Introduction
Angioid streaks represent disruptions in a deteriorated and calcified Bruch membrane linked with retinal bleeding, either occurring spontaneously or following blunt injury.[1] Typically, they develop around the optic disc and extend outward linearly. These streaks can be idiopathic or associated with systemic conditions such as the rare disease pseudoxanthoma elasticum (PXE, MIM #264800), Ehlers-Danlos syndrome, sickle cell disease, and Paget disease of the bone. The invasion of choroidal fibrovascular tissue into the sub-retinal epithelial space may result in bleeding, neovascularization, and scarring, ultimately leading to symptoms like metamorphopsia or decreased visual acuity. Individuals with angioid streaks face the risk of subretinal bleeding, even from minor injuries.[2]
Affected patients are often asymptomatic. In asymptomatic patients, continued observation is the only necessary management. In cases where neovascularization occurs, early intervention with anti-vascular endothelial growth factor (VEGF) forms the mainstay of treatment. Combining photodynamic therapy with medications like bevacizumab can also help regress choroidal neovascularization. Timely intervention, coupled with the use of protective eyewear to prevent future trauma, plays a crucial role in enhancing overall outcomes and reducing the likelihood of future choroidal rupture and bleeding.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Angioid streaks are idiopathic or associated with a systemic underlying illness. The most commonly associated systemic condition is PXE or Grönblad–Strandberg syndrome, which is associated with elastic fiber degeneration in connective tissue followed by secondary calcium deposition. PXE is an autosomal recessive disease caused by a variation in ATP-binding cassette, subfamily C, member 6 gene, ABCC6, on chromosome 16p13.1.[3][4] ABCC6 encodes the multidrug resistance associated protein 6 (MRP6), which is in part responsible for regulating the amount of calcium and other minerals used by connective tissues. Additional common systemic associations include these conditions:
- Paget disease of the bone: A disease of osteoclasts that causes increased rates of bone remodeling. Paget disease of the bone is affected by both genetic and environmental factors. Currently, 15 different genetic loci are associated with Paget disease of the bone. Viral infections may also play a role in the development.
- Hemoglobinopathies: Sickle cell disease, among other hemoglobinopathies, is commonly associated with angioid plaques.
- Ehlers-Danlos syndrome: Historically, angioid streaks have been associated with Ehlers-Danlos syndrome. More recent literature reveals angioid streaks are not as common as previously thought.
Angioid streaks may be seen in conjunction with a variety of additional systemic conditions:[1][5]
- Marfan syndrome
- Acromegaly
- Hemochromatosis
- Diabetes
- Sturge Weber syndrome with facial angiomatosis
- Myopia
- Hyperphosphatemia
- Neurofibromatosis
- Hypercalcemia
- Abetalipoproteinemia
- Familial polyposis of the colon
- Congenital hypertrophy of retinal pigment epithelium
- Diffuse lipomatosis
- Cutaneous calcinosis
- Microsomia
- Myopia
- Trauma
- Hypertensive coronary artery disease
- Senile elastosis
- Epilepsy
Epidemiology
Idiopathic angioid streaks typically appear in the sixth decade of life, constituting roughly half of all cases, whereas patients with underlying conditions tend to show symptoms earlier. The occurrence of angioid streaks is notably high in specific conditions: approximately 87% of patients with PXE, 10% with Paget disease of the bone, and 1% to 2% with sickle cell disease.
Patients with PXE may present in their thirties, with a mean of 51.7, while those with sickle cell disease may exhibit symptoms in the second and third decades of life, with an average age of 41.7. On the other hand, patients with Paget disease tend to develop angioid streaks at a later age, typically around 67. Although no gender predilection exists, angioid streaks more commonly affect White individuals compared to their Black and Asian counterparts. Choroidal neovascularization accounts for central vision loss in 70% to 86% of affected patients.
Pathophysiology
Angioid streaks result from cracks in an abnormally thickened and calcified Bruch membrane, but the exact pathophysiology remains unclear. A combination of elastic degeneration, iron deposition from hemolysis, impaired nutrition, and the deposition of calcium, magnesium, or iron salts from disturbed metabolism may contribute to membrane fragility.[6]
Experts believe that the Bruch membrane undergoes calcification and becomes brittle, leading to crack development in patients affected by PXE and Paget disease of the bone. An abnormal accumulation and metabolism of glycosaminoglycans and glycoproteins within the Bruch membrane precedes the calcification in PXE.[7][8] Elastic tissue damage due to oxidative damage from hypoxia and cytokines may be the underlying cause in patients with sickle cell disease. Researchers also feel iron deposition in conditions like hereditary spherocytosis may contribute to breaks in the Bruch membrane.
The orientation of angioid streaks may be related to force lines due to the pull of intrinsic and extrinsic muscles of the eye on the fixed optic nerve. When full-thickness breaks occur, disruptions of the choriocapillaris occur, causing atrophy of the retinal pigment epithelium and loss of the overlying photoreceptor cells. Angioid streaks may cause vision change in 3 different ways. Trauma can lead to rupture of the choroid and submacular hemorrhage. Breaks in the mechanical integrity of the Bruch membrane allow space for abnormal neovascularization. Choroidal neovascularization can cause hemorrhage and macular edema and is the primary cause of vision loss.[9] The streak may travel through the fovea, causing retinal pigment epithelial loss. Angioid streaks also predispose the tissue to localized rupture that may occur spontaneously or with trauma.
