Introduction
Barrett esophagus (BE) is a pre-malignant condition characterized by the conversion of the normal esophageal squamous epithelium into metaplastic columnar epithelium. A worldwide consensus on the exact requirements for the diagnosis, however, has not yet been reached. The majority of BE cases are acquired, with the precipitant being long-standing gastroesophageal reflux (GERD). Rare families have an increased incidence of developing BE through autosomal dominant inheritance of certain susceptibility alleles, known as the familial Barrett esophagus phenotype. BE predisposes patients to the development of dysplasia and esophageal adenocarcinoma (EAC), a cancer with high morbidity and mortality. Surveillance programs have been developed to aid management decisions based on the presence of non-dysplastic BE, low-grade dysplasia, high-grade dysplasia, or invasive adenocarcinoma. These continue to change as new studies and new data are evaluated and included in newer guidelines. When to apply various endoscopic and surgical therapies is another topic that has undergone recent change. Advances in technologies and further studies for patient risk stratification are research areas of interest with the potential for affecting the future guidelines for diagnosing and managing BE.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The majority of patients with BE have a known history of chronic GERD. GERD is the reflux of stomach and bile acids through the lower esophageal sphincter and into the distal esophagus. The stomach lining is mucinous columnar epithelium made to withstand the acidic environment required for digestion; however, the esophagus is lined by squamous epithelium. As a response to the acidic irritant, the squamous epithelium becomes inflamed. Continued exposure to acids leads to persistent inflammation and a columnar metaplasia reaction with the eventual development of an intestinal-type phenotype characterized by the presence of goblet cells.[4]
Risk factors for GERD include conditions which weaken the lower esophageal sphincter, such as a hiatal hernia or pregnancy, conditions causing increased pressure on the stomach, including obesity, pregnancy, and asthma, and conditions which affect transit of food from the stomach to the small intestine, such as diabetes, peptic ulcer disease, and connective tissue disorders.[5]
Why BE develops in some patients with GERD and not in others remains unclear. However, research has identified a few risk factors. BE has a male predominance of 3:1, is more common in those of white ethnicity, and has a prevalence that increases with age. Obesity, more specifically, abdominal adiposity rather than BMI, is another strong risk factor for both GERD and BE.
Epidemiology
GERD is an extremely common condition with prevalence rates ranging from 8-40% worldwide. BE is found in 1.3 to 1.6% of the general population and 5 to 15% of symptomatic GERD patients undergoing endoscopy. The prevalence of BE among children is much less, only up to 4.8%. The incidence of GERD, BE, and EAC has been increasing significantly over the last four decades, though this may partially be attributable to increased numbers of endoscopies, it remains an alarming indication of how current management of these conditions is in great need of improvement and further research. More than 95% of patients with BE do not develop cancer. Studies indicate the absolute annual risk of EAC in nondysplastic BE is 0.1 to 0.5%/year, a highly variable 1 to 43%/year for low-grade dysplasia, and 23-60%/year for high-grade dysplasia. A greater extent of dysplasia has a significantly higher risk of cancer as well as the presence of an endoscopic abnormality.[6][7]
Pathophysiology
The exact pathogenesis of BE remains to be elucidated; however, some progress has been made in determining the molecular alterations and primary cell type involved. Studies have indicated that the exposure to acids induces the squamous epithelial cells to secrete inflammatory cytokines such as IL8 and IL1b, which act to mediate the inflammatory response and signal T lymphocytes and neutrophils to migrate into the epithelium. Bile acids, specifically, have been shown to upregulate CDX2, the intestinal differentiation factor, and MUC2, the goblet cell-specific gene, within normal columnar and esophageal cancer cell lines. Up to 90% of BE patients have a detectable clonal aberration of p16. CDX2 and TP53 mutations are early molecular alterations found to be present in metaplastic columnar epithelium even before morphologic dysplasia is recognized. However, immunohistochemical staining to evaluate these markers is not currently recommended in routine diagnostic cases of BE. A recent study described a population of possible embryonic stem cells at the squamocolumnar junction, while other studies suggest that stem cells derived from undifferentiated mesenchymal cells in the lamina propria of the esophagus or the bone marrow may be the cell of origin for BE. It is agreed upon that differentiation of the epithelium toward an intestinal-type phenotype is multifactorial and a multi-step process. There is evidence of a type of epithelium known as “multilayered epithelium (ME),” which shows combined squamous and columnar features, likely representing an intermediate phase within the metaplastic reaction. ME is strongly associated with GERD esophagitis and expresses a mucin and cytokeratin profile similar to BE columnar epithelium as well as intestinal transcription factors. Further studies are needed to understand the complete mechanism of the pathogenesis of BE.[4][3]
History and Physical
Most patients with BE will exhibit symptoms of GERD, such as the retrosternal burning sensation known as heartburn, especially after eating. Acid regurgitation is another likely symptom. Other possible symptoms, though less common, include dysphagia and a globus sensation where patients feel as if there is a “lump” or some obstruction in their throat when there is none. Rarely, patients may be asymptomatic. Commonly, patients will have a chronic history of GERD symptoms with no other specific physical examination findings.
