Introduction
Anagen effluvium is a form of nonscarring alopecia, with two distinct types of anagen effluvium—dystrophic anagen effluvium and loose anagen syndrome. Dystrophic anagen effluvium is most commonly associated with chemotherapy; however, it can also manifest in cases of protein-energy deficiency, pemphigus, alopecia areata, and different forms of heavy metal poisoning. In this disorder, affected anagen hairs suffer a toxic or inflammatory insult, resulting in a hair shaft fracture. Loose anagen hair syndrome is an inherited condition characterized by loosely anchored anagen hairs that can be easily and painlessly pulled from the scalp. This condition arises due to hereditary keratin defects in the inner root sheath, the opposed companion layer, or both.[1]
The more commonly observed form of anagen effluvium is often called chemotherapy-induced alopecia due to its susceptibility to the effects of antimetabolites, alkylating drugs, and mitotic inhibitors used in chemotherapy. Hair shedding often occurs within 14 days of chemotherapy administration. Trichoscopy can reveal tapered fractures of anagen hairs, frequently resulting in hair shaft injury.[2][3][4] In contrast to telogen effluvium, where the hairs are shed, the hairs in anagen effluvium are broken. Therefore, it might be argued that the term anagen effluvium is linguistically misleading, as effluvium refers to the act of shedding.[5]
Understandably, the hair loss associated with this disorder can be emotionally and psychologically distressing to the patient. The prognosis for a patient with anagen effluvium is guarded. No treatment has proven entirely effective in preventing or stopping hair loss in anagen effluvium. Patient education and aesthetic advice in managing hair loss are crucial. Expectations should be managed so that patients understand the unfortunate inevitability of the disorder; however, they should also be assured that most patients with anagen effluvium experience hair restoration following the completion of chemotherapy, although it often takes months or years before full recovery is attained. Unfortunately, hair thinning may persist in a few patients without complete recovery.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The pathogenesis of anagen effluvium can be attributed to multiple etiologies, including medicines, heavy metal or radiation exposures, chronic disease, autoimmune conditions, poor nutritional status, and secondary to specific surgeries.
The most common etiology involves several antimetabolites, alkylating agents, and mitotic inhibitors administered in chemotherapy treatment for cancer. The frequency of anagen effluvium is primarily attributed to antimicrotubule agents such as paclitaxel 80% of the time, topoisomerase inhibitors such as doxorubicin 60% to 100% of the time, alkylating agents such as cyclophosphamide more than 60%, and antimetabolites such as 5-fluorouracil at 10% to 20%.
Several drugs, such as isoniazid, doxorubicin, nitrosoureas, cyclophosphamide, levodopa, colchicine, and cyclosporine, have been implicated in triggering anagen effluvium. Although methotrexate is generally considered a safe medication for the long-term treatment of psoriasis, there have been instances of idiosyncratic reactions observed in clinical settings. Although albendazole typically has a mild side effect profile, occasional reports of anagen and telogen effluvium have been associated with this medicine.[6]
Heavy metal toxicity associated with thallium, mercury, boron, bismuth, copper, and cadmium has also been implicated. Radiation has also been shown to cause both reversible and permanent alopecia. Permanent destruction of the hair follicle occurs when hair follicle stem cells are damaged, typically induced by exposure to greater than 30 Gy of deep x-rays.[7][8][9] Endovascular embolization of cerebral aneurysms can result in nonscarring scalp baldness in the treated area due to radiation exposure.[10]
Anagen effluvium can also be observed in inflammatory disorders such as alopecia areata and syphilis, secondary to an inflammatory insult to the hair bulb. Pemphigus vulgaris, an autoimmune disorder targeting desmosomal proteins, can also cause anagen effluvium, as desmosomal proteins are expressed in the epithelium of the hair follicle.
There have been reports of instances of anagen effluvium following reduction surgery for scarring alopecic regions, nevi excision, and scalp graft.[11] Anagen effluvium has been observed in a 2-year-old boy with severe hypotension and hypoxia requiring extracorporeal membrane oxygenation. Anagen effluvium has also been documented following severe protein-energy malnutrition states, such as kwashiorkor. Any of these factors may cause an abrupt cessation of mitosis in the hair matrix.[7]
Several concurrent factors can influence the risk and severity of chemotherapy-induced alopecia. These factors include impaired drug metabolism, such as in patients with liver dysfunction who may experience unexpected and significant alopecia. In addition, prior exposure to scalp irradiation, advanced age, the presence of androgenetic alopecia, previous chemotherapy-induced alopecia, and the occurrence of graft-versus-host disease in patients who have undergone hematopoietic cell transplantation can also contribute to the development of alopecia.[12]
Epidemiology
Anagen effluvium has no gender or regional preference; it is equally prevalent among men and women worldwide. Furthermore, there is no observed association between hair type, ethnicity, and race and changes in the severity of alopecia or the rate and pattern of hair regrowth.
