Introduction
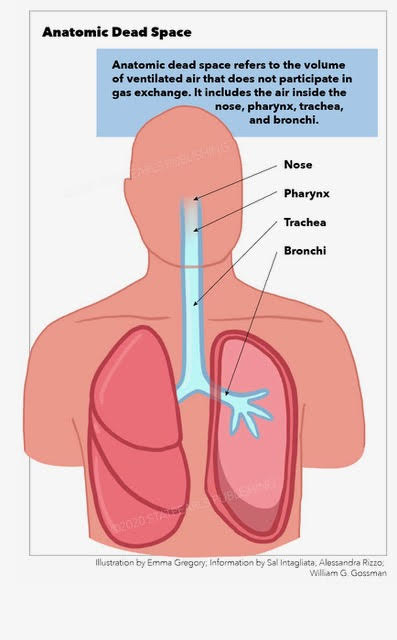
Anatomic dead space is an important phenomenon in respiratory physiology whereby, owing to the fact that upper airways do not function as locations for gas exchange, and because of the tidal nature of ventilation, there is always a fraction of the inspired air that does not perform a physiologic function of exchanging carbon dioxide for oxygen.[1] This is therefore termed anatomical dead space as it serves no respiratory function. This phenomenon has clinical significance because, both in healthy and impaired lungs, properly calculating and accounting for this non-physiological space is important for the proper respiratory care of ventilated patients. Indeed, it may serve as a prognostic factor in patients with acute repository distress syndrome (ARDS) who require ventilation.[2] However, differences in the exact way of measuring this space result in clinically significant different results and, therefore, debate remains about the true value of this measured parameter.[3]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
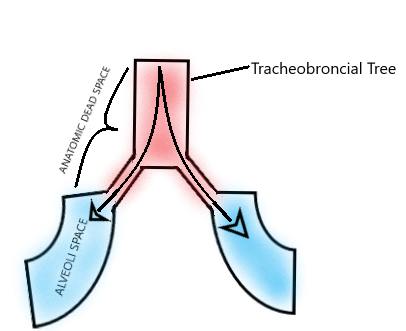
Dead space of the respiratory system refers to the volume of inspired air in a given breath in which oxygen (O2) and carbon dioxide (CO2) gasses are not exchanged across the alveolar membrane in the respiratory tract.[1] This is comprised of two segments: the anatomic dead space (parts of the airway that are not alveolar exchange membranes) and the alveolar dead space (alveoli that are ventilated but not perfused with pulmonary capillary blood flow).[4] See Figure. Anatomical Dead Space Diagram.
Anatomic dead space specifically refers to the volume of air located in the respiratory tract segments that are responsible for conducting air to the alveoli and respiratory bronchioles but do not take part in the process of gas exchange itself. These segments of the respiratory tract include the upper airways, trachea, bronchi, and terminal bronchioles. On the other hand, alveolar dead space refers to the volume of air in alveoli that are ventilated but not perfused, and thus gas exchange does not take place.[5][6] See Figure. Anatomic Dead Space.
Physiologic dead space (VD-Phys) is the sum of the anatomic (VD-Ana) and alveolar (VD-Alv) dead space. Thus:
VD-Phys = VD-Ana + VD-Alv (L)
Dead space ventilation (VD) is then calculated by multiplying VD-Phys by the respiratory rate (RR):
VD = VD-Phys x RR (L/min)
Total ventilation (VE) is, therefore, the sum of alveolar ventilation (Valv) and VD:
VE = Valv + VD (L/min)
Enghoff's equation compiles these variables with PaCO2, tidal volume (TV), and expired CO2 (PECO2). It is then implied that V/VT represents the portion of a tidal volume that does not participate in gas exchange [7]:
V/VT = (PaCO2 - PECO2)/PaCO2q
Dead space has particular significance in the concept of ventilation (V) and perfusion (Q) in the lung, represented by the V/Q ratio. Alveoli with no perfusion have a V/Q of infinity (Q=0), whereas alveoli with no ventilation have a V/Q of 0 (V=0). Therefore, in situations (i.e., V/Q =infinity) in which the alveoli are ventilated but not perfused, gas exchange cannot occur, such as when pulmonary embolism increases alveolar dead space.