Histopathology
Deterioration and fragmentation of the elastic lamina within the midsegment of the Bruch membrane characterize angioid streaks. Abundant granulomatous material in the elastic lamina is evident on electron microscopy. Additional findings are the absence of choriocapillaris beneath the streaks and thinning or discoloration of the retinal pigment epithelium. Patients with PXE and angioid streaks demonstrate calcium deposition in the Bruch membrane, accompanied by distinct breaks.[5]
History and Physical
Angioid streaks are usually bilateral and asymptomatic visually. Symptoms develop when the streaks involve the fovea or choroidal neovascularization involving the macular region. Metamorphopsia and micropsia can be early signs of macular involvement. The history and clinical examination findings may vary based on the systemic illness. Visual fields should be normal unless the central macula is affected. As vision becomes affected, patients lose color perception.
Clinical Features
The evaluation begins with a comprehensive ocular examination, including visual acuity, refraction, intraocular pressure, ocular motility, examination of the ocular adnexa, lacrimal system, cornea, anterior chamber, iris, lens, fundus, and pupillary reaction, including evaluation for the presence of a relative afferent pupillary defect.[10]
Clinicians may overlook angioid streaks on cursory retinal examination due to their similar appearance to retinal blood vessels.
Clinical characteristics of angioid streaks include these:
- Jagged irregular lines with serrated margins radiating from the optic disc
- Similar variable caliber to retinal vessels of 50 to 500 µm
- Subretinal location
- Streaks may interconnect and usually form a ring around the optic disc
- Gray, black, reddish, or pink color
- Macula may be involved
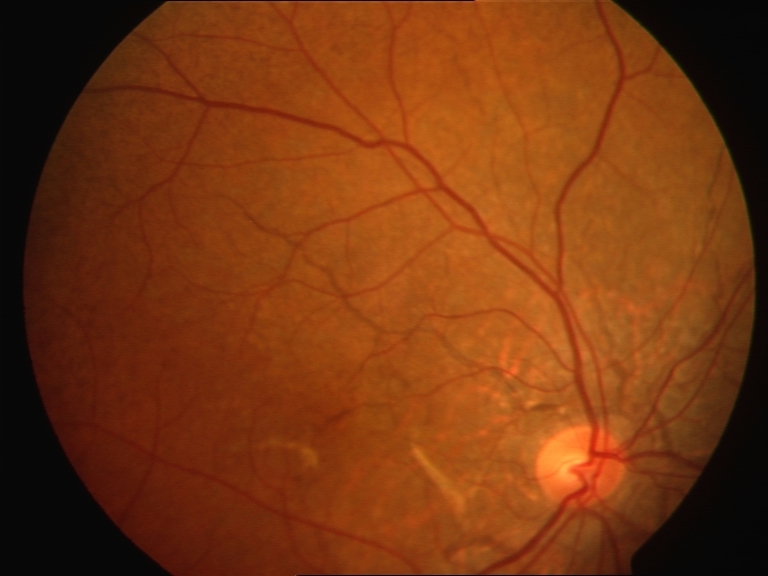
- Abrupt tapering at the end away from the optic disc (see Image. Fundus with Angioid Streaks).[11]
Angioid streaks may increase in number, width, and length as individuals age. These streaks may fade over time and appear as areas of hyperpigmentation of the retinal pigment epithelium or chorioretinal atrophy.[11] Some cases may have equally spread depigmentation around the angioid streak, which can be on either side. The width of this depigmented area may vary, extending up to 3 times the width of the angioid streak on either side.[12]
Associated features found in patients with angioid streaks include the following:
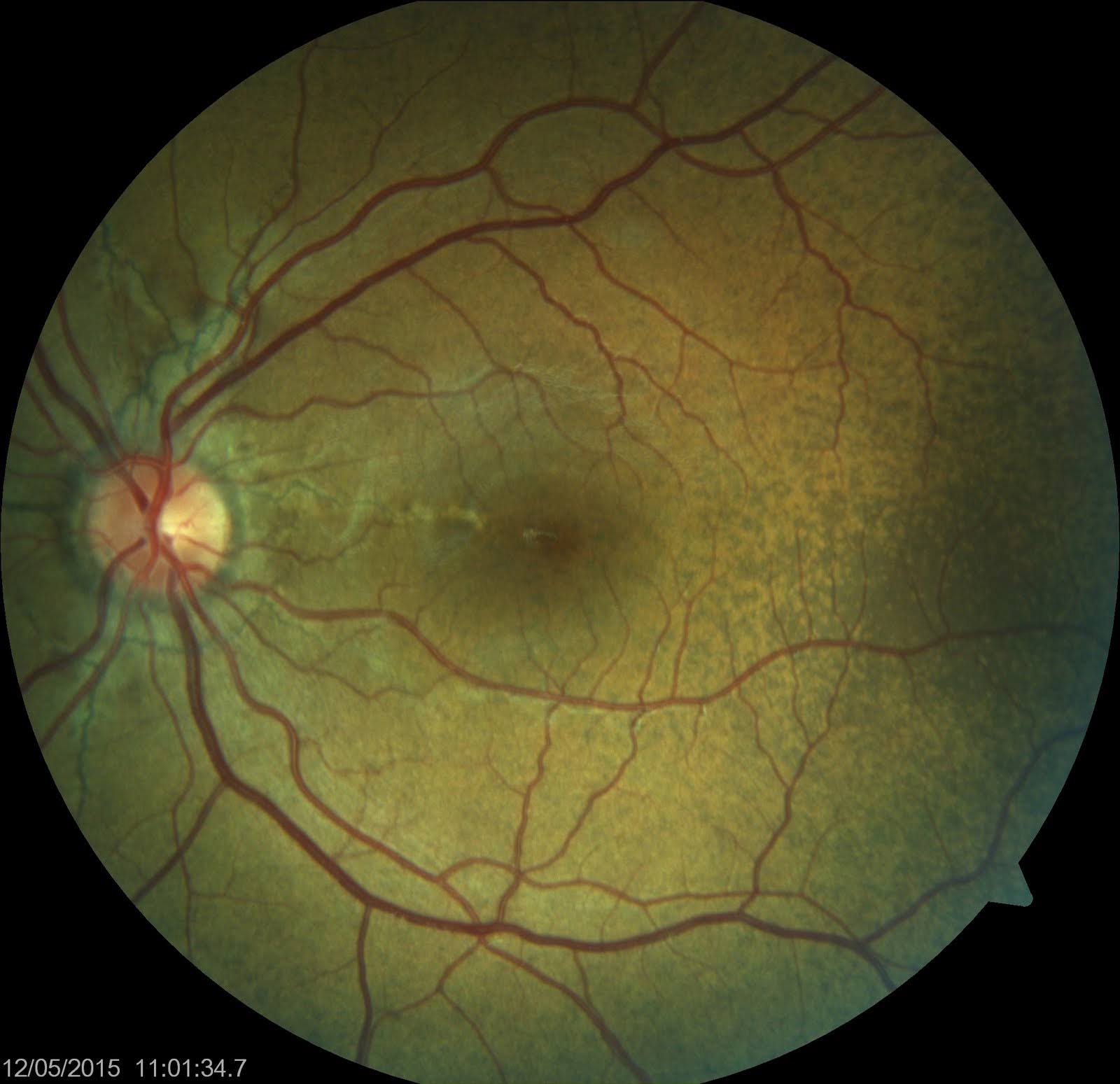
- Peau d'orange appearance of the fundus: Also known as leopard skin spotting, peau d'orange is primarily seen temporal to the macula and manifests as speckled yellowish mottling, which is sometimes confluent and resembles the skin of an orange (see Image. Angioid Streaks and Peau d'orange of Fundus). Peau d'orange can precede angioid streaks and is commonly associated with PXE due to alterations in the Bruch membrane, noted in nearly all patients diagnosed with skin lesions.