Pathophysiology
Understanding the anatomy of the hair follicle and the cyclical phases of hair growth is crucial for understanding anagen effluvium. There are roughly 100,000 hairs on the scalp, and each hair goes through 4 stages of growth—anagen, catagen, telogen, and kenogen.
Anagen is a growth phase lasting between 2 and 6 years, with an average of 3 years. Approximately 90% of the hairs taken from a normal scalp are anagen hairs. The anagen phase is a period of epithelial proliferation in which bulb matrix cells undergo mitosis and proliferation to form the hair shaft. Severe insult to the hair bulb or hair matrix in the form of medications, toxin exposure, or inflammation that causes a cessation of this mitotic activity can cause damage to the hair shaft, resulting in breakage and, if the bulb is affected, complete hair loss.
Catagen is a transitional phase between anagen and telogen. In this phase, all growth ceases. Less than 1% of scalp hairs are in catagen at any time.
Telogen is a resting phase lasting approximately 3 to 5 months, occurring immediately before the hair falls out, a process known as teloptosis. Telogen effluvium, a separate entity from anagen effluvium, occurs when anagen hairs are prematurely shifted into the telogen phase and can be triggered by various causes, including medications, physical or psychological stressors, hospitalization, and pregnancy.
Kenogen is the lag phase between telogen hair loss and new hair growth.[13][14][15]
The target of cytotoxic chemotherapy is cells undergoing accelerated division, including hair matrix cells in the body. Alopecia may ensue through either one or both of the following mechanisms:
- Significant suppression of keratinocyte growth in the hair follicle matrix can result in hair separation at the bulb and subsequent shedding. The impact on hair matrix keratinocytes depends on the level of toxicity. Agents or schedules with lower toxicity cause a dystrophic anagen effluvium, leading to less hair loss and delayed hair regrowth. On the other hand, agents with higher toxicity cause severe hair loss but have the potential for faster hair regrowth.[12]
- The hair shaft may undergo thinning during the peak of chemotherapy efficacy, leading to Pohl-Pinkus constrictions. These constrictions result from the abrupt and repeated arrest of the hair follicle's metabolic and mitotic activities. Consequently, the hair shaft may fracture at the follicular opening of these constrictions while the hair cycle is at rest.
The reversibility of alopecia is proportional to the extent of injury to the hair follicle stem cell. Chemotherapy-induced alopecia is generally reversible, but not always, due to its selective impact on proliferative cells located in the bulb while sparing the quiescent stem cells in the bulge that play a crucial role in reinitiating follicle growth.[12]
Several signaling molecules, including Wnt, sonic hedgehog, notch, and bone morphogenic proteins, have been linked to the hair follicle's initial formation and subsequent cycling.[16] Transient overexpression of sonic hedgehog in a mouse model of chemotherapy-induced alopecia expedited the regeneration of hair fibers.[17] The downregulation of sonic hedgehog transcription has been demonstrated to mediate chemotherapy-induced damage to rapidly proliferating epithelial tissue in feather follicles. Consequently, the sonic hedgehog holds potential as a prospective target for preventing alopecia.[18]
Histopathology
A punch biopsy of a normal scalp exhibits approximately up to 15% telogen phase hair follicles. In anagen effluvium, a punch biopsy of the scalp exhibits a normal anagen-to-telogen ratio, which is less than 15% telogen hair follicles.