Although initially counter-intuitive, there are multiple functions performed by the non-gas exchanging upper airway, including the anatomical dead space, that are important to normal respiratory function. Carbon dioxide is retained, resulting in bicarbonate-buffered blood and interstitium. Inspired air is raised or lowered to body temperature, increasing the affinity of hemoglobin for O2 and improving O2 uptake.[8] Particulate matter is trapped in the mucus that lines the conducting airways, allowing it to be removed by mucociliary transport and thus performing a first-line barrier function to foreign matter. Finally, inspired air is humidified in the upper airways, which is important to its temperature and gas exchange function.[9]
Alveolar dead space is typically negligible in a healthy adult. Anatomic, and therefore physiological, dead space normally is estimated at 2 mL/kg of body weight and comprises 1/3 of the TV in a healthy adult patient; it is even higher in pediatric patients.[10] Effectively, 1/3 of a TV of inhaled air is rebreathed due to dead space. At the end of expiration, the dead volume consists of a gas mixture high in CO2 and low in O2 compared to ambient air. The composition of end-expiratory dead volume air is 5 to 6% carbon dioxide and 15 to 16% oxygen. In comparison, ambient air is comprised of 0.04% carbon dioxide and 21% oxygen.
Physiologic Variants
Numerous physiologic factors can influence the anatomic dead space, owing to variations in its function from posture, sleep, and the anatomy of the upper airway itself, as well as the associated bony and soft tissue structures.
Respiratory Cycle
Inhalation increases bronchial diameter and length, effectively increasing the anatomic dead space. Likewise, exhalation decreases the amount of anatomic dead space by "deflating" the bronchial tree.
Positioning
Dead space decreases with the supine position and increases during a sitting position. The upright position allows a mismatched ratio of ventilation (V) and perfusion (Q) to occur, in which the apices of the lungs can not be as well perfused as ventilated (due to gravity's greater effect on blood than air), so wasted ventilation occurs and effectively increases dead space volume.[11]
Sleep
Anatomic dead space is believed to decrease during sleep and be the primary physiologic cause of observed decreases in tidal volume, minute ventilation, and respiratory rate during sleep.[12][13]
Maxilla
Variation also can occur in patients with maxillary defects or those who have undergone maxillectomy procedures. These patients have an increased anatomic dead space due to communication between the nasal and oral cavities, ultimately affecting respiratory function.
Surgical Considerations
In patients with disease-free lungs who are undergoing general anesthesia for procedures non-affective of the thoracic cavity or diaphragm, dead space and compliance of the lungs have enabled physicians to tailor patients' PEEP to optimal levels, with the reasoning that the point of minimum dead space with maximum compliance represents the point at which the maximum amount of alveoli are opened for ventilation. Increasing VD, however, can signify that alveoli may be over-distending from overly-aggressive ventilation parameters. Lung recruitment maneuvers in adjunct to PEEP in mechanical ventilation have been shown to significantly increase functional residual capacity, compliance, and PaO2 with decreases in dead space compared to PEEP alone.[14][15][16]
Clinical Significance
Dead space can be affected by various clinical scenarios, both in terms of lung and airway pathology that change the anatomical dead space or secondary to healthcare intervention that affects physiology, such as mechanical ventilation.
Lung Disease
Chronic obstructive pulmonary disease (COPD) destroys alveolar tissue and leads to air trapping and decreased diffusion surface area, thereby increasing dead space volume.[17][18] Acute respiratory distress syndrome (ARDS) creates disturbances in the pulmonary microvasculature, theoretically increasing dead space. However, it is poorly understood if these portions of the lung are ventilated sufficiently to be considered dead space. VDphys/VT measured by Enghoff's equation increases in ARDS; however, due to the ratio being reflective of V/Q changes, which occur in pulmonary shunting mechanisms (perfusion without ventilation). Elevated dead-space fractions have been observed in critical care cohorts of patients with ARDS to be associated with an increased likelihood of death, both in the early and intermediate stages of the disease.[2][19]
Pulmonary Embolism
Clinical trials have demonstrated that in patients where the diagnosis of pulmonary embolism is suspected, dead space and capnography findings can be utilized, along with the biochemical test D-dimer, to exclude the diagnosis. The clinical diagnosis of pulmonary embolism when not critically unwell is difficult because common signs such as shortness of breath, chest pain, and mild hypoxia may be caused by various clinical entities. Furthermore, the diagnostic imaging technique of CT pulmonary angiography is a relatively expensive technique and therefore requires appropriate clinical triage. In this setting, volumetric capnography was found to be a sensitive but not specific test for pulmonary embolism, demonstrating a sensitivity of 98.4% (95% confidence interval [CI], 91.6%-100.0%).[20] Furthermore, capnography can be used for periodic monitoring of thrombolysis treatment in pulmonary embolism by trending changes in dead space measurements.[21] Dead space and capnography can thus prove to be useful tools, minimizing unnecessary tests by ruling out pulmonary embolism with simple capnography measurements.