Evaluation
In January 2016, the American College of Gastroenterology (ACG) published its new clinical guideline for the diagnosis and management of BE. They now recommend screening for BE in men with at least five years of chronic GERD symptoms who also have at least two (2) additional risk factors including greater than 50 years of age, history of smoking, white ethnicity, central obesity, or a confirmed family history of BE. Due to the extremely low prevalence of EAC in women, this population has no indications for screening except for the presence of multiple risk factors.[1][8][9]
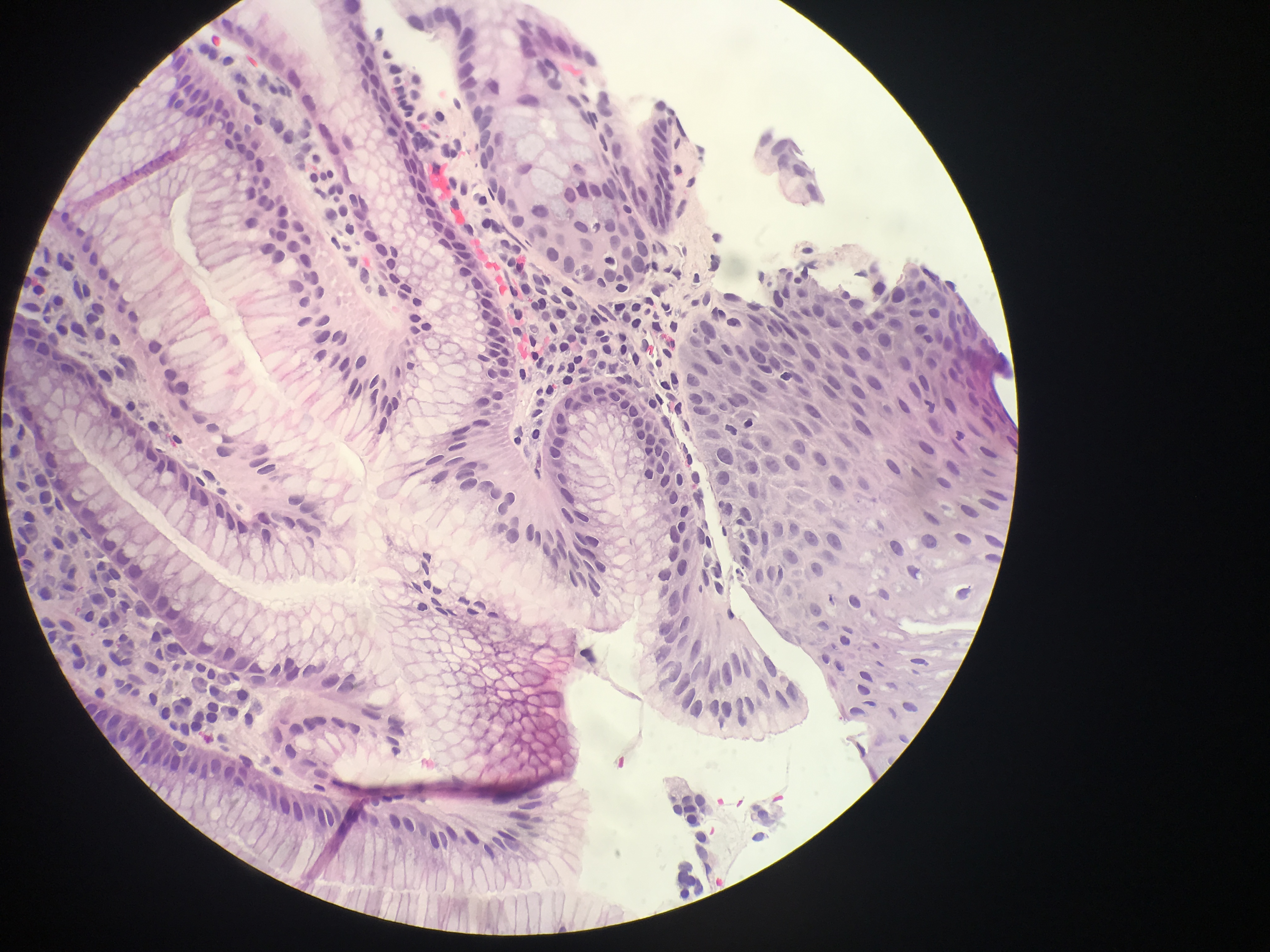
Diagnosis of BE, as defined by the American College of Gastroenterology, requires gross endoscopic identification of columnar metaplasia, generally described as “salmon-pink” tongues of mucosal tissue, as well as pathological confirmation of intestinal metaplasia with goblet cells on biopsy evaluation. However, the British Society of Gastroenterology, as well as the GERD Society Study Committee in Japan, do not require the presence of goblet cells to diagnose BE and base the diagnosis solely on the presence of columnar metaplasia. Due to the controversy over the significance of goblet cells, another alternative classification has been proposed, which allows the pathologist to state that there is columnar metaplasia and then further specify whether goblet cells are present or are not present. To maximize the possibility of finding BE, dysplasia, and/or carcinoma, a minimum of 8 biopsies is recommended by the ACG. The Prague C & M criteria are recommended for endoscopic grading of BE, with the most proximal extent of circumferential columnar mucosa from the GEJ being the C value, and the maximal extent of non-circumferential columnar mucosa above the GEJ being the M value.[10][11]
In cases of BE, the pathologist must determine whether dysplasia or carcinoma is present. As studies suggest that the extent of dysplasia also correlates to the risk of cancer, it may be valuable for pathologists to not only comment on the presence but also the extent of dysplasia (such as focal or diffuse). Sometimes there may be a specific concern for dysplasia if an area of nodularity or ulceration is noted on endoscopy. However, BE-related dysplasia is often flat and not apparent on endoscopy. The major histologic pattern of dysplasia is intestinal, therefore resembling an adenoma of the colon. However, non-intestinal dysplasia also exists, including foveolar and serrated patterns. Validated criteria for classification as either low or high-grade only exists for intestinal-type dysplasia, however. Low-grade dysplasia may have up to mild architectural abnormalities with cytologic atypia consisting of nuclear