History and Physical
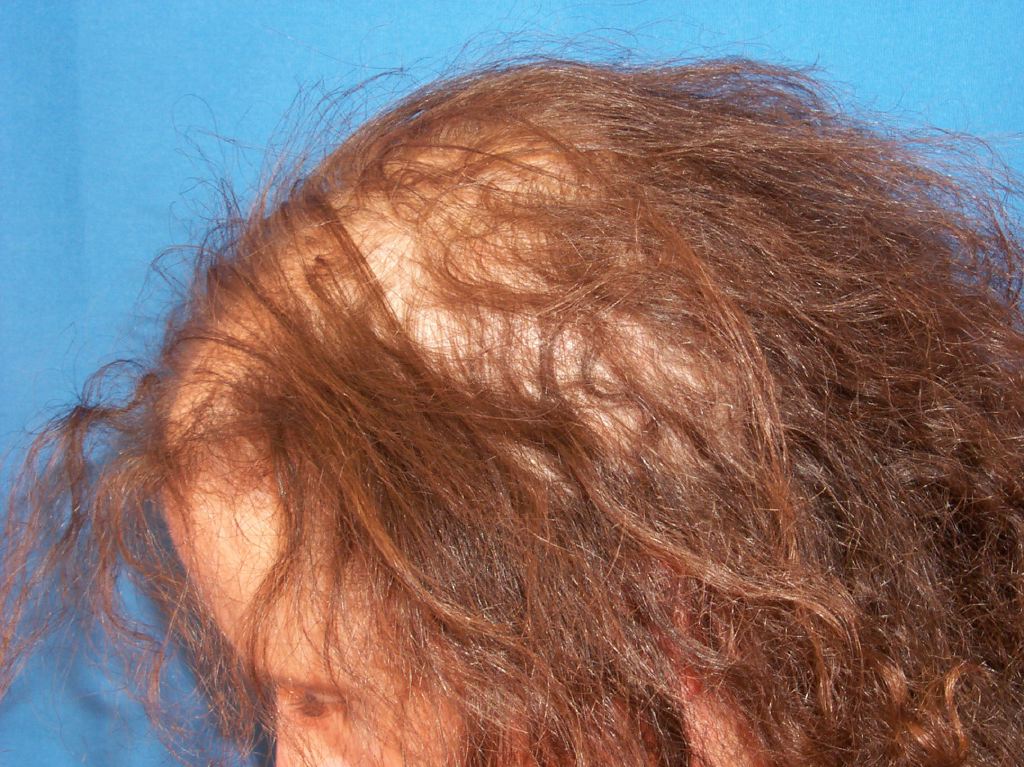
The key physical examination finding in anagen effluvium is a tapered hair shaft fracture. An overlap syndrome of telogen effluvium and anagen effluvium may be observed in patients undergoing chemotherapy, as the onset of chemotherapy is a significant stressor on the patient. Identifying anagen and telogen hairs with the naked eye is possible. Nevertheless, it can be helpful to observe hair microscopically. Anagen hairs demonstrate full pigment with roots covered with inner and outer root sheaths, as opposed to telogen hairs that possess club-shaped roots, no inner or outer root sheaths, and depigmentation of the proximal part of the shaft (see Image. Anagen Effluvium). The patient does not display signs of an active inflammatory scarring process, such as erythema, scaling, or pigmentation associated with cicatricial alopecia; anagen effluvium is non-cicatricial.
Chemotherapy-induced alopecia primarily affects the scalp, especially in areas with low total hair densities, such as the crown and the frontal regions, where hair regrows slowly.[19] Hair loss in chemotherapy is not always limited to the scalp. Madarosis, a condition characterized by the loss of eyebrows, eyelashes, and hair in the extremities, axillary region, and pubic area, can vary and occur even after the final chemotherapy dose. Hair in these specific regions typically exhibits a faster recovery rate compared to hair located on the scalp. The temporal onset of alopecia is contingent upon the specific systemic treatment agent, dosage, and regimen employed. Alopecia typically develops approximately 2 to 3 weeks after the initial administration of most chemotherapy regimens and is entirely eradicated by the end of the second cycle. Weekly chemotherapy administration typically results in a decelerated and occasionally partial occurrence of alopecia, although sustained treatment may lead to hair regrowth. The administration of high-dose chemotherapy in the context of hematopoietic cell transplantation results in the rapid and extensive occurrence of alopecia.[20]
Alopecia is not the sole effect chemotherapy, immune treatment, molecularly targeted therapies, and endocrine agents can have on hair. Methotrexate and other targeted biological therapies can have a short-term effect on follicular melanocytes, resulting in the darkening of eyebrows, eyelashes, and hair on the scalp. This hyperpigmentation is typically observed as bands that alternate with the typical color, commonly referred to as the flag sign. This phenomenon arises due to the cyclic nature of treatment and nontreatment periods. Partial alopecia, curling of the hair, and depigmentation may be observed as a consequence of the administration of small-molecule inhibitors and monoclonal antibodies that specifically target epidermal growth factor receptor (EGFR), BRAF, Bruton tyrosine kinase (BTK), Bcr/Abl, cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), KIT, and platelet-derived growth factor receptor (PDGFR) or vascular endothelial growth factor receptor (VEGFR).[21] The administration of targeted biological medicines, antibody-drug conjugates, and routine endocrine therapy, specifically tamoxifen and aromatase inhibitors, have the potential to induce partial or mild alopecia.[22] In immunotherapy, immune checkpoint inhibitors can lead to sporadic loss of pigmentation in the skin, a condition known as vitiligo, and hair, a condition known as poliosis. Furthermore, they may lead to hair loss, known as alopecia.[23]
Evaluation
The diagnosis of anagen effluvium can typically be established based on the findings from history and physical examinations alone, often eliminating the need for a biopsy. However, if a biopsy is requested or necessary for diagnosis, it can help exclude telogen effluvium.