[13][14] This appearance occurs less frequently in angioid streaks related to sickle cell disease or Paget disease of the bone. The pigmentary mottling may extend to the equator, occasionally affecting the retina nasal to the optic disc. Areas of opacification, termed "coquille d’oeuf" or eggshell, result from calcium deposition within the Bruch membrane, weakening its structure and leading to a cracked eggshell appearance.[15] Coquille d’oeuf presents as confluent opacification around the optic disc, with non-confluent peau d'orange areas peripheral to this lesion and remaining confined within the area of the confluent lesion.[15]
- Optic disc drusen: Drusen are hyaline bodies that may also precede the presence of angioid streaks. Drusen are often the first evidence of PXE. Optic head drusen are related to neovascularization in the peripapillary area and may cause visual loss due to pressure on the head of the optic nerve.
- Peripapillary chorioretinal degeneration, focal peripheral chorioretinal scars, and reticular pigment dystrophy of the macula: These changes may be present at diagnosis.
- Optic nerve atrophy: Atrophy may be present in patients with Paget disease of the bone.
- Macular thinning or pigmentary changes: These changes are usually bilateral, without hemorrhage or hard exudates.
- Crystalline bodies and comet-shaped lesions: Multiple, small, round, subretinal lesions in the mid-peripheral fundus or inferior to the optic nerve that cause some atrophy of the retinal pigment epithelium.
- Subretinal or submacular hemorrhage: The patient usually presents with a visual decline due to subfoveal hemorrhage, which may occur in the absence of choroidal neovascularization in cases of blunt ocular trauma.
Severe visual impairment occurs in 70% of affected patients due to 1 of the following findings:
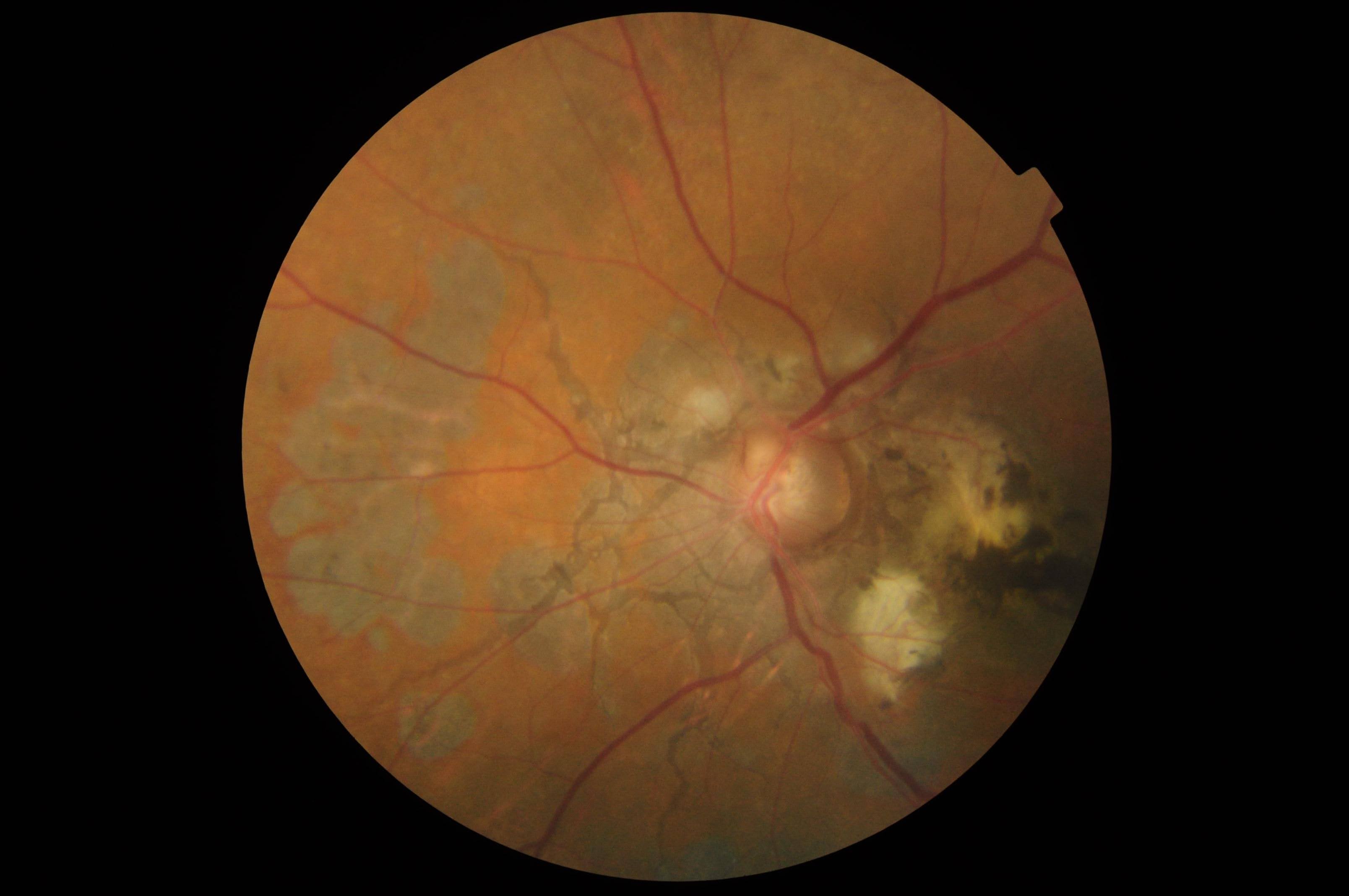
- Choroidal neovascularization with subsequent serous and hemorrhagic detachment of the fovea: This finding is the most serious complication (see Image. Choroidal Neovascularization). Usually, an associated angioid streak is present near choroidal neovascularization, which subretinal fluid, hard exudates, or blood may characterize. The incidence of choroidal neovascularization associated with angioid streaks is nearly 86%. Seventy-one percent of cases are bilateral.[1] Choroidal neovascularization is typically subretinal and recurrent. Choroidal neovascularization causes poor vision in patients with angioid streaks, and vision loss occurs earlier than neovascularization due to wet age-related macular degeneration. The chances of developing choroidal neovascularization are highest if the angioid streaks are wide and long or involve an area within 1 disc diameter of the foveola.[11] A cracked eggshell appearance or diffuse type of angioid steaks also predisposes patients to choroidal neovascularization.[16] PXE has a higher risk of macular neovascularization.
- Choroidal rupture: Generally, due to trivial trauma, choroidal rupture can result in secondary hemorrhage involving the fovea.
