secondary Analysis of a Randomized Clinical Trial. JAMA network open. 2022 Feb 1:5(2):e2148329. doi: 10.1001/jamanetworkopen.2021.48329. Epub 2022 Feb 1 [PubMed PMID: 35171260]
Level 1 (high-level) evidenceParekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, Weizer AZ, Konety BR, Tollefson M, Krupski TL, Smith ND, Shabsigh A, Barocas DA, Quek ML, Dash A, Kibel AS, Shemanski L, Pruthi RS, Montgomery JS, Weight CJ, Sharp DS, Chang SS, Cookson MS, Gupta GN, Gorbonos A, Uchio EM, Skinner E, Venkatramani V, Soodana-Prakash N, Kendrick K, Smith JA Jr, Thompson IM. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet (London, England). 2018 Jun 23:391(10139):2525-2536. doi: 10.1016/S0140-6736(18)30996-6. Epub [PubMed PMID: 29976469]
Level 1 (high-level) evidenceBochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, Coleman JA, Mathew S, Vickers A, Schnorr GC, Feuerstein MA, Rapkin B, Parra RO, Herr HW, Laudone VP. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. European urology. 2015 Jun:67(6):1042-1050. doi: 10.1016/j.eururo.2014.11.043. Epub 2014 Dec 8 [PubMed PMID: 25496767]
Level 1 (high-level) evidenceBochner BH, Dalbagni G, Marzouk KH, Sjoberg DD, Lee J, Donat SM, Coleman JA, Vickers A, Herr HW, Laudone VP. Randomized Trial Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: Oncologic Outcomes. European urology. 2018 Oct:74(4):465-471. doi: 10.1016/j.eururo.2018.04.030. Epub 2018 May 18 [PubMed PMID: 29784190]
Level 1 (high-level) evidenceBui T, Bordoni B. Anatomy, Abdomen and Pelvis: Inguinal Lymph Node. StatPearls. 2024 Jan:(): [PubMed PMID: 32491571]
Qi W, Zhong M, Jiang N, Zhou Y, Lv G, Li R, Shi B, Chen S. Which lymph node dissection template is optimal for radical cystectomy? A systematic review and Bayesian network meta-analysis. Frontiers in oncology. 2022:12():986150. doi: 10.3389/fonc.2022.986150. Epub 2022 Nov 25 [PubMed PMID: 36505883]
Level 1 (high-level) evidenceMuilwijk T, Akand M, Gevaert T, Joniau S. No survival difference between super extended and standard lymph node dissection at radical cystectomy: what can we learn from the first prospective randomized phase III trial? Translational andrology and urology. 2019 Mar:8(Suppl 1):S112-S115. doi: 10.21037/tau.2018.12.09. Epub [PubMed PMID: 31143684]
Level 1 (high-level) evidenceMøller MK, Høyer S, Jensen JB. Extended versus superextended lymph-node dissection in radical cystectomy: subgroup analysis of possible recurrence-free survival benefit. Scandinavian journal of urology. 2016 Jun:50(3):175-80. doi: 10.3109/21681805.2015.1132473. Epub 2016 Apr 6 [PubMed PMID: 27049808]
Svatek R, Zehnder P. Role and extent of lymphadenectomy during radical cystectomy for invasive bladder cancer. Current urology reports. 2012 Apr:13(2):115-21. doi: 10.1007/s11934-012-0235-3. Epub [PubMed PMID: 22328190]
Bruins HM, Veskimae E, Hernandez V, Imamura M, Neuberger MM, Dahm P, Stewart F, Lam TB, N'Dow J, van der Heijden AG, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Sherif A, Witjes JA. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: a systematic review. European urology. 2014 Dec:66(6):1065-77. doi: 10.1016/j.eururo.2014.05.031. Epub 2014 Jul 26 [PubMed PMID: 25074764]
Level 1 (high-level) evidenceGschwend JE, Heck MM, Lehmann J, Rübben H, Albers P, Wolff JM, Frohneberg D, de Geeter P, Heidenreich A, Kälble T, Stöckle M, Schnöller T, Stenzl A, Müller M, Truss M, Roth S, Liehr UB, Leißner J, Bregenzer T, Retz M. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. European urology. 2019 Apr:75(4):604-611. doi: 10.1016/j.eururo.2018.09.047. Epub 2018 Oct 15 [PubMed PMID: 30337060]