In anagen effluvium, histopathological evaluation of a punch biopsy of the scalp reveals a normal anagen-to-telogen ratio, which is less than 15% of hair follicles in the telogen phase; more than 15% of the hair follicles in the telogen phase strongly supports a diagnosis of telogen effluvium. A trichogram, which measures the ratio of anagen to telogen hair by forcibly extracting hairs within a unit area, reveals a significant proportion of dystrophic anagen hairs. Anagen effluvium is characterized by a tapered, narrowed, irregular, or fractured hair apex. Long, pigmented roots encased in inner and outer root sheaths characterize anagen hairs. In contrast, telogen hair resembles a club or spherical shape. Follicular openings persist under both circumstances. Additional diagnostic tests may be scheduled to exclude alternative etiologies of alopecia, such as iron deficiency, thyroid disorders, systemic lupus, and infectious conditions, including syphilis.
Trichoscopy involves examining the scalp and hair using a handheld or video dermoscopy device. The phenomenon of proximal hair shaft tapering, often referred to as exclamation hair, and the occurrence of acute constrictions in response to consecutive doses of chemotherapy have been documented. Tapering hair in chemotherapy-induced anagen effluvium is caused by cytotoxic medications, which promote apoptosis, a gradual reduction in cellular production from the hair matrix, and premature follicle entrance into telogen. The presence of black spots in the hair shaft suggests a sudden halt in mitotic activity, leading to localized hair shaft thinning and subsequent breakage at the thinning site. Pohl-Pinkus constriction and coudability hair refer to hair thinning at the proximal end. Pigtail hairs are multiple regrowing hairs. In the advanced stages of the disease, the hair pull test exclusively reveals the spared telogen hair.[24]
The Dean Scale, devised by Dean, categorizes hair loss into five grades—Grade 0: no hair loss; Grade 1: >0% to ≤25% hair loss; Grade 2: >25% to ≤50% hair loss; Grade 3: >50% to ≤75% hair loss; and Grade 4: >75% hair loss. This scale is a comprehensive grading scale utilized for evaluating the efficacy of alopecia prevention treatments.[25] The hair mass index can be used to scientifically quantify the amount of hair for research purposes.[26] Furthermore, it is possible to assess the psychosocial consequences of alopecia on individuals by employing the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) scale.[27]
Treatment / Management
The primary objective in managing anagen effluvium is to minimize the duration of alopecia experienced by the patient. Understandably, the hair loss associated with this disorder can be emotionally and psychologically distressing to the patient.
Researchers have studied several agents, but unfortunately, no treatment appears to be effective in preventing or stopping hair loss.[5] The lack of a pharmacological agent effective in treating and preventing anagen effluvium necessitates the importance of patient education and aesthetic advice in managing hair loss. Patients should be encouraged to obtain hairpieces or protective scarves before hair loss and educated on such garments' benefits, including cold protection and the aesthetic component.
Therapeutic approaches include reducing scalp blood flow to physically diminish the quantity of drug delivered to the dividing hair bulb and pharmacological or biological interventions to obstruct the chemotherapy's effects on the hair follicle. Several studies have described using a scalp tourniquet during chemotherapy. However, it is essential to note that if scalp or brain metastases are possible, this method should not be used so that the chemotherapeutic agent can penetrate.
Another successful method involves inducing scalp hypothermia to a scalp temperature below 24 °C during chemotherapy with daunorubicin, doxorubicin, paclitaxel, vincristine, vinblastine, mechlorethamine, actinomycin D, and epirubicin. Scalp hypothermia has demonstrated successful application in patients with various solid tumors who are undergoing bolus or short-term infusional chemotherapy regimens that are linked to a moderate-to-high likelihood of experiencing complete alopecia; this includes cases involving ovarian and prostate cancers. The empirical support for the efficacy of scalp hypothermia is particularly strong in individuals undergoing treatment for breast cancer. The option of scalp hypothermia may be available to individuals with advanced cancer who experience alopecia as an adverse effect of palliative chemotherapy in the advanced stage.
