Introduction
Basophilic stippling is one example of several clinically significant erythrocyte inclusions identified on peripheral blood smears. The presence of basophilic stippling is attributed to aggregates of ribosomes or fragments of ribosomal RNA precipitated throughout the cytoplasm of circulating erythrocytes. This finding is associated with acquired and heritable hematologic disorders affecting erythropoiesis and erythrocyte maturation.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Basophilic stippling is a frequent manifestation of hematologic disease in the peripheral blood, and it is also observable in bone marrow aspirates. It is implicated in cases of lead poisoning but can be an indicator of various heavy metal toxicities. Alternative causes of basophilic stippling such as hemoglobinopathies, nutritional deficiencies, and myelodysplasia warrant consideration as well in the context of appropriate clinical history.
Structure
Ribosomes and fragments of ribosomal RNA/ribonuclear proteins can form aggregates in circulating reticulocytes and erythrocytes as a consequence of disease. These aggregates precipitate in the cytoplasm similar to other erythrocyte inclusions like Pappenheimer bodies and Howell-Jolly bodies, but the composition of basophilic stippling is distinctive. Further discussion of the functional, histochemical, and pathologic implications of basophilic stippling follows in subsequent sections.
Function
Immature erythrocytes require the same organelles as other human cells to function and synthesize materials necessary to proceed with maturation. Ribosomes facilitate the translation of mRNA to produce proteins utilized in numerous cellular functions and remain present in reticulocytes alongside other essential organelles, such as mitochondria, following enucleation of erythroblasts. Terminal maturation of reticulocytes into erythrocytes involves the elimination of these remaining organelles. Clearance of ribosomes is thought to occur during later phases of maturation in which reticulocytes enter circulation from the bone marrow to complete their conversion into erythrocytes. Enzymatic degradation via Ulk1 protein kinase signaling appears to be attributable to ribosome clearance; however, a detailed mechanism is not clear at this time.[1]
Incomplete or failure of ribosomal degradation leads to precipitation of ribosomes or ribosomal remnants in circulating erythrocytes, which is visible as basophilic stippling on microscopy. Aberrant terminal maturation is involved in several hematologic disorders but is of particular interest in heavy metal toxicity and anemia – especially those associated with heritable enzymopathies. For example, pyrimidine nucleotidase is a key enzyme involved in the catabolism of ribosomal and messenger RNA during terminal maturation. Deficiency or inhibition of this enzyme causes anemia with marked basophilic stippling on peripheral smears.[1][2]
Tissue Preparation
Most specimens consist of whole blood collected via the appropriate protocol for hematologic studies, typically in a lavender top tube with potassium EDTA anticoagulant. A peripheral blood smear is then made from the specimen and prepared with Wright-Giemsa stain for light microscopy. Bone marrow aspirate with Wright-Giemsa staining may also demonstrate this histologic finding.
Histochemistry and Cytochemistry
No specific histochemical markers or staining methodologies yet exist for basophilic stippling. That said, basophilic stippling can be differentiated from other erythrocyte inclusions – namely Pappenheimer bodies – by its lack of special staining. Pappenheimer bodies have a similar appearance to basophilic stippling in peripheral blood with Wright-Giemsa staining; however, the former is composed of iron aggregates rather than ribosomal material. Prussian blue stain preferentially binds iron in tissues; therefore, Pappenheimer bodies rather than basophilic stippling will be visible on a specimen treated with Prussian blue.[3]