Level 1 (high-level) evidenceSasaki Y, Izumi K, Fukuta K, Kadoriku F, Atagi Y, Daizumoto K, Shiozaki K, Tomida R, Kusuhara Y, Fukawa T, Yanagihara Y, Yamaguchi K, Yamamoto Y, Izaki H, Takahashi M, Okamoto K, Yamanaka M, Furukawa J. Impact of lymph node dissection on surgical and oncological outcomes in patients undergoing robot-assisted radical cystectomy for bladder cancer: a multicenter retrospective study. Journal of robotic surgery. 2024 Mar 30:18(1):141. doi: 10.1007/s11701-024-01893-y. Epub 2024 Mar 30 [PubMed PMID: 38554230]
Level 2 (mid-level) evidenceInternational Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, Australian Bladder Cancer Study Group, National Cancer Institute of Canada Clinical Trials Group, Finnbladder, Norwegian Bladder Cancer Study Group, Club Urologico Espanol de Tratamiento Oncologico Group, Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jun 1:29(16):2171-7. doi: 10.1200/JCO.2010.32.3139. Epub 2011 Apr 18 [PubMed PMID: 21502557]
Level 1 (high-level) evidenceAdvanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet (London, England). 2003 Jun 7:361(9373):1927-34 [PubMed PMID: 12801735]
Level 1 (high-level) evidenceWinquist E, Kirchner TS, Segal R, Chin J, Lukka H, Genitourinary Cancer Disease Site Group, Cancer Care Ontario Program in Evidence-based Care Practice Guidelines Initiative. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. The Journal of urology. 2004 Feb:171(2 Pt 1):561-9 [PubMed PMID: 14713760]
Level 1 (high-level) evidenceAdvanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. European urology. 2005 Aug:48(2):202-5; discussion 205-6 [PubMed PMID: 15939524]
Level 1 (high-level) evidenceMalmström PU, Rintala E, Wahlqvist R, Hellström P, Hellsten S, Hannisdal E. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. The Nordic Cooperative Bladder Cancer Study Group. The Journal of urology. 1996 Jun:155(6):1903-6 [PubMed PMID: 8618283]
. Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet (London, England). 1999 Aug 14:354(9178):533-40 [PubMed PMID: 10470696]
Level 1 (high-level) evidenceGalsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, Yu EY, Powles T, Moshier EL, Ladoire S, Hussain SA, Agarwal N, Vaishampayan UN, Recine F, Berthold D, Necchi A, Theodore C, Milowsky MI, Bellmunt J, Rosenberg JE, Retrospective International Study of Cancers of the Urothelial Tract (RISC) Investigators. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015 Aug 1:121(15):2586-93. doi: 10.1002/cncr.29387. Epub 2015 Apr 14 [PubMed PMID: 25872978]
Level 2 (mid-level) evidenceSternberg CN, Collette L. What has been learned from meta-analyses of neoadjuvant and adjuvant chemotherapy in bladder cancer? BJU international. 2006 Sep:98(3):487-9 [PubMed PMID: 16925739]
van de Putte EE, Mertens LS, Meijer RP, van der Heijden MS, Bex A, van der Poel HG, Kerst JM, Bergman AM, Horenblas S, van Rhijn BW. Neoadjuvant induction dose-dense MVAC for muscle invasive bladder cancer: efficacy and safety compared with classic MVAC and gemcitabine/cisplatin. World journal of urology. 2016 Feb:34(2):157-62. doi: 10.1007/s00345-015-1636-y. Epub 2015 Jul 17 [PubMed PMID: 26184106]
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 Aug 10:41(23):3881-3890. doi: 10.1200/JCO.22.02763. Epub [PubMed PMID: 37549482]
Level 1 (high-level) evidenceYuh BE, Ruel N, Wilson TG, Vogelzang N, Pal SK. Pooled analysis of clinical outcomes with neoadjuvant cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer. The Journal of urology. 2013 May:189(5):1682-6. doi: 10.1016/j.juro.2012.10.120. Epub 2012 Nov 1 [PubMed PMID: 23123547]