The process of scalp hypothermia involves the local vasoconstriction of blood vessels, leading to a decrease in the transport of chemotherapy to the scalp. As a result, the metabolic rate of follicle cells decreases, and there is a decrease in the uptake of cellular drugs.[28] Typically, scalp hypothermia leads to modest adverse effects such as a patient's cold sensation, headache, nausea, dry skin, and claustrophobia. According to reports, manual caps have been associated with scalp thermal injury. However, using an inner protective cap or a band at the hair's edge could possibly mitigate this issue.[29] The DigniCap and Paxman scalp hypothermia systems, both automated scalp cooling cap devices, have recently received clearance from the United States Food and Drug Administration (FDA). The FDA granted this approval based on the results of two prospective clinical trials conducted on patients undergoing (neo)adjuvant chemotherapy for breast cancer. The FDA has expanded its approval to include patients with all types of solid tumors. In the United States, there is a lack of prospective controlled trials examining the effectiveness and safety of manual caps despite their availability.
Scalp hypothermia should not be utilized by pediatric patients, adult patients with solid tumors undergoing continuous infusion chemotherapy for 24 hours or brain radiotherapy, patients diagnosed with leukemia or certain types of lymphoma, and patients undergoing bone marrow or stem cell transplantation with myeloablative doses of chemotherapy and radiation therapy. Patients with cold agglutinin illness, cryoglobulinemia, or post-traumatic cold dystrophy should avoid scalp hypothermia.
Preclinical investigations indicate that utilizing small molecules and biological medicines can mitigate or prevent alopecia by safeguarding the hair bulb against the harmful consequences of chemotherapy. Human-specific therapies that have undergone testing include:
- Minoxidil: A trial of minoxidil for patients who are experiencing endocrine therapy-associated hair loss or chemotherapy-induced alopecia with a delayed recovery is recommended. Minoxidil should be reserved for treating chemotherapy-induced alopecia rather than used for prevention. Oral minoxidil is recommended over topical minoxidil for the majority of patients with chemotherapy-induced alopecia or significant alopecia related to endocrine therapy, with or without targeted agents. For patients with less significant hair loss due to endocrine therapy, topical minoxidil is an acceptable option. Despite its limited effectiveness in stopping or preventing hair loss, topical minoxidil has been shown to reduce baldness on average by 50 days.[30][31][32] (A1)
- Topical bimatoprost: Conducting a trial of topical bimatoprost is a justifiable approach for treating eyelash or eyebrow hypotrichosis caused by chemotherapy. However, evidence currently supports its preventive benefits, and its safety during chemotherapy remains unassessed. A randomized controlled trial involving 130 patients with idiopathic or chemotherapy-induced alopecia revealed a positive outcome for patients with chemotherapy-induced eyelid hypotrichosis.[33] (A1)
- Finasteride: The data are insufficient to support the use of finasteride for treating or preventing alopecia in patients undergoing chemotherapy or in women with breast cancer and endocrine therapy-associated alopecia. Nevertheless, research has demonstrated that finasteride has the potential to elevate serum estrogen levels in 34% of individuals, and it has been linked to the development of gynecomastia in males.[34] The potential risks associated with finasteride appear to outweigh any potential advantages.
- Spironolactone: Despite the scarcity of available data, spironolactone is a relatively nontoxic treatment option that could be considered for managing persistent alopecia following cancer treatment. The available data on the efficacy and safety of spironolactone, both as a standalone treatment and in conjunction with topical minoxidil, in women with alopecia following chemotherapy or endocrine therapy are currently limited[35]. The potential for elevated estrogen levels and perhaps elevated rates of breast cancer associated with spironolactone has been a subject of concern. However, individuals without a prior history of the illness have not consistently demonstrated this association.[36] (A1)
- Topical calcitriol: The available data are inadequate to substantiate the efficacy of topical calcitriol as a preventive measure against chemotherapy-induced alopecia. According to a study, the administration of calcitriol prior to chemotherapy did not modify the cytotoxic properties of the treatment. However, it did effectively mitigate the occurrence of substantial alopecia.[37] However, a phase I trial involving 12 patients did not demonstrate the efficacy of anthracycline- and cyclophosphamide-containing chemotherapy in preventing chemotherapy-induced alopecia.[38] Moreover, there have been concerns raised over the potential safeguarding of cancer cells against the detrimental effects of chemotherapy.[39]
Differential Diagnosis
The differential diagnosis of anagen effluvium includes other nonscarring alopecias, such as telogen effluvium, trichotillomania, and androgenetic alopecia. These conditions can be differentiated through a detailed examination of the patient's history, the hair pull test, and trichoscopy. Hair loss may be a prominent sign of an underlying disorder. A thorough review of systems should be completed to exclude other causes of hair loss, such as nutritional deficiencies, metabolic or endocrine disorders, and infections.