elongation, dense and hyperchromatic nuclei, an increased nuclear:cytoplasmic (N:C) ratio, stratification limited to the basal half of the epithelium, increased mitoses and up to the mild loss of polarity. Features distinguishing high-grade dysplasia from low-grade include crypt crowding, full-thickness stratification, a prominent loss of polarity, and mild to marked nuclear pleomorphism. If there is extensive regenerative change so much that the atypia present slightly overlaps with low-grade dysplasia, the pathologist may give a diagnosis of “Indefinite for Dysplasia.” Distinction between high-grade dysplasia and intramucosal carcinoma suffers from a high degree of interobserver variability, however, recommended criteria for a diagnosis of intramucosal carcinoma include the lamina propria being invaded by individual neoplastic cells with no connection to the crypts, sheeting out of malignant cells, irregular and angulated glands located in the lamina propria or muscularis mucosae, a complex anastomosing pattern of glands within the lamina propria, and/or back-to-back glands or cells in an irregular architectural pattern.[12][13]
Treatment / Management
Surveillance plan strategies have a history of being arbitrary and not validated by strong prospective clinical studies with a 2014 study, even concluding that there is no association between surveillance and decreased EAC-related deaths. Based on their systematic review of the literature and evaluation of the level of evidence, the ACG made several changes to the recommended management of BE in its newest guidelines. High-definition white-light endoscopy is a novel endoscopic technology that is now recommended for routine use in surveillance beyond electronic chromoendoscopy. For non-dysplastic BE, the current ACG recommendation after the initial diagnosis is to follow up with surveillance every 3 to 5 years. The patient should also be placed on once-daily proton pump inhibitor therapy, regardless of the presence of reflux symptoms, due to evidence of a chemopreventive effect of proton pump inhibitors, where the risk of progression to neoplastic BE is reduced compared to no acid suppression or H2 blockers. Due to the significant impact on management, if BE is associated with dysplasia, the ACG emphasizes the importance of confirming a dysplasia diagnosis with a second pathologist with extensive experience in BE-related neoplasia. Patients with confirmed low-grade dysplasia can now be considered for endoscopic ablative therapy or can follow a 6 to 12-month interval surveillance plan. For patients with confirmed high-grade dysplasia with endoscopic mucosal abnormalities, endoscopic mucosal resection followed by ablation of the remaining BE mucosa is recommended. If no mucosal