Level 2 (mid-level) evidenceLee FC, Harris W, Cheng HH, Shenoi J, Zhao S, Wang J, Champion T, Izard J, Gore JL, Porter M, Yu EY, Wright JL. Pathologic Response Rates of Gemcitabine/Cisplatin versus Methotrexate/Vinblastine/Adriamycin/Cisplatin Neoadjuvant Chemotherapy for Muscle Invasive Urothelial Bladder Cancer. Advances in urology. 2013:2013():317190. doi: 10.1155/2013/317190. Epub 2013 Dec 8 [PubMed PMID: 24382958]
Level 3 (low-level) evidenceRosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullén A, Nilsson S, Malmström PU, Nordic Urothelial Cancer Group. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. European urology. 2012 Jun:61(6):1229-38. doi: 10.1016/j.eururo.2011.12.010. Epub 2011 Dec 13 [PubMed PMID: 22189383]
Voskuilen CS, Oo HZ, Genitsch V, Smit LA, Vidal A, Meneses M, Necchi A, Colecchia M, Xylinas E, Fontugne J, Sibony M, Rouprêt M, Lenfant L, Côté JF, Buser L, Saba K, Furrer MA, van der Heijden MS, Daugaard M, Black PC, van Rhijn BWG, Hendricksen K, Poyet C, Seiler R. Multicenter Validation of Histopathologic Tumor Regression Grade After Neoadjuvant Chemotherapy in Muscle-invasive Bladder Carcinoma. The American journal of surgical pathology. 2019 Dec:43(12):1600-1610. doi: 10.1097/PAS.0000000000001371. Epub [PubMed PMID: 31524642]
Level 1 (high-level) evidenceBrodtkorb TH, Knight C, Kamgar F, Teitsson S, Kurt M, Patel MY, Poretta T, Mamtani R, Palmer S. Cost-effectiveness of nivolumab versus surveillance for the adjuvant treatment of patients with urothelial carcinoma who are at high risk of recurrence: a US payer perspective. Journal of medical economics. 2024 Jan-Dec:27(1):543-553. doi: 10.1080/13696998.2024.2329019. Epub 2024 Apr 4 [PubMed PMID: 38470512]
Level 3 (low-level) evidencevan der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J, Oldenburg J, Chatta G, Ürün Y, Ye D, He Z, Valderrama BP, Ku JH, Tomita Y, Filian J, Wang L, Purcea D, Patel MY, Nasroulah F, Galsky MD, CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. The New England journal of medicine. 2023 Nov 9:389(19):1778-1789. doi: 10.1056/NEJMoa2309863. Epub 2023 Oct 22 [PubMed PMID: 37870949]
Madersbacher S, Schmidt J, Eberle JM, Thoeny HC, Burkhard F, Hochreiter W, Studer UE. Long-term outcome of ileal conduit diversion. The Journal of urology. 2003 Mar:169(3):985-90 [PubMed PMID: 12576827]
Sumfest JM, Burns MW, Mitchell ME. The Mitrofanoff principle in urinary reconstruction. The Journal of urology. 1993 Dec:150(6):1875-7; discussion 1877-8 [PubMed PMID: 8230523]
Bihrle R. The Indiana pouch continent urinary reservoir. The Urologic clinics of North America. 1997 Nov:24(4):773-9 [PubMed PMID: 9391530]
Jonsson MN, Adding LC, Hosseini A, Schumacher MC, Volz D, Nilsson A, Carlsson S, Wiklund NP. Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder. European urology. 2011 Nov:60(5):1066-73. doi: 10.1016/j.eururo.2011.07.035. Epub 2011 Aug 4 [PubMed PMID: 21852033]
Lavallée E, Wiklund P. The Studer Neobladder: An Established and Reproducible Technique for Intracorporeal Urinary Diversion. European urology open science. 2022 Jan:35():18-20. doi: 10.1016/j.euros.2021.09.019. Epub 2021 Nov 24 [PubMed PMID: 34888533]
Hussein AA, Ahmed YE, Kozlowski JD, May PR, Nyquist J, Sexton S, Curtin L, Peabody JO, Abol-Enein H, Guru KA. Robot-assisted approach to 'W'-configuration urinary diversion: a step-by-step technique. BJU international. 2017 Jul:120(1):152-157. doi: 10.1111/bju.13824. Epub 2017 Mar 22 [PubMed PMID: 28220593]