Prognosis
Chemotherapy-induced alopecia is typically reversible due to its selective targeting of proliferating cells in the bulb, sparing the quiescent stem cells in the bulge responsible for restarting follicle growth. Once treatment is discontinued, the hair follicle promptly returns to its regular cycle and exhibits visible regrowth within 3 to 6 months. Approximately 65% of patients encounter graying, curling, or straightening effects in their newly regrown hair, which is plausibly attributed to chemotherapy's differential effects on hair follicle melanocytes and inner root sheath epithelia. Importantly, these adverse effects often decrease over time.[28]
The occurrence of permanent alopecia following standard-dose chemotherapy for breast cancer is infrequent. However, there is now compelling evidence indicating the presence of permanent or prolonged alopecia following standard-dose chemotherapy, particularly with docetaxel. The dosage per infusion and the duration of exposure closely correlate with this phenomenon.[41] Furthermore, a study linked a genetic predisposition, specifically in the ABCB1 gene, to the development of permanent docetaxel-related alopecia in individuals with breast cancer.[42] A single case series has indicated instances of delayed recovery associated with paclitaxel, although such occurrences are infrequent given the current dose regimens.[35]
Complications
Alopecia is commonly recognized as a substantial hindrance to the well-being of cancer patients undergoing chemotherapy. The sudden loss of hair due to chemotherapy can severely impact mental health and quality of life, particularly in young women.[43] Some patients may undergo considerable emotional distress, leading them to choose suboptimal treatment or refuse or delay treatment that could otherwise be beneficial.
In most cases, hair regrowth follows a typical pattern, but in certain instances, individuals with straight hair may develop curly hair upon regrowth. The color of the hair may also undergo alterations. Although chemotherapy-induced anagen effluvium typically leads to complete hair regrowth and is reversible, specific chemotherapy treatments can cause persistent alopecia, depending on the administered dosage. This alopecia has histological characteristics comparable to nonscarring alopecia, similar to androgenetic alopecia.[44]
Deterrence and Patient Education
Before initiating chemotherapy that may result in alopecia, each patient must be informed about this potential adverse effect and be provided with information regarding alternative chemotherapy approaches. Implementing this proactive strategy is crucial to mitigate the psychological anguish linked to hair loss. Patients with breast cancer undergoing docetaxel infusions at doses of 75 mg/m² or higher should be provided with appropriate information regarding the potential for permanent or protracted alopecia. Cooling the scalp may prevent permanent alopecia, although data on this topic are limited.
Pearls and Other Issues
The application of a pressure cuff around the scalp and local hypothermia can retard anagen arrest if these measures are implemented during the infusion of the causative medication. The discontinuation or avoidance of the causative drug reverses the anagen effluvium. The objective of pharmacotherapy is to shorten the period of alopecia resulting from chemotherapy. Unfortunately, no treatment appears to be generally effective in preventing this secondary effect of chemotherapy.
Enhancing Healthcare Team Outcomes
Anagen effluvium is a form of nonscarring alopecia commonly associated with chemotherapy. In this disorder, affected anagen hairs suffer a toxic or inflammatory insult, resulting in a hair shaft fracture. The hair shaft is commonly damaged, and tapered fractures of anagen hairs can be appreciated on trichoscopy.
Hair shedding typically occurs within 14 days of administration of the offending drug. The prognosis for individuals with anagen effluvium is often guarded, highlighting the uncertainty and potential challenges associated with this condition. The condition is reversible in most instances, with hair regrowth occurring upon discontinuing the offending chemotherapeutic agent.
Despite this potential for recovery, achieving full hair restoration can be a lengthy process, often taking months or even years. During this period, individuals may experience varying degrees of emotional distress and adjustment challenges due to changes in their appearance. Healthcare professionals should offer comprehensive support and guidance throughout this journey.