abnormality is visible, in other words, the mucosa is flat; radiofrequency ablation therapy is considered sufficient for low- or high-grade dysplasia as well as carcinoma. Cryotherapy is another option of ablative therapy. It is necessary to continue surveillance of patients after ablative therapy, as multiple treatments are often required to ensure complete elimination of the lesional tissue. Surgical therapies such as esophagectomy are not the preferred initial approach to management for high-grade dysplasia or intramucosal cancer, as the procedure has a high risk of morbidities, though should be considered if intramucosal cancer has poor prognostic factors, such as lymphovascular invasion or poor differentiation or if cancer involves the submucosa. Patients with significant comorbidities that limit their likely survival may not benefit from endoscopic therapies, with risks of the procedures being greater than potential gains.[14][15]
Differential Diagnosis
- Acute gastritis
- Antral web Cholelithiasis
- Chronic gastritis
- Coronary artery atherosclerosis
- Esophageal cancer
- Esophageal mobility disorders
- Esophageal spasm
- Esophagitis
- Gall stones
- Helicobacter pylori infections
Prognosis
There is no longer any question that if Barrett esophagus is left untreated, it can develop into an adenocarcinoma. However, the risk of progression is very slow, and most patients will not develop a malignancy. However, at the same time, it should be noted that the number of cases of adenocarcinoma has steadily increased over the past three decades.[16]
Deterrence and Patient Education
The role of diet in the etiology of Barrett esophagus remains inconclusive but it is recommended that patients lower the intake of meat. In addition, patients should avoid oily foods, alcohol, caffeinated beverages, chocolate, acidic juices, vinegar and carbonated drinks.
The American College of Gastroenterology recommends upper endoscopy to detect or screen for Barrett esophagus after age 50.
Pearls and Other Issues
Since not all patients will progress from BE to cancer, an essential area of research is the development of reliable risk-profiling methods to identify patients at higher risk and who will benefit more from therapies. The use of biomarkers to risk-stratify patients is currently undergoing extensive research, with DNA content abnormalities and TP53 and CDKN2A genetic alterations showing the most promise to date. At this time, though, there is not enough evidence to recommend them for use in routine practice. Novel sampling techniques for screening that show promise include transnasal endoscopy and cytosponge. Many more on-going and future studies should add to our current knowledge base regarding BE and its best management.