Otaola-Arca H, Seetharam Bhat KR, Patel VR, Moschovas MC, Orvieto M. Totally intracorporeal robot-assisted urinary diversion for bladder cancer (part 2). Review and detailed characterization of the existing intracorporeal orthotopic ileal neobladder. Asian journal of urology. 2021 Jan:8(1):63-80. doi: 10.1016/j.ajur.2020.05.013. Epub 2020 Jun 8 [PubMed PMID: 33569273]
Becerra MF, Venkatramani V, Reis IM, Soodana-Prakash N, Punnen S, Gonzalgo ML, Raolji S, Castle EP, Woods ME, Svatek RS, Weizer AZ, Konety BR, Tollefson M, Krupski TL, Smith ND, Shabsigh A, Barocas DA, Quek ML, Dash A, Parekh DJ. Health Related Quality of Life of Patients with Bladder Cancer in the RAZOR Trial: A Multi-Institutional Randomized Trial Comparing Robot versus Open Radical Cystectomy. The Journal of urology. 2020 Sep:204(3):450-459. doi: 10.1097/JU.0000000000001029. Epub 2020 Apr 9 [PubMed PMID: 32271690]
Level 1 (high-level) evidenceGerharz EW, Månsson A, Hunt S, Skinner EC, Månsson W. Quality of life after cystectomy and urinary diversion: an evidence based analysis. The Journal of urology. 2005 Nov:174(5):1729-36 [PubMed PMID: 16217273]
Level 2 (mid-level) evidenceAli AS, Hayes MC, Birch B, Dudderidge T, Somani BK. Health related quality of life (HRQoL) after cystectomy: comparison between orthotopic neobladder and ileal conduit diversion. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015 Mar:41(3):295-9. doi: 10.1016/j.ejso.2014.05.006. Epub 2014 May 24 [PubMed PMID: 24913090]
Level 2 (mid-level) evidenceGhosh A, Somani BK. Recent Trends in Postcystectomy Health-related Quality of Life (QoL) Favors Neobladder Diversion: Systematic Review of the Literature. Urology. 2016 Jul:93():22-6. doi: 10.1016/j.urology.2015.12.079. Epub 2016 Mar 22 [PubMed PMID: 27015941]
Level 1 (high-level) evidencePhilip J, Manikandan R, Venugopal S, Desouza J, Javlé PM. Orthotopic neobladder versus ileal conduit urinary diversion after cystectomy--a quality-of-life based comparison. Annals of the Royal College of Surgeons of England. 2009 Oct:91(7):565-9. doi: 10.1308/003588409X432293. Epub 2009 Jun 25 [PubMed PMID: 19558757]
Level 2 (mid-level) evidenceHolzbeierlein JM, Lopez-Corona E, Bochner BH, Herr HW, Donat SM, Russo P, Dalbagni G, Sogani PC. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. The Journal of urology. 2004 Sep:172(3):878-81 [PubMed PMID: 15310988]
Henry K, Miller J, Mori M, Loening S, Fallon B. Comparison of transurethral resection to radical therapies for stage B bladder tumors. The Journal of urology. 1988 Nov:140(5):964-7 [PubMed PMID: 3172367]
Thomas DJ, Roberts JT, Hall RR, Reading J. Radical transurethral resection and chemotherapy in the treatment of muscle-invasive bladder cancer: a long-term follow-up. BJU international. 1999 Mar:83(4):432-7 [PubMed PMID: 10210567]
Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, Bostrom PJ, Kuk C, Li K, Templeton AJ, Sridhar SS, van der Kwast TH, Chung P, Bristow RG, Milosevic M, Warde P, Fleshner NE, Jewett MAS, Bashir S, Zlotta AR. Propensity Score Analysis of Radical Cystectomy Versus Bladder-Sparing Trimodal Therapy in the Setting of a Multidisciplinary Bladder Cancer Clinic. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Jul 10:35(20):2299-2305. doi: 10.1200/JCO.2016.69.2327. Epub 2017 Apr 14 [PubMed PMID: 28410011]
Coppin CM, Gospodarowicz MK, James K, Tannock IF, Zee B, Carson J, Pater J, Sullivan LD. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996 Nov:14(11):2901-7 [PubMed PMID: 8918486]
Zietman AL, Grocela J, Zehr E, Kaufman DS, Young RH, Althausen AF, Heney NM, Shipley WU. Selective bladder conservation using transurethral resection, chemotherapy, and radiation: management and consequences of Ta, T1, and Tis recurrence within the retained bladder. Urology. 2001 Sep:58(3):380-5 [PubMed PMID: 11549485]