Mechanical Ventilation
When patients require mechanical ventilation, the necessary tubing from the ventilator machine to the endotracheal tube increases dead space volume by adding length to the space between inhaled air and the alveolar gas exchange space. In other words, the tubing of mechanical ventilators obviously does not participate in gas exchange, and minimizing this space is important when considering the optimal care of mechanically ventilated patients, particularly during critical illness.[22]
Positive end-expiratory pressure (PEEP) is commonly used in the ventilation of critically ill respiratory patients, for example, in ARDS. The modality of ventilation increases the anatomic dead space by expanding the conducting airways.[23] Excessive PEEP can over-distend alveoli and result in lung barotrauma, increasing the dead space volume.
Furthermore, in patients who have been mechanically ventilated during a critical care illness, the dead space to tidal volume ratio (Vd/Vt) has been demonstrated to be of clinical use when considering extubation, both in the adult and pediatric populations. In adults, one study demonstrated that a poor Vd/Vt ratio was powerfully predictive of extubation failure, with an area under the ROC curve value of 0.94 (95%CI 0.86 to 0.98, p<0.0001).[24] Similarly, in the pediatric population, a Vd/Vt ratio of less than or equal to 0.50 was found to be significantly associated with successful intubation.[25] However, another large prospective study found that although there was no specific value that predicted extubation success, a high Vd/Vt was predictive of the need for a high level of respiratory support post-extubation among critically ill pediatric patients.[10]
Specifically, within the pediatric population who are mechanically ventilated, studies have shown that preterm infants have higher anatomical dead space volumes compared to neonates born at term. The median anatomical dead space volume was found to be 3.7 ml/kg in infants born prematurely and 2.4 ml/kg in infants born at term.[26] This is clinically important because of the effect it likely has on the work of breathing and the efficacy of low-volume mechanical ventilation in infants who have a proportionally larger fraction of their tidal volume occupied by anatomic dead space.
Anesthesia
Bronchodilation from inhalational anesthetic agents, particularly isoflurane, increases dead space volume.[27] Estimating the dead space can be of significant value in clinical situations for diagnostic, prognostic, and therapeutic value. Dead space is an integral part of volume capnography, which measures expired CO2 and dead space (VDphys/VT) on a breath-by-breath basis for efficient monitoring of patient ventilation. Even though the VDphys/VT ratio measured by Enghoff's equation is adversely affected by pulmonary shunting in ARDS, VDphys/VT has been shown to be a significant predictor of mortality during early-phase acute respiratory distress syndrome (ARDS), and increases in the VDphys/VT ratio correlated with poorer patient outcomes. Measurement of this dead space provides a quantifiable indicator of overall lung function for physicians to assess throughout the course of ARDS patients' hospital course. PEEP, an integral part of ARDS ventilation management, can be titrated to a patient's specific need based on capnography and dead space monitoring, but this finding has not been consistently shown in multiple studies.
High-flow nasal oxygen
Clearance of the anatomic dead space is believed to play a significant role in using nasal high-flow cannulas. It is believed that high nasal flow allows dead space to be cleared more rapidly and subsequently decreases the portion of dead space that is rebreathed, increasing alveolar ventilation.[28] A recent study demonstrated that the administration of nasal high-flow oxygen cleared expired air, thus reducing the physiologic dead space, and this extended below the soft palate.[29] This may reduce the amount of reinspired air, improve alveolar ventilation, and reduce respiratory rate.