- Foveal involvement by a streak: Damage to retinal pigment epithelium and the choriocapillaris may result in permanent loss of central visual acuity.
Pseudoxanthoma Elasticum
Ectopic mineralization and fragmentation of elastic fibers in various body systems, including the skin, eyes, vasculature, and gastrointestinal tract, characterize PXE. Cutaneous and ocular manifestations typically emerge in the second or third decades. Skin lesions initially appear as yellowish or darker papules on the neck, extending to other areas such as the axillae, chest, abdomen, and flexural surfaces, leading to lax and redundant skin and a "plucked chicken" appearance.[17] Symptoms of acute retinopathy are unilateral visual decline, metamorphopsia, glare, relative scotoma, scintillations, and flashes.[18]
In addition to ocular involvement, peripheral vascular manifestations include diminished peripheral pulses, intermittent claudication, renovascular hypertension, premature coronary artery disease, and cerebrovascular disease. Aneurysms in the brain, kidney, and mesentery may cause bleeding and, rarely, intestinal angina and gastrointestinal bleeding due to mesenteric artery involvement.[3][19]
Nearly all patients with PXE show angioid streaks within 20 years following their diagnosis.[5][14] Some crystalline bodies exhibit a comet-like tail of depigmentation, often localized inferior to the optic disc, creating a comet-rain-like appearance. Comet lesions are considered pathognomonic of PXE.[13][20][21] Clinicians may note punched-out scars similar to presumed ocular histoplasmosis syndrome. These scars that do not have a tail have been called "salmon spots."[22] Hyperpigmentation on either side of the angioid streak can give rise to a "wing sign," which may be noted in approximately half of patients with PXE.[13] This wing sign is also present in angioid streaks associated with sickle cell disease. Refer to "Clinical Features" for more information regarding the ocular manifestations associated with PXE.
Paget Disease of the Bone
Most patients with Paget disease of the bone are asymptomatic. When symptomatic, pain, bone deformity, arthritis, and fracture are the main symptoms and findings. Some patients may experience radiculopathy due to nerve root compression, hearing loss, kyphoscoliosis, or headache. Refer to "Clinical Features" for more information regarding the ocular manifestations associated with PXE.
Sickle Cell Disease
The major features associated with sickle cell disease are related to hemolytic anemia and vaso-occlusion, which can lead to acute and chronic pain and tissue ischemia or infarction. The clinical history and physical findings are dependent on the organ system affected. They can include acute chest syndrome, infection, myocardial infarction, stroke, priapism, anemia, and kidney infarction, among a variety of other afflictions. Refer to "Clinical Features" for more information regarding the ocular manifestations associated with PXE.