Enhancing Healthcare Team Outcomes
The incidence of adenocarcinoma of the esophagus is increasing in most western nations, and most believe this is due to Barrett's esophagus. The progression of the lesion to cancer is slow, but once cancer occurs, the prognosis is very poor. Over the years, multiple societies have developed guidelines for screening, surveillance, and management of Barrett's esophagus.[9][17] Since most patients may have no symptoms or symptoms of reflux, suspicion of Barrett should always be high, especially in high-risk populations. Unfortunately, many gastroenterologists and surgeons have been performing upper endoscopies, but there is no clear-cut evidence revealing that this procedure can detect esophageal cancer early or prevents cancer. Unnecessary endoscopies also lead to high healthcare costs. Thus, the recommendations are that one follow strict guidelines for surveillance based on patient risk factors, rather than empirically performing endoscopy in all patients. An interprofessional approach with a team composed of pathologists, thoracic surgeons, radiologists, gastroenterologists, and general surgeons will permit individualization of care and the best results. The nurse's role is vital in educating the patient about reflux and ways to prevent it. The pharmacist should ensure that the patient is adequately treated for reflux and has a referral to a gastroenterologist.[18][19] Pharmacists will verify all anti-acid medication strategies (e.g., PPIs, H@ receptor blockers, etc.), verifying dosing, drug interactions, counseling patients on administration and adverse events, and reporting back to the prescriber with any concerns. [Level V]
Outcomes
There are many case series reporting on Barrett's outcomes, which are good to excellent. However, there are very few studies on long-term outcomes. Even though there are several types of treatments for Barrett and GERD, there is no solid evidence to indicate which one is the best or most effective. With the ability to deliver laser and radio-frequency wave therapy via the endoscope, more long-term studies are needed to determine not only the safety of these procedures but also their effectiveness in preventing the progression of Barrett's esophagus to a malignancy.[20] [Level V]
Media
(Click Image to Enlarge)
References
Michopoulos S. Critical appraisal of guidelines for screening and surveillance of Barrett's esophagus. Annals of translational medicine. 2018 Jul:6(13):259. doi: 10.21037/atm.2018.05.09. Epub [PubMed PMID: 30094245]
Nowicki A, Kula Z, Świerszczyńska A. Barrett's esophagus and gland cancer - the experience of one center. Polski przeglad chirurgiczny. 2018 May 16:90(3):19-24. doi: 10.5604/01.3001.0011.8166. Epub [PubMed PMID: 30015321]
Dewan KR, Patowary BS, Bhattarai S, Shrestha G. Barrett's Esophagus in Patients with Gastroesophageal Reflux Disease. Journal of Nepal Health Research Council. 2018 Jul 3:16(2):144-148 [PubMed PMID: 29983427]
Inadomi J,Alastal H,Bonavina L,Gross S,Hunt RH,Mashimo H,di Pietro M,Rhee H,Shah M,Tolone S,Wang DH,Xie SH, Recent advances in Barrett's esophagus. Annals of the New York Academy of Sciences. 2018 Jul 5 [PubMed PMID: 29974975]
Level 3 (low-level) evidenceMikolašević I, Bokun T, Filipec Kanižaj T. Gastroesophageal reflux disease, Barrett esophagus, and esophageal adenocarcinoma - where do we stand? Croatian medical journal. 2018 Jun 30:59(3):97-99 [PubMed PMID: 29972731]
Erőss B, Farkas N, Vincze Á, Tinusz B, Szapáry L, Garami A, Balaskó M, Sarlós P, Czopf L, Alizadeh H, Rakonczay Z Jr, Habon T, Hegyi P. Helicobacter pylori infection reduces the risk of Barrett's esophagus: A meta-analysis and systematic review. Helicobacter. 2018 Aug:23(4):e12504. doi: 10.1111/hel.12504. Epub 2018 Jun 25 [PubMed PMID: 29938864]