Media
(Click Image to Enlarge)
References
Robertson HT. Dead space: the physiology of wasted ventilation. The European respiratory journal. 2015 Jun:45(6):1704-16. doi: 10.1183/09031936.00137614. Epub 2014 Nov 13 [PubMed PMID: 25395032]
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. The New England journal of medicine. 2002 Apr 25:346(17):1281-6 [PubMed PMID: 11973365]
Doorduin J, Nollet JL, Vugts MP, Roesthuis LH, Akankan F, van der Hoeven JG, van Hees HW, Heunks LM. Assessment of dead-space ventilation in patients with acute respiratory distress syndrome: a prospective observational study. Critical care (London, England). 2016 May 5:20(1):121. doi: 10.1186/s13054-016-1311-8. Epub 2016 May 5 [PubMed PMID: 27145818]
Level 2 (mid-level) evidenceVerscheure S, Massion PB, Verschuren F, Damas P, Magder S. Volumetric capnography: lessons from the past and current clinical applications. Critical care (London, England). 2016 Jun 23:20(1):184. doi: 10.1186/s13054-016-1377-3. Epub 2016 Jun 23 [PubMed PMID: 27334879]
Hedenstierna G, Sandhagen B. Assessing dead space. A meaningful variable? Minerva anestesiologica. 2006 Jun:72(6):521-8 [PubMed PMID: 16682925]
Farkas EA, Detterbeck FC. Airway complications after pulmonary resection. Thoracic surgery clinics. 2006 Aug:16(3):243-51 [PubMed PMID: 17004552]
Bourgoin P, Baudin F, Brossier D, Emeriaud G, Wysocki M, Jouvet P. Assessment of Bohr and Enghoff Dead Space Equations in Mechanically Ventilated Children. Respiratory care. 2017 Apr:62(4):468-474. doi: 10.4187/respcare.05108. Epub 2017 Feb 21 [PubMed PMID: 28223465]
Keck T, Leiacker R, Heinrich A, Kühnemann S, Rettinger G. Humidity and temperature profile in the nasal cavity. Rhinology. 2000 Dec:38(4):167-71 [PubMed PMID: 11190750]
Plotnikow GA, Accoce M, Navarro E, Tiribelli N. Humidification and heating of inhaled gas in patients with artificial airway. A narrative review. Revista Brasileira de terapia intensiva. 2018 Mar:30(1):86-97. doi: 10.5935/0103-507x.20180015. Epub [PubMed PMID: 29742220]
Level 3 (low-level) evidenceGehlbach JA, Miller AG, Hornik CP, Cheifetz IM. Dead Space to Tidal Volume Ratio Is Associated With Higher Postextubation Support in Children. Respiratory care. 2020 Nov:65(11):1721-1729. doi: 10.4187/respcare.07351. Epub 2020 Jun 30 [PubMed PMID: 32606073]
Smith LJ, Macleod KA, Collier GJ, Horn FC, Sheridan H, Aldag I, Taylor CJ, Cunningham S, Wild JM, Horsley A. Supine posture changes lung volumes and increases ventilation heterogeneity in cystic fibrosis. PloS one. 2017:12(11):e0188275. doi: 10.1371/journal.pone.0188275. Epub 2017 Nov 27 [PubMed PMID: 29176899]
Biselli P, Fricke K, Grote L, Braun AT, Kirkness J, Smith P, Schwartz A, Schneider H. Reductions in dead space ventilation with nasal high flow depend on physiological dead space volume: metabolic hood measurements during sleep in patients with COPD and controls. The European respiratory journal. 2018 May:51(5):. pii: 1702251. doi: 10.1183/13993003.02251-2017. Epub 2018 May 30 [PubMed PMID: 29724917]
Mündel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. Journal of applied physiology (Bethesda, Md. : 1985). 2013 Apr:114(8):1058-65. doi: 10.1152/japplphysiol.01308.2012. Epub 2013 Feb 14 [PubMed PMID: 23412897]
Level 1 (high-level) evidenceTang Y, Turner MJ, Baker AB. Effects of lung time constant, gas analyser delay and rise time on measurements of respiratory dead-space. Physiological measurement. 2005 Dec:26(6):1103-14 [PubMed PMID: 16311457]
Blanch L, Romero PV, Lucangelo U. Volumetric capnography in the mechanically ventilated patient. Minerva anestesiologica. 2006 Jun:72(6):577-85 [PubMed PMID: 16682932]