Evaluation
General Investigation
A general evaluation is necessary to evaluate for potential associated systemic conditions and their complications, including gastrointestinal hemorrhage, heart disease, anemia, and pathological fractures. Serum calcium, phosphorous, and alkaline phosphatase levels may be abnormal in patients with Paget disease of the bone, though the diagnosis is generally radiologic. Characteristic radiographic findings are thickened cortices marked by tunneling and accentuated trabeculae. Following plain radiographs, patients with Paget disease of the bone should undergo a baseline radionuclide bone scan to determine the extent of skeletal involvement. A bone biopsy is only necessary if the appearance of the lesion is unusual and a concern exists for metastatic disease.
Clinicians diagnose PXE based on the presence of major and minor criteria. Patients must have 2 of the following:
- Two major skin criteria
- One major ocular criterion
- Two allelic pathogenic mutations in ABCC6
Skin major criteria are pseudoxanthomatous papules, plaques on the neck, flexural creases, and histopathology showing calcification of clumped, pleomorphic elastic fibers in the mid- or lower dermis. Ocular major criteria are 1 or more angioid streaks larger than 1 disc diameter in length and peau d'orange.
Minor diagnostic criteria are a definite diagnosis of PXE in a first-degree relative, finding of pathogenic mutation on only 1 allele on genetic testing, and histopathologic changes of PXE on skin with no lesions.
The diagnostic test for sickle cell disease depends on the patient's age. Clinicians use DNA-based testing for prenatal diagnosis. After birth, high-performance liquid chromatography or electrophoresis is the preferred method of diagnosis. High-performance liquid chromatography identifies and quantifies hemoglobins. In the United States, newborns undergo screening for sickle cell disease.
Ocular Investigations
Angioid streaks may be very subtle and require multimodal imaging for proper evaluation.[23]
Fluorescein angiography
Fluorescein angiography (FA) uses fluorescein to visualize the blood vessels in the choroid and retina. Hypofluorescence, a reduction from the normally expected fluorescence, hyperfluorescence, or an increased fluorescence, indicates an abnormal result. Hypofluorescence may occur due to a blocking effect or a vascular filling defect. Fluorescein leakage, staining, pooling, transmission defects, and autofluorescence cause hyperfluorescence.
Under normal circumstances, the capillary-free zone appears dark due to the blockage of choroidal fluorescence by xanthophyll pigment and tightly packed retinal pigment epithelial cells. Atrophic retinal pigment epithelium or atrophy and separation of underlying choriocapillaries cause hyperfluorescence in early FA. Late FA may reveal staining of the edges of the streaks and in the center of the macula due to the Bruch membrane crack extension.
Indocyanine green angiography
Indocyanine green angiography (ICGA) is superior to FA in detecting choroidal neovascularization. In addition, the peau d'orange appearance of the temporal macula on ICGA presents as a speckled pattern in the midperiphery.[24]
Optical coherence tomography and optical coherence tomography angiography
Optical coherence tomography identifies neovascular leakage and monitors treatment efficacy, which is helpful as distinguishing between low-grade leakage, which would mean choroidal neovascularization activity, and staining on fluorescein angiography can be challenging. Optical coherence tomography angiography (OCTA) provides noninvasive information on retinal structures and vascular flow.
Ultrasound
Ultrasound can detect optic nerve head drusen in approximately 25% of patients with PXE and angioid streaks.[25]
Treatment / Management
Angioid streaks are usually asymptomatic and do not require treatment. However, eyes with angioid streaks are more prone to develop subretinal hemorrhage after trivial trauma, so clinicians should advise patients to use protective eyewear.[2] All patients with angioid streaks should be screened for potential systemic associations. Examination of family members may provide clues to any underlying systemic illnesses. Clinicians should perform fundus FA to exclude choroidal neovascularization when subretinal hemorrhage is present.[26] If choroidal neovascularization is absent, the hemorrhage usually resolves independently.(B3)
Choroidal neovascularization associated with angioid streaks is aggressive and may lead to disciform scar or atrophy at the macula despite treatment. As such, early diagnosis and management are of crucial importance.[27][28] One large series reveals that final visual outcomes are worse in subfoveal choroid neovascularization than in juxtafoveal or extrafoveal neovascularization.[9] Despite treatment, patients may continue to lose vision within 5 to 10 years, along with a high propensity for recurrence of choroidal neovascularization.[9][29] If choroidal neovascularization is detected, the management options include laser photocoagulation, photodynamic therapy (PDT), macular translocation surgery, autologous retinal pigment epithelium and choroid transplantation, and anti-VEGF agents.[9][30][31][32] Recurrence of choroidal neovascularization causing vision loss is a concern for patients with angioid streaks.(B2)
Among anti-VEGF agents, ranibizumab, aflibercept, brolucizumab, and bevacizumab, approved by the United States Food and Drug Administration (FDA) for age-related macular degeneration, diabetic macular edema, diabetic retinopathy, macular edema following retinal vein occlusion and myopic choroidal neovascularization have been used successfully and may improve or stabilize visual acuity.[33] All anti-VEGF agents have efficacy against both macular and juxtapapillary choroidal neovascularization and may rapidly improve the retinal anatomy on OCT. Anti-VEGF agents may help stop the activity of choroidal neovascularization without forming a scar.[1] Anti-VEGF therapy improves visual acuity and central macular thickness in patients with choroid neovascularization related to pattern dystrophy-like deposits in PXE.[34] Anti-VEGF agents are safe and effective and are considered first-line therapy for choroid neovascularization associated with angioid streaks.[9][29][35] (B2)
In the past, attempts to use laser photocoagulation and photodynamic therapy resulted in poor outcomes and frequent recurrences. Recent advances now allow the use of photocoagulation to slow the progression of choroid neovascularization toward the fovea and stabilize vision without causing significant damage to the retinal pigment epithelium and neurosensory retina. Recurrence rates may still reach 77%. Patients often require multiple treatments and close monitoring with FA.