Microscopy, Light
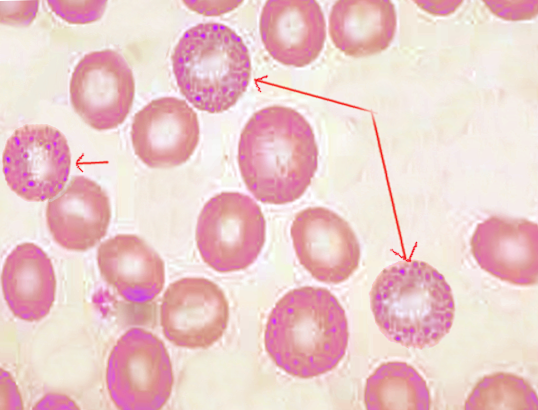
The appearance of basophilic stippling on light microscopy of peripheral blood and bone marrow aspirates is characteristic of its namesake. Affected erythrocytes demonstrate small, basophilic (purple-blue) punctate inclusions. Unlike other erythrocyte inclusions, basophilic stippling does not show polarity and is diffusely and evenly scattered throughout the cell. The inclusions may be coarse or fine in appearance, and these different morphologies can be associated with particular disease processes as discussed later.[4]
Microscopy, Electron
Electron microscopy is not routine for the examination of peripheral blood smears. However, a study from 1965 comparing supravital staining (light microscopy) and electron microscopy of peripheral blood from rodents with induced lead/phenylhydrazine toxicity or post-phlebotomy anemia noted that the structure and general appearance of stippled material in reticulocytes was consistent with ribosomes.[5] Overall, the visualization of basophilic stippling in more mature erythrocytes was subject to limitations by preparation of peripheral blood for electron microscopy; therefore, researchers extrapolated findings in reticulocytes to other affected erythrocytes.[5]
Clinical Significance
Many hematologic disorders involve disrupted erythropoiesis and erythrocyte maturation, which introduces the potential for basophilic stippling on peripheral smears. Coarse basophilic stippling is always clinically significant. Fine basophilic stippling may be seen artifactually, as well as in a variety of clinical instances mentioned below. Classically, coarse basophilic stippling is associated with heavy metal toxicity with a predominant emphasis on lead poisoning. Lead toxicity is attributed to coarse-appearing basophilic stippling due to inhibition of pyrimidine-5'-nucleotidase, thereby preventing degradation of ribosomal RNA in circulating erythrocytes.[4] Basophilic stippling is considered a pathognomonic finding in lead poisoning in conjunction with hypochromic microcytic anemia (although normocytic anemia can be appreciated as well) and deposition of lead in the gingiva and joints.[6]
Supporting clinical history classically entails environmental or occupational exposure to lead. Basophilic stippling can occur in cases of lead intoxication secondary to foreign bodies such as bullet fragments and retained shrapnel in active-duty military personnel.[7][8] Patients demonstrating anemia with basophilic stippling and toxic blood lead concentrations have also been connected to the use of adulterated illicit drugs (ex. opium) and contaminated herbal remedies/supplements imported from Asian countries.[4][9][6][10] Toxic levels of other heavy metals such as zinc may produce basophilic stippling as well but are considerably rare compared to lead.[11]
Patients with anemia secondary to hemoglobinopathies such as thalassemia and sickle cell disease can exhibit basophilic stippling that may be finer in appearance than observed in lead toxicity.[4][12] The presence of basophilic stippling in the context of hemoglobinopathies is likely related to increased erythrocyte hemolysis and release of immature cells into the bloodstream to compensate for relatively rapid erythrocyte turnover.[12] Moreover, pyrimidine-5'-nucleotidase (P5'N) deficiency is a rare autosomal recessive enzymopathy characterized by hemolytic anemia with significant basophilic stippling on peripheral smear. P5'N deficiency may have links to a mutation in the NT53C gene, but additional studies are needed to characterize the full scope of genetic factors related to this condition.[2]