Media
(Click Image to Enlarge)
References
Price VH, Gummer CL. Loose anagen syndrome. Journal of the American Academy of Dermatology. 1989 Feb:20(2 Pt 1):249-56 [PubMed PMID: 2915060]
Malakar SS, Mehta PR, Malakar SS. Tulipoid Hair: Anagen Effluvium Marker! International journal of trichology. 2018 Jul-Aug:10(4):188-190. doi: 10.4103/ijt.ijt_98_17. Epub [PubMed PMID: 30386082]
Concha JSS, Werth VP. Alopecias in lupus erythematosus. Lupus science & medicine. 2018:5(1):e000291. doi: 10.1136/lupus-2018-000291. Epub 2018 Oct 25 [PubMed PMID: 30397497]
Sant'Anna Addor FA, Donato LC, Melo CSA. Comparative evaluation between two nutritional supplements in the improvement of telogen effluvium. Clinical, cosmetic and investigational dermatology. 2018:11():431-436. doi: 10.2147/CCID.S173082. Epub 2018 Sep 10 [PubMed PMID: 30237729]
Level 2 (mid-level) evidenceKanwar AJ, Narang T. Anagen effluvium. Indian journal of dermatology, venereology and leprology. 2013 Sep-Oct:79(5):604-12. doi: 10.4103/0378-6323.116728. Epub [PubMed PMID: 23974578]
Ghias MH, Amin BD, Kutner AJ. Albendazole-induced anagen effluvium. JAAD case reports. 2020 Jan:6(1):54-56. doi: 10.1016/j.jdcr.2019.08.010. Epub 2019 Dec 26 [PubMed PMID: 31909140]
Level 3 (low-level) evidenceCotter L, Cheng K, Kirkorian AY. Anagen Effluvium in Association With Extracorporeal Membrane Oxygenation. Pediatric dermatology. 2017 Jul:34(4):e201-e202. doi: 10.1111/pde.13183. Epub 2017 Jun 13 [PubMed PMID: 28612487]
Fonia A, Cota C, Setterfield JF, Goldberg LJ, Fenton DA, Stefanato CM. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: Clinicopathologic findings in a cohort of 10 patients. Journal of the American Academy of Dermatology. 2017 May:76(5):948-957. doi: 10.1016/j.jaad.2016.12.027. Epub 2017 Mar 8 [PubMed PMID: 28284826]
Sonthalia S, Daulatabad D. Azathioprine-associated anagen effluvium. Indian journal of dermatology, venereology and leprology. 2016 May-Jun:82(3):322-4. doi: 10.4103/0378-6323.174411. Epub [PubMed PMID: 27088942]
Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, Figueroa-Sanchez JA, Tosti A, Garza-Rodríguez V. Anagen Effluvium after Neurointerventional Radiation: Trichoscopy as a Diagnostic Ally. Skin appendage disorders. 2022 Mar:8(2):102-107. doi: 10.1159/000518743. Epub 2021 Sep 14 [PubMed PMID: 35419426]
Contin LA, Santos LDN, Pereira IJN, Rocha VB. Anagen Effluvium after Therapeutic Scalp Surgery: Unreported Phenomenon. Skin appendage disorders. 2021 Jun:7(4):311-314. doi: 10.1159/000513087. Epub 2021 Mar 23 [PubMed PMID: 34307480]
Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. The Lancet. Oncology. 2013 Feb:14(2):e50-9. doi: 10.1016/S1470-2045(12)70553-3. Epub [PubMed PMID: 23369683]
Seol JE, Kim DH, Park SH, Cho GJ, Kim H. Three Cases of Radiation-induced Temporary Alopecia with Hair Microscopic Examination: "Coudability Hair" Might Not be Specific for Alopecia Areata. International journal of trichology. 2018 Jan-Feb:10(1):40-43. doi: 10.4103/ijt.ijt_74_17. Epub [PubMed PMID: 29440860]
Level 3 (low-level) evidenceFreites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, Paus R, Lacouture ME. Hair disorders in patients with cancer. Journal of the American Academy of Dermatology. 2019 May:80(5):1179-1196. doi: 10.1016/j.jaad.2018.03.055. Epub 2018 Apr 14 [PubMed PMID: 29660422]
Biswal SG, Mehta RD. Cutaneous Adverse Reactions of Chemotherapy in Cancer Patients: A Clinicoepidemiological Study. Indian journal of dermatology. 2018 Jan-Feb:63(1):41-46. doi: 10.4103/ijd.IJD_65_17. Epub [PubMed PMID: 29527024]