Transpupillary thermotherapy (TTT) does appear to maintain visual acuity and produce an initial decrease in the size of angioid streaks, but they increase within 3 months. For this reason, TTT is not an acceptable option in treating choroidal neovascularization associated with angioid streaks. PDT is approved by the FDA to treat choroid neovascularization due to age-related macular degeneration. Small studies reveal a short-term benefit of PDT in treating choroid neovascularization due to angioid streaks. However, the long-term effects of PDT, especially in patients who may need multiple treatments, are unknown. Centers around the world use a combination of photodynamic therapy and ranibizumab. Surgical options, including macular translocation surgery, transplantation of an autologous full-thickness retinal pigment epithelium and choroidal patch, and surgical removal of choroidal neovascular membranes, might be a rescue option in patients with refractory subfoveal CNV, despite anti-VEGF treatment.
Differential Diagnosis
The following list includes the differential diagnoses of angioid streaks:
- Normal retinal vessels
- Myopic lacquer cracks
- These cracks are mechanical breaks of the retinal pigment epithelium, Bruch membrane, and choriocapillaris complex that have healed and are found in the posterior pole of highly myopic eyes caused by stretching of the coats of the eyeball with increasing axial myopia.
- Reticular dystrophy of the retinal pigment epithelium
- Retinal pigment epithelium changes that cause pigmentation in a "fishnet with knot" pattern characterize this condition. The changes are located at the posterior pole and may cause a circle similar to angioid streaks.
- Subretinal tracks due to ophthalmomyiasis interna are smooth and may cross each other multiple times.[36]
- Subretinal bands associated with long-standing retinal detachment or surgically repaired retinal detachments may simulate angioid streaks.
- Peripheral punched-out scars may simulate presumed ocular histoplasmosis syndrome.
- Choroidal rupture
- Central serous retinopathy
- Choroidal sclerosis
- Exudative age-related macular degeneration
- Metastatic choroidal tumor
- Toxoplasmosis
Prognosis
The visual and long-term prognosis of treated and untreated patients with choroidal neovascularization is generally poor.[37] Choroidal neovascularization develops in 72% to 86% of patients with angioid streaks over time. Once neovascularization develops in 1 eye, it becomes bilateral in the other eye within 18 months in 50% of patients.[11] Fifteen percent of patients with choroidal neovascularization develop clinically significant visual loss from minor trauma. By 50, most patients with PXE have vision that is 20/200 or worse. Angioid streaks associated with sickle cell disease have a much more favorable prognosis. The course is typically benign, and choroid neovascularization is rare.
Complications
The complications of angioid streaks include the following:
Deterrence and Patient Education
Angioid streaks can either occur without an identifiable cause or be linked to an underlying systemic illness, necessitating a comprehensive evaluation to uncover any potential associated conditions. While many patients show no symptoms, clinicians must regularly monitor their condition. Affected individuals need to recognize the possibility of vision loss due to foveal involvement or choroidal neovascularization. They must understand the need to promptly seek medical attention if they notice a decline in visual acuity, experience central vision impairment, encounter difficulties with depth perception, or perceive distortion in lines and objects. Given the risk of choroidal rupture and subretinal hemorrhage resulting from even minor blunt trauma, patients should wear protective eyewear, such as rigid goggles, during activities like sports or any other endeavors that may pose a risk of head or eye injury.
Clinicians should reinforce the importance of choroid neovascularization through early detection with regular ophthalmologic examinations. The Amsler grid helps with in-home monitoring of metamorphopsia and scotoma and may prompt the patients to seek medical care sooner.[38] Family members of patients with angioid streaks should also receive a comprehensive ophthalmic evaluation for early detection of angioid streaks and to rule out systemic associations.
Enhancing Healthcare Team Outcomes
Angioid streaks manifest as disruptions in the Bruch membrane due to the mineralization and fragmentation of its elastic fibers. They may arise spontaneously or are commonly associated with conditions such as pseudoxanthoma elasticum, Paget disease of the bone, and sickle cell anemia. These streaks pose a significant risk to affected individuals, potentially leading to choroidal neovascularization, subretinal hemorrhage, and detachment of retinal photoreceptors, which can result in permanent central vision loss, especially when involving the macula. Managing patients with angioid streaks requires a collaborative effort among healthcare professionals to ensure prompt diagnosis and intervention to preserve vision.