Balar AV, Castellano DE, Grivas P, Vaughn DJ, Powles T, Vuky J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Necchi A, Petrylak DP, Plimack ER, Xu JZ, Imai K, Moreno BH, Bellmunt J, de Wit R, O'Donnell PH. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2023 Mar:34(3):289-299. doi: 10.1016/j.annonc.2022.11.012. Epub 2022 Dec 6 [PubMed PMID: 36494006]
Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. 2024 Jan:(): [PubMed PMID: 30725602]
Bamias A, Tiliakos I, Karali MD, Dimopoulos MA. Systemic chemotherapy in inoperable or metastatic bladder cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2006 Apr:17(4):553-61 [PubMed PMID: 16303860]
Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, Necchi A, Valderrama BP, Ravaud A, Shariat SF, Szabados B, van der Heijden MS, Gillessen S, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology. 2024 Jun:35(6):485-490. doi: 10.1016/j.annonc.2024.03.001. Epub 2024 Mar 13 [PubMed PMID: 38490358]
Level 1 (high-level) evidencePowles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. The New England journal of medicine. 2020 Sep 24:383(13):1218-1230. doi: 10.1056/NEJMoa2002788. Epub 2020 Sep 18 [PubMed PMID: 32945632]
Level 3 (low-level) evidenceGrivas P, Park SH, Voog E, Caserta C, Gurney H, Bellmunt J, Kalofonos H, Ullén A, Loriot Y, Sridhar SS, Yamamoto Y, Petrylak DP, Sternberg CN, Gupta S, Huang B, Costa N, Laliberte RJ, di Pietro A, Valderrama BP, Powles T. Avelumab First-line Maintenance Therapy for Advanced Urothelial Carcinoma: Comprehensive Clinical Subgroup Analyses from the JAVELIN Bladder 100 Phase 3 Trial. European urology. 2023 Jul:84(1):95-108. doi: 10.1016/j.eururo.2023.03.030. Epub 2023 Apr 28 [PubMed PMID: 37121850]
Level 3 (low-level) evidenceMassari F, Santoni M, Takeshita H, Okada Y, Tapia JC, Basso U, Maruzzo M, Scagliarini S, Büttner T, Fornarini G, Myint ZW, Galli L, Souza VC, Pichler R, De Giorgi U, Gandur N, Lam ET, Gilbert D, Popovic L, Grande E, Mammone G, Berardi R, Crabb SJ, Kemp R, Molina-Cerrillo J, Freitas M, Luz M, Iacovelli R, Calabrò F, Tural D, Atzori F, Küronya Z, Chiari R, Campos S, Caffo O, Fay AP, Kucharz J, Zucali PA, Rinck JA, Zeppellini A, Bastos DA, Aurilio G, Mota A, Trindade K, Ortega C, Sade JP, Rizzo M, Fiala O, Vau N, Giannatempo P, Barillas A, Monteiro FSM, Dauster B, Mennitto A, Nogueira L, de Carvalho Fernandes R, Seront E, Aceituno LG, Grillone F, Cutuli HJ, Fernandez M, Bassanelli M, Kopp RM, Roviello G, Abahssain H, Procopio G, Milella M, Kopecky J, Martignetti A, Messina C, Caitano M, Inman E, Kanesvaran R, Herchhorn D, Santini D, Bamias A, Bisonni R, Mosca A, Morelli F, Maluf F, Soares A, Nunes F, Pinto A, Zgura A, Incorvaia L, Ansari J, Zabalza IO, Landmesser J, Rizzo A, Mollica V, Marchetti A, Rosellini M, Sorgentoni G, Battelli N, Buti S, Porta C, Bellmunt J. Global real-world experiences with pembrolizumab in advanced urothelial carcinoma after platinum-based chemotherapy: the ARON-2 study. Cancer immunology, immunotherapy : CII. 2024 Apr 18:73(6):106. doi: 10.1007/s00262-024-03682-w. Epub 2024 Apr 18 [PubMed PMID: 38634928]
Sassine AG, Cakir Y, Della Vecchia L, Ehlers JP. Erdafitinib-associated retinal alterations and rapid onset bilateral white cataracts. American journal of ophthalmology case reports. 2024 Jun:34():102028. doi: 10.1016/j.ajoc.2024.102028. Epub 2024 Feb 29 [PubMed PMID: 38572298]
Level 3 (low-level) evidenceYuan T, Li F, Hou Y, Guo H. Adverse events in patients with advanced urothelial carcinoma treated with erdafitinib: a retrospective pharmacovigilance study. Frontiers in pharmacology. 2023:14():1266890. doi: 10.3389/fphar.2023.1266890. Epub 2023 Nov 21 [PubMed PMID: 38074150]