Miller DM, Adams AP, Light D. Dead space and paediatric anaesthetic equipment: a physical lung model study. Anaesthesia. 2004 Jun:59(6):600-6 [PubMed PMID: 15144302]
Chuang ML. Combining Dynamic Hyperinflation with Dead Space Volume during Maximal Exercise in Patients with Chronic Obstructive Pulmonary Disease. Journal of clinical medicine. 2020 Apr 15:9(4):. doi: 10.3390/jcm9041127. Epub 2020 Apr 15 [PubMed PMID: 32326507]
Romero PV, Rodriguez B, de Oliveira D, Blanch L, Manresa F. Volumetric capnography and chronic obstructive pulmonary disease staging. International journal of chronic obstructive pulmonary disease. 2007:2(3):381-91 [PubMed PMID: 18229577]
Raurich JM, Vilar M, Colomar A, Ibáñez J, Ayestarán I, Pérez-Bárcena J, Llompart-Pou JA. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respiratory care. 2010 Mar:55(3):282-7 [PubMed PMID: 20196876]
Kline JA, Israel EG, Michelson EA, O'Neil BJ, Plewa MC, Portelli DC. Diagnostic accuracy of a bedside D-dimer assay and alveolar dead-space measurement for rapid exclusion of pulmonary embolism: a multicenter study. JAMA. 2001 Feb 14:285(6):761-8 [PubMed PMID: 11176914]
Level 2 (mid-level) evidenceVerschuren F, Heinonen E, Clause D, Roeseler J, Thys F, Meert P, Marion E, El Gariani A, Col J, Reynaert M, Liistro G. Volumetric capnography as a bedside monitoring of thrombolysis in major pulmonary embolism. Intensive care medicine. 2004 Nov:30(11):2129-32 [PubMed PMID: 15378240]
Level 3 (low-level) evidenceHinkson CR, Benson MS, Stephens LM, Deem S. The effects of apparatus dead space on P(aCO2) in patients receiving lung-protective ventilation. Respiratory care. 2006 Oct:51(10):1140-4 [PubMed PMID: 17005059]
Level 1 (high-level) evidenceTusman G, Gogniat E, Madorno M, Otero P, Dianti J, Ceballos IF, Ceballos M, Verdier N, Böhm SH, Rodriguez PO, San Roman E. Effect of PEEP on Dead Space in an Experimental Model of ARDS. Respiratory care. 2020 Jan:65(1):11-20. doi: 10.4187/respcare.06843. Epub 2019 Oct 15 [PubMed PMID: 31615922]
González-Castro A, Suárez-Lopez V, Gómez-Marcos V, González-Fernandez C, Iglesias-Posadilla D, Burón-Mediavilla J, Rodríguez-Borregan JC, Miñambres E, Llorca J. [Utility of the dead space fraction (Vd/Vt) as a predictor of extubation success]. Medicina intensiva. 2011 Dec:35(9):529-38. doi: 10.1016/j.medin.2011.05.016. Epub 2011 Jul 22 [PubMed PMID: 21782289]
Hubble CL, Gentile MA, Tripp DS, Craig DM, Meliones JN, Cheifetz IM. Deadspace to tidal volume ratio predicts successful extubation in infants and children. Critical care medicine. 2000 Jun:28(6):2034-40 [PubMed PMID: 10890660]
Dassios T, Dixon P, Hickey A, Fouzas S, Greenough A. Physiological and anatomical dead space in mechanically ventilated newborn infants. Pediatric pulmonology. 2018 Jan:53(1):57-63. doi: 10.1002/ppul.23918. Epub 2017 Nov 20 [PubMed PMID: 29152912]
Praetel C, Banner MJ, Monk T, Gabrielli A. Isoflurane inhalation enhances increased physiologic deadspace volume associated with positive pressure ventilation and compromises arterial oxygenation. Anesthesia and analgesia. 2004 Oct:99(4):1107-1113. doi: 10.1213/01.ANE.0000131727.52766.F7. Epub [PubMed PMID: 15385359]
Level 1 (high-level) evidenceSpoletini G, Alotaibi M, Blasi F, Hill NS. Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Chest. 2015 Jul:148(1):253-261. doi: 10.1378/chest.14-2871. Epub [PubMed PMID: 25742321]
Möller W, Feng S, Domanski U, Franke KJ, Celik G, Bartenstein P, Becker S, Meyer G, Schmid O, Eickelberg O, Tatkov S, Nilius G. Nasal high flow reduces dead space. Journal of applied physiology (Bethesda, Md. : 1985). 2017 Jan 1:122(1):191-197. doi: 10.1152/japplphysiol.00584.2016. Epub 2016 Nov 17 [PubMed PMID: 27856714]