Diagnosis typically entails a thorough ocular examination and imaging techniques such as FA and OCT. Treatment primarily focuses on managing complications, often involving interventions such as anti-VEGF injections or photocoagulation. Regular monitoring, patient education, and emotional support are essential aspects of comprehensive care for individuals with angioid streaks, vital for optimizing outcomes and minimizing the risk of vision loss. Embracing a coordinated, interprofessional approach involving ophthalmology, primary care, advanced practice clinicians, pharmacists, and nurses is crucial for delivering patient-centered care, enhancing team performance, and improving patient outcomes in managing angioid streaks.[39]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Chatziralli I, Saitakis G, Dimitriou E, Chatzirallis A, Stoungioti S, Theodossiadis G, Theodossiadis P. ANGIOID STREAKS: A Comprehensive Review From Pathophysiology to Treatment. Retina (Philadelphia, Pa.). 2019 Jan:39(1):1-11. doi: 10.1097/IAE.0000000000002327. Epub [PubMed PMID: 30260918]
Chraibi F, Salima B, Meryem A, Idriss BA, Tahri H. Subretinal hemorrhages associated with angioid streaks following a mild ocular trauma. Oman journal of ophthalmology. 2012 Sep:5(3):200-2. doi: 10.4103/0974-620X.106109. Epub [PubMed PMID: 23436976]
Level 3 (low-level) evidenceRoach ES, Islam MP. Pseudoxanthoma elasticum. Handbook of clinical neurology. 2015:132():215-21. doi: 10.1016/B978-0-444-62702-5.00015-9. Epub [PubMed PMID: 26564082]
Uitto J, Jiang Q, Váradi A, Bercovitch LG, Terry SF. PSEUDOXANTHOMA ELASTICUM: DIAGNOSTIC FEATURES, CLASSIFICATION, AND TREATMENT OPTIONS. Expert opinion on orphan drugs. 2014 Jun 1:2(6):567-577 [PubMed PMID: 25383264]
Level 3 (low-level) evidenceGeorgalas I, Papaconstantinou D, Koutsandrea C, Kalantzis G, Karagiannis D, Georgopoulos G, Ladas I. Angioid streaks, clinical course, complications, and current therapeutic management. Therapeutics and clinical risk management. 2009 Feb:5(1):81-9 [PubMed PMID: 19436620]
KLIEN BA. Angloid streaks; a clinical and histopathologic study. American journal of ophthalmology. 1947 Aug:30(8):955-68 [PubMed PMID: 20256332]
Hosen MJ, Lamoen A, De Paepe A, Vanakker OM. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica. 2012:2012():598262. doi: 10.6064/2012/598262. Epub 2012 Jul 25 [PubMed PMID: 24278718]
Passi A, Albertini R, Baccarani Contri M, de Luca G, de Paepe A, Pallavicini G, Pasquali Ronchetti I, Tiozzo R. Proteoglycan alterations in skin fibroblast cultures from patients affected with pseudoxanthoma elasticum. Cell biochemistry and function. 1996 Jun:14(2):111-20 [PubMed PMID: 8640951]
Ramakrishnan T, Chandra S, Sivaprasad S. Long-term follow-up of management of choroidal neovascularisation secondary to angioid streaks with intravitreal anti-vascular endothelial growth factor. Eye (London, England). 2021 Mar:35(3):853-857. doi: 10.1038/s41433-020-0979-9. Epub [PubMed PMID: 32461565]
Simakurthy S, Tripathy K. Marcus Gunn Pupil. StatPearls. 2024 Jan:(): [PubMed PMID: 32491607]
Mansour AM, Ansari NH, Shields JA, Annesley WH Jr, Cronin CM, Stock EL. Evolution of angioid streaks. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1993:207(2):57-61 [PubMed PMID: 8272342]
Level 3 (low-level) evidenceShields JA, Federman JL, Tomer TL, Annesley WH Jr. Angioid streaks. I. Ophthalmoscopic variations and diagnostic problems. The British journal of ophthalmology. 1975 May:59(5):257-66 [PubMed PMID: 1138852]
Plomp AS, Toonstra J, Bergen AA, van Dijk MR, de Jong PT. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. American journal of medical genetics. Part A. 2010 Apr:152A(4):1049-58. doi: 10.1002/ajmg.a.33329. Epub [PubMed PMID: 20358627]
Neldner KH. Pseudoxanthoma elasticum. Clinics in dermatology. 1988 Jan-Mar:6(1):1-159 [PubMed PMID: 3359381]
Spaide RF. Peau d'orange and angioid streaks: manifestations of Bruch membrane pathology. Retina (Philadelphia, Pa.). 2015 Mar:35(3):392-7. doi: 10.1097/IAE.0000000000000420. Epub [PubMed PMID: 25526100]
Mansour AM, Shields JA, Annesley WH Jr, el-Baba F, Tasman W, Tomer TL. Macular degeneration in angioid streaks. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1988:197(1):36-41 [PubMed PMID: 2460816]
Level 2 (mid-level) evidenceTripathy K, Das A, Chawla R, Temkar S. A young female with subretinal thread-like structures. Oman journal of ophthalmology. 2019 Jan-Apr:12(1):67. doi: 10.4103/ojo.OJO_152_2016. Epub [PubMed PMID: 30787543]
Gliem M, Birtel J, Müller PL, Hendig D, Faust I, Herrmann P, Holz FG, Adamus G, Charbel Issa P. Acute Retinopathy in Pseudoxanthoma Elasticum. JAMA ophthalmology. 2019 Oct 1:137(10):1165-1173. doi: 10.1001/jamaophthalmol.2019.2910. Epub [PubMed PMID: 31393536]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Terry SF, Uitto J. Pseudoxanthoma Elasticum. GeneReviews(®). 1993:(): [PubMed PMID: 20301292]