Level 2 (mid-level) evidenceCatto JWF, Tran B, Rouprêt M, Gschwend JE, Loriot Y, Nishiyama H, Redorta JP, Daneshmand S, Hussain SA, Cutuli HJ, Procopio G, Guadalupi V, Vasdev N, Naini V, Crow L, Triantos S, Baig M, Steinberg G, THOR-2 Cohort 1 Investigators. Erdafitinib in BCG-treated high-risk non-muscle-invasive bladder cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2024 Jan:35(1):98-106. doi: 10.1016/j.annonc.2023.09.3116. Epub 2023 Oct 21 [PubMed PMID: 37871701]
Loriot Y, O'Hagan A, Siefker-Radtke AO. Plain language summary of erdafitinib in locally advanced or metastatic urothelial carcinoma: a phase 2 study with long-term follow-up. Future oncology (London, England). 2024 Feb:20(5):231-243. doi: 10.2217/fon-2023-0596. Epub 2023 Nov 2 [PubMed PMID: 37916514]
Tang M, Garg A, Bonate PL, Rosenberg JE, Matsangou M, Kadokura T, Yamada A, Choules M, Pavese J, Nagata M, Tenmizu D, Koibuchi A, Heo N, Wang L, Wojtkowski T, Hanley WD, Poondru S. Clinical Pharmacology of the Antibody-Drug Conjugate Enfortumab Vedotin in Advanced Urothelial Carcinoma and Other Malignant Solid Tumors. Clinical pharmacokinetics. 2024 Apr:63(4):423-438. doi: 10.1007/s40262-024-01369-0. Epub 2024 Apr 12 [PubMed PMID: 38609704]
Benjamin DJ, Rezazadeh Kalebasty A, Prasad V. The Overall Survival Benefit in EV-302: Is Enfortumab Vedotin plus Pembrolizumab the New Frontline Standard of Care for Metastatic Urothelial Carcinoma? European urology oncology. 2024 Jun:7(3):313-315. doi: 10.1016/j.euo.2024.02.010. Epub 2024 Mar 13 [PubMed PMID: 38485615]
Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS, EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. The New England journal of medicine. 2024 Mar 7:390(10):875-888. doi: 10.1056/NEJMoa2312117. Epub [PubMed PMID: 38446675]
Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, Srinivas S, Merchan JR, McKay RR, Petrylak DP, Sasse C, Moreno BH, Yu Y, Carret AS, Rosenberg JE. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 Jan 1:41(1):22-31. doi: 10.1200/JCO.22.01643. Epub 2022 Aug 30 [PubMed PMID: 36041086]
Utz DC, Hanash KA, Farrow GM. The plight of the patient with carcinoma in situ of the bladder. The Journal of urology. 1970 Feb:103(2):160-4 [PubMed PMID: 5410591]
Stanisic TH, Donovan JM, Lebouton J, Graham AR. 5-year experience with intravesical therapy of carcinoma in situ: an inquiry into the risks of "conservative" management. The Journal of urology. 1987 Nov:138(5):1158-61 [PubMed PMID: 3118055]
Lamm D, Herr H, Jakse G, Kuroda M, Mostofi FK, Okajima E, Sakamoto A, Sesterhenn I, da Silva FC. Updated concepts and treatment of carcinoma in situ. Urologic oncology. 1998 Jul-Oct:4(4-5):130-8 [PubMed PMID: 21227218]
Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. The Journal of urology. 2002 Nov:168(5):1964-70 [PubMed PMID: 12394686]
Level 1 (high-level) evidenceVaidya SR, Aeddula NR. Chronic Kidney Disease. StatPearls. 2024 Jan:(): [PubMed PMID: 30571025]
Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, Raj G, Bochner BH, Dalbagni G, Herr HW, Donat SM. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. European urology. 2009 Jan:55(1):164-74. doi: 10.1016/j.eururo.2008.07.031. Epub 2008 Jul 18 [PubMed PMID: 18675501]
Level 2 (mid-level) evidenceRosenberg S, Gofrit ON, Hidas G, Landau EH, Pode D. Diagnosing spontaneous ileal neobladder perforation: Too often delayed. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2013 Nov-Dec:7(11-12):E817-9. doi: 10.5489/cuaj.1531. Epub [PubMed PMID: 24475003]