Megaloblastic anemia due to vitamin B12/folate deficiency or chronic alcohol use disorder can present with basophilic stippling as well.[4] Furthermore, myelodysplastic syndrome (MDS) acquired from variable gene mutations over time induces faulty erythropoiesis and hematopoietic stem cell differentiation. Numerous morphological defects are present on peripheral smears from patients with MDS, and basophilic stippling is a common finding.[13]
Media
References
Moras M, Lefevre SD, Ostuni MA. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Frontiers in physiology. 2017:8():1076. doi: 10.3389/fphys.2017.01076. Epub 2017 Dec 19 [PubMed PMID: 29311991]
Santos Ad, Dantas LE, Traina F, Albuquerque DM, Chaim EA, Saad ST. Pyrimidine-5'-nucleotidase Campinas, a new mutation (p.R56G) in the NT5C3 gene associated with pyrimidine-5'-nucleotidase type I deficiency and influence of Gilbert's Syndrome on clinical expression. Blood cells, molecules & diseases. 2014 Dec:53(4):246-52. doi: 10.1016/j.bcmd.2014.05.009. Epub 2014 Aug 18 [PubMed PMID: 25153905]
Level 3 (low-level) evidenceMeguro R, Asano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K. Nonheme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Archives of histology and cytology. 2007 Apr:70(1):1-19 [PubMed PMID: 17558140]
Level 3 (low-level) evidenceChan NCN, Chan KP. Coarse basophilic stippling in lead poisoning. Blood. 2017 Jun 15:129(24):3270. doi: 10.1182/blood-2017-03-773499. Epub [PubMed PMID: 28620106]
JENSEN WN, MORENO GD, BESSIS MC. AN ELECTRON MICROSCOPIC DESCRIPTION OF BASOPHILIC STIPPLING IN RED CELLS. Blood. 1965 Jun:25():933-43 [PubMed PMID: 14294770]
Level 3 (low-level) evidenceTsai MT, Huang SY, Cheng SY. Lead Poisoning Can Be Easily Misdiagnosed as Acute Porphyria and Nonspecific Abdominal Pain. Case reports in emergency medicine. 2017:2017():9050713. doi: 10.1155/2017/9050713. Epub 2017 May 29 [PubMed PMID: 28630774]
Level 3 (low-level) evidenceGrasso IA, Blattner MR, Short T, Downs JW. Severe Systemic Lead Toxicity Resulting From Extra-Articular Retained Shrapnel Presenting as Jaundice and Hepatitis: A Case Report and Review of the Literature. Military medicine. 2017 Mar:182(3):e1843-e1848. doi: 10.7205/MILMED-D-16-00231. Epub [PubMed PMID: 28290970]
Level 3 (low-level) evidenceWeiss D, Lee D, Feldman R, Smith KE. Severe lead toxicity attributed to bullet fragments retained in soft tissue. BMJ case reports. 2017 Mar 8:2017():. doi: 10.1136/bcr-2016-217351. Epub 2017 Mar 8 [PubMed PMID: 28275014]
Level 3 (low-level) evidenceFakoor M, Akhgari M, Shafaroodi H. Lead Poisoning in Opium-Addicted Subjects, Its Correlation with Pyrimidine 5'-Nucleotidase Activity and Liver Function Tests. International journal of preventive medicine. 2019:10():36. doi: 10.4103/ijpvm.IJPVM_490_18. Epub 2019 Mar 5 [PubMed PMID: 30967922]
Zhao Y, Lv J. Basophilic Stippling and Chronic Lead Poisoning. Turkish journal of haematology : official journal of Turkish Society of Haematology. 2018 Nov 13:35(4):298-299. doi: 10.4274/tjh.2018.0195. Epub 2018 Sep 5 [PubMed PMID: 30182925]
Vander Meeren S, Van Damme A, Jochmans K. Prominent basophilic stippling and hemochromatosis in glucose-6-phosphate dehydrogenase deficiency. International journal of hematology. 2015 Feb:101(2):112-3. doi: 10.1007/s12185-014-1716-6. Epub 2014 Dec 9 [PubMed PMID: 25487752]
Level 3 (low-level) evidenceThom CS, Dickson CF, Gell DA, Weiss MJ. Hemoglobin variants: biochemical properties and clinical correlates. Cold Spring Harbor perspectives in medicine. 2013 Mar 1:3(3):a011858. doi: 10.1101/cshperspect.a011858. Epub 2013 Mar 1 [PubMed PMID: 23388674]
Level 3 (low-level) evidenceZahid MF, Khan N, Pei J, Testa JR, Dulaimi E. Genomic imbalances in peripheral blood confirm the diagnosis of myelodysplastic syndrome in a patient presenting with non-immune hemolytic anemia. Leukemia research reports. 2016:5():23-6. doi: 10.1016/j.lrr.2016.05.001. Epub 2016 May 13 [PubMed PMID: 27298759]