Gass JD. "Comet" lesion: an ocular sign of pseudoxanthoma elasticum. Retina (Philadelphia, Pa.). 2003 Oct:23(5):729-30 [PubMed PMID: 14574271]
Barteselli G, Viola F. Comet lesions in pseudoxanthoma elasticum: a spectral domain optical coherence tomography analysis. Retina (Philadelphia, Pa.). 2015 May:35(5):1051-3. doi: 10.1097/IAE.0000000000000396. Epub [PubMed PMID: 25360788]
Krill AE, Klien BA, Archer DB. Precursors of angioid streaks. American journal of ophthalmology. 1973 Dec:76(6):875-9 [PubMed PMID: 4128186]
Sampson DM, Alonso-Caneiro D, Chew AL, Lamey T, McLaren T, De Roach J, Chen FK. Enhanced Visualization of Subtle Outer Retinal Pathology by En Face Optical Coherence Tomography and Correlation with Multi-Modal Imaging. PloS one. 2016:11(12):e0168275. doi: 10.1371/journal.pone.0168275. Epub 2016 Dec 13 [PubMed PMID: 27959968]
Muraleedharan S, Tripathy K. Indocyanine Green (ICG) Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35593804]
Pierro L, Brancato R, Minicucci M, Pece A. Echographic diagnosis of Drusen of the optic nerve head in patients with angioid streaks. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1994:208(5):239-42 [PubMed PMID: 7816413]
Ruia S, Tripathy K. Fluorescein Angiography. StatPearls. 2024 Jan:(): [PubMed PMID: 35015403]
Iacono P, Battaglia Parodi M, La Spina C, Bandello F. Intravitreal Bevacizumab for Nonsubfoveal Choroidal Neovascularization Associated With Angioid Streaks: 3-Year Follow-up Study. American journal of ophthalmology. 2016 May:165():174-8. doi: 10.1016/j.ajo.2016.03.017. Epub 2016 Mar 21 [PubMed PMID: 27013066]
Finger RP, Charbel Issa P, Ladewig M, Götting C, Holz FG, Scholl HP. Fundus autofluorescence in Pseudoxanthoma elasticum. Retina (Philadelphia, Pa.). 2009 Nov-Dec:29(10):1496-505. doi: 10.1097/IAE.0b013e3181aade47. Epub [PubMed PMID: 19823106]
Kumar K, Balasubramaniam S, Agarwal A. Clinical characteristics and treatment outcomes of angioid streak associated choroidal neovascular membrane (AS-CNV): a Zambian case series. The Pan African medical journal. 2020:36():294. doi: 10.11604/pamj.2020.36.294.25065. Epub 2020 Aug 17 [PubMed PMID: 33117488]
Level 2 (mid-level) evidenceOzdek S, Bozan E, Gürelik G, Hasanreisoglu B. Transpupillary thermotherapy for the treatment of choroidal neovascularization secondary to angioid streaks. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2007 Feb:42(1):95-100 [PubMed PMID: 17361248]
Eckstein M, Wells JA, Aylward B, Gregor Z. Surgical removal of non-age-related subfoveal choroidal neovascular membranes. Eye (London, England). 1998:12 ( Pt 5)():775-80 [PubMed PMID: 10070507]
Parolini B, Alkabes M, Baldi A, Pinackatt S. VISUAL RECOVERY AFTER AUTOLOGOUS RETINAL PIGMENT EPITHELIUM AND CHOROIDAL PATCH IN A PATIENT WITH CHOROIDAL NEOVASCULARIZATION SECONDARY TO ANGIOID STREAKS: LONG-TERM RESULTS. Retinal cases & brief reports. 2016 Fall:10(4):368-72. doi: 10.1097/ICB.0000000000000265. Epub [PubMed PMID: 26679062]
Level 3 (low-level) evidenceChakraborty S, Sheth JU. Intravitreal Brolucizumab for Choroidal Neovascularization Associated to Angioid Streaks. Case reports in ophthalmological medicine. 2022:2022():3442306. doi: 10.1155/2022/3442306. Epub 2022 Jul 14 [PubMed PMID: 35874928]
Level 3 (low-level) evidenceBattaglia Parodi M, Romano F, Marchese A, Arrigo A, Llorenç V, Cicinelli MV, Bandello F, Adán A. Anti-VEGF treatment for choroidal neovascularization complicating pattern dystrophy-like deposit associated with pseudoxanthoma elasticum. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2019 Feb:257(2):273-278. doi: 10.1007/s00417-018-4190-7. Epub 2018 Nov 23 [PubMed PMID: 30470876]
Benitez-Herreros J, Camara-Gonzalez C, Lopez-Guajardo L, Beckford-Torngren C, Pareja-Esteban J. [Choroidal neovascularization secondary to angioid streaks: A familial case report]. Archivos de la Sociedad Espanola de Oftalmologia. 2014 May:89(5):190-3. doi: 10.1016/j.oftal.2012.11.005. Epub 2012 Dec 23 [PubMed PMID: 24269391]
Level 3 (low-level) evidenceSingh P, Tripathy K. Ophthalmomyiasis. StatPearls. 2024 Jan:(): [PubMed PMID: 35015433]
Rohart C, Le HM, Estrada-Walker J, Giocanti-Auregan A, Cohen SY. LONG-TERM PROGNOSIS OF CHOROIDAL NEOVASCULARIZATION COMPLICATING ANGIOID STREAKS. Retina (Philadelphia, Pa.). 2023 Jun 1:43(6):882-887. doi: 10.1097/IAE.0000000000003746. Epub [PubMed PMID: 36727798]
Tripathy K, Salini B. Amsler Grid. StatPearls. 2024 Jan:(): [PubMed PMID: 30844168]
Stumpf MJ, Schahab N, Nickenig G, Skowasch D, Schaefer CA. Therapy of Pseudoxanthoma Elasticum: Current Knowledge and Future Perspectives. Biomedicines. 2021 Dec 13:9(12):. doi: 10.3390/biomedicines9121895. Epub 2021 Dec 13 [PubMed PMID: 34944710]
Level 3 (low-level) evidence