Level 1 (high-level) evidenceThrift AP. Barrett's Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Digestive diseases and sciences. 2018 Aug:63(8):1988-1996. doi: 10.1007/s10620-018-5068-6. Epub [PubMed PMID: 29671158]
Bellizzi AM, Hafezi-Bakhtiari S, Westerhoff M, Marginean EC, Riddell RH. Gastrointestinal pathologists' perspective on managing risk in the distal esophagus: convergence on a pragmatic approach. Annals of the New York Academy of Sciences. 2018 Dec:1434(1):35-45. doi: 10.1111/nyas.13680. Epub 2018 May 11 [PubMed PMID: 29749623]
Level 3 (low-level) evidenceClermont M, Falk GW. Clinical Guidelines Update on the Diagnosis and Management of Barrett's Esophagus. Digestive diseases and sciences. 2018 Aug:63(8):2122-2128. doi: 10.1007/s10620-018-5070-z. Epub [PubMed PMID: 29671159]
Akın H, Aydın Y. How should we describe, diagnose and observe the Barrett's esophagus? The Turkish journal of gastroenterology : the official journal of Turkish Society of Gastroenterology. 2017 Dec:28(Suppl 1):S26-S30. doi: 10.5152/tjg.2017.08. Epub [PubMed PMID: 29199163]
Beg S, Ragunath K, Wyman A, Banks M, Trudgill N, Pritchard DM, Riley S, Anderson J, Griffiths H, Bhandari P, Kaye P, Veitch A. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut. 2017 Nov:66(11):1886-1899. doi: 10.1136/gutjnl-2017-314109. Epub 2017 Aug 18 [PubMed PMID: 28821598]
Level 2 (mid-level) evidenceDunbar KB, Souza RF. Beyond Dysplasia Grade: The Role of Biomarkers in Stratifying Risk. Gastrointestinal endoscopy clinics of North America. 2017 Jul:27(3):447-459. doi: 10.1016/j.giec.2017.02.003. Epub [PubMed PMID: 28577766]
Ooi J, Wilson P, Walker G, Blaker P, DeMartino S, O'Donohue J, Reffitt D, Lanaspre E, Chang F, Meenan J, Dunn JM. Dedicated Barrett's surveillance sessions managed by trained endoscopists improve dysplasia detection rate. Endoscopy. 2017 Jun:49(6):524-528. doi: 10.1055/s-0043-103410. Epub 2017 Apr 11 [PubMed PMID: 28399610]
Markar SR, Arhi C, Leusink A, Vidal-Diez A, Karthikesalingam A, Darzi A, Lagergren J, Hanna GB. The Influence of Antireflux Surgery on Esophageal Cancer Risk in England: National Population-based Cohort Study. Annals of surgery. 2018 Nov:268(5):861-867. doi: 10.1097/SLA.0000000000002890. Epub [PubMed PMID: 30048317]
Ali Khan M, Howden CW. The Role of Proton Pump Inhibitors in the Management of Upper Gastrointestinal Disorders. Gastroenterology & hepatology. 2018 Mar:14(3):169-175 [PubMed PMID: 29928161]
Bresalier RS. Chemoprevention of Barrett's Esophagus and Esophageal Adenocarcinoma. Digestive diseases and sciences. 2018 Aug:63(8):2155-2162. doi: 10.1007/s10620-018-5149-6. Epub [PubMed PMID: 29948566]
Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017 Mar:152(4):706-715. doi: 10.1053/j.gastro.2017.01.031. Epub [PubMed PMID: 28257716]
Schmidt HM, Mohiuddin K, Bodnar AM, El Lakis M, Kaplan S, Irani S, Gan I, Ross A, Low DE. Multidisciplinary treatment of T1a adenocarcinoma in Barrett's esophagus: contemporary comparison of endoscopic and surgical treatment in physiologically fit patients. Surgical endoscopy. 2016 Aug:30(8):3391-401. doi: 10.1007/s00464-015-4621-z. Epub 2015 Nov 5 [PubMed PMID: 26541725]
Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N. Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health technology assessment (Winchester, England). 2006 Mar:10(8):1-142, iii-iv [PubMed PMID: 16545207]
Level 1 (high-level) evidenceCodipilly DC, Chandar AK, Singh S, Wani S, Shaheen NJ, Inadomi JM, Chak A, Iyer PG. The Effect of Endoscopic Surveillance in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Gastroenterology. 2018 Jun:154(8):2068-2086.e5. doi: 10.1053/j.gastro.2018.02.022. Epub 2018 Feb 16 [PubMed PMID: 29458154]
Level 1 (high-level) evidence