Hautmann RE, de Petriconi RC, Volkmer BG. 25 years of experience with 1,000 neobladders: long-term complications. The Journal of urology. 2011 Jun:185(6):2207-12. doi: 10.1016/j.juro.2011.02.006. Epub 2011 Apr 16 [PubMed PMID: 21497841]
Kyriakidis A. Fournier's gangrene following delayed rupture of an ileal neobladder (Hautmann). British journal of urology. 1995 Nov:76(5):668 [PubMed PMID: 8535701]
Parsons JK, Schoenberg MP. Successful conservative management of perforated ileal neobladder. The Journal of urology. 2001 Apr:165(4):1214-5 [PubMed PMID: 11257680]
Khalil N, Lilly E, Alkassis M, Mansour R, Tayeh GA, Sarkis J, Aoun F. Late Spontaneous Rupture of the Orthotopic Neobladder, an Unusual Complication. Indian journal of surgical oncology. 2022 Jun:13(2):260-261. doi: 10.1007/s13193-021-01446-x. Epub 2021 Sep 12 [PubMed PMID: 35782804]
Mari A, Kimura S, Foerster B, Abufaraj M, D'Andrea D, Hassler M, Minervini A, Rouprêt M, Babjuk M, Shariat SF. A systematic review and meta-analysis of the impact of lymphovascular invasion in bladder cancer transurethral resection specimens. BJU international. 2019 Jan:123(1):11-21. doi: 10.1111/bju.14417. Epub 2018 Jun 29 [PubMed PMID: 29807387]
Level 1 (high-level) evidenceSeisen T, Compérat E, Léon P, Roupret M. Impact of histological variants on the outcomes of nonmuscle invasive bladder cancer after transurethral resection. Current opinion in urology. 2014 Sep:24(5):524-31. doi: 10.1097/MOU.0000000000000086. Epub [PubMed PMID: 25051021]
Level 3 (low-level) evidenceSylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European urology. 2006 Mar:49(3):466-5; discussion 475-7 [PubMed PMID: 16442208]
Isharwal S, Konety B. Non-muscle invasive bladder cancer risk stratification. Indian journal of urology : IJU : journal of the Urological Society of India. 2015 Oct-Dec:31(4):289-96. doi: 10.4103/0970-1591.166445. Epub [PubMed PMID: 26604439]
Chan Y, Fisher P, Tilki D, Evans CP. Urethral recurrence after cystectomy: current preventative measures, diagnosis and management. BJU international. 2016 Apr:117(4):563-9. doi: 10.1111/bju.13370. Epub 2015 Dec 12 [PubMed PMID: 26556525]
Nieder AM, Sved PD, Gomez P, Kim SS, Manoharan M, Soloway MS. Urethral recurrence after cystoprostatectomy: implications for urinary diversion and monitoring. Urology. 2004 Nov:64(5):950-4 [PubMed PMID: 15533484]
Knapik JA, Murphy WM. Urethral wash cytopathology for monitoring patients after cystoprostatectomy with urinary diversion. Cancer. 2003 Dec 25:99(6):352-6 [PubMed PMID: 14681943]
Clark PE, Hall MC. Contemporary management of the urethra in patients after radical cystectomy for bladder cancer. The Urologic clinics of North America. 2005 May:32(2):199-206 [PubMed PMID: 15862617]
Sherwood JB, Sagalowsky AI. The diagnosis and treatment of urethral recurrence after radical cystectomy. Urologic oncology. 2006 Jul-Aug:24(4):356-61 [PubMed PMID: 16818191]
Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman AL. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Dec 1:32(34):3801-9. doi: 10.1200/JCO.2014.57.5548. Epub 2014 Nov 3 [PubMed PMID: 25366678]
Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, Coen JJ, Skowronski RY, Paly JJ, McGovern FJ, Zietman AL. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. European urology. 2012 Apr:61(4):705-11. doi: 10.1016/j.eururo.2011.11.010. Epub 2011 Nov 12 [PubMed PMID: 22101114]
Quek ML, Stein JP, Daneshmand S, Miranda G, Thangathurai D, Roffey P, Skinner EC, Lieskovsky G, Skinner DG. A critical analysis of perioperative mortality from radical cystectomy. The Journal of urology. 2006 Mar:175(3 Pt 1):886-9; discussion 889-90 [PubMed PMID: 16469572]