Level 2 (mid-level) evidenceCotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends in molecular medicine. 2001 Jul:7(7):293-301 [PubMed PMID: 11425637]
Level 3 (low-level) evidenceSato N, Leopold PL, Crystal RG. Effect of adenovirus-mediated expression of Sonic hedgehog gene on hair regrowth in mice with chemotherapy-induced alopecia. Journal of the National Cancer Institute. 2001 Dec 19:93(24):1858-64 [PubMed PMID: 11752010]
Xie G, Wang H, Yan Z, Cai L, Zhou G, He W, Paus R, Yue Z. Testing chemotherapeutic agents in the feather follicle identifies a selective blockade of cell proliferation and a key role for sonic hedgehog signaling in chemotherapy-induced tissue damage. The Journal of investigative dermatology. 2015 Mar:135(3):690-700. doi: 10.1038/jid.2014.409. Epub 2014 Sep 18 [PubMed PMID: 25233072]
Chon SY, Champion RW, Geddes ER, Rashid RM. Chemotherapy-induced alopecia. Journal of the American Academy of Dermatology. 2012 Jul:67(1):e37-47. doi: 10.1016/j.jaad.2011.02.026. Epub 2011 Dec 16 [PubMed PMID: 22178150]
Kanti V, Nuwayhid R, Lindner J, Hillmann K, Stroux A, Bangemann N, Kleine-Tebbe A, Blume-Peytavi U, Garcia Bartels N. Analysis of quantitative changes in hair growth during treatment with chemotherapy or tamoxifen in patients with breast cancer: a cohort study. The British journal of dermatology. 2014 Mar:170(3):643-50. doi: 10.1111/bjd.12716. Epub [PubMed PMID: 24641211]
Belum VR, Marulanda K, Ensslin C, Gorcey L, Parikh T, Wu S, Busam KJ, Gerber PA, Lacouture ME. Alopecia in patients treated with molecularly targeted anticancer therapies. Annals of oncology : official journal of the European Society for Medical Oncology. 2015 Dec:26(12):2496-502. doi: 10.1093/annonc/mdv390. Epub 2015 Sep 19 [PubMed PMID: 26387145]
Gallicchio L, Calhoun C, Helzlsouer KJ. Aromatase inhibitor therapy and hair loss among breast cancer survivors. Breast cancer research and treatment. 2013 Nov:142(2):435-43. doi: 10.1007/s10549-013-2744-2. Epub 2013 Nov 7 [PubMed PMID: 24197658]
Zarbo A, Belum VR, Sibaud V, Oudard S, Postow MA, Hsieh JJ, Motzer RJ, Busam KJ, Lacouture ME. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. The British journal of dermatology. 2017 Jun:176(6):1649-1652. doi: 10.1111/bjd.15237. Epub 2017 Apr 24 [PubMed PMID: 27943234]
Gupta S, Khandpur S, Bhari N. Anagen Effluvium: A Trichoscopic Analysis. Indian dermatology online journal. 2021 Sep-Oct:12(5):786-787. doi: 10.4103/idoj.IDOJ_18_20. Epub 2020 Sep 19 [PubMed PMID: 34667780]
Dean JC, Salmon SE, Griffith KS. Prevention of doxorubicin-induced hair loss with scalp hypothermia. The New England journal of medicine. 1979 Dec 27:301(26):1427-9 [PubMed PMID: 514322]
Komen MMC, van den Hurk CJG, Nortier JWR, van der Ploeg T, Smorenburg CH, van der Hoeven JJM. Patient-reported outcome assessment and objective evaluation of chemotherapy-induced alopecia. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2018 Apr:33():49-55. doi: 10.1016/j.ejon.2018.01.001. Epub 2018 Feb 3 [PubMed PMID: 29551177]
Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting. 2016:35():67-73 [PubMed PMID: 27249687]
Dorr VJ. A practitioner's guide to cancer-related alopecia. Seminars in oncology. 1998 Oct:25(5):562-70 [PubMed PMID: 9783595]
Rugo HS, Klein P, Melin SA, Hurvitz SA, Melisko ME, Moore A, Park G, Mitchel J, Bågeman E, D'Agostino RB Jr, Ver Hoeve ES, Esserman L, Cigler T. Association Between Use of a Scalp Cooling Device and Alopecia After Chemotherapy for Breast Cancer. JAMA. 2017 Feb 14:317(6):606-614. doi: 10.1001/jama.2016.21038. Epub [PubMed PMID: 28196257]
Rodriguez R, Machiavelli M, Leone B, Romero A, Cuevas MA, Langhi M, Romero Acuña L, Romero Acuña J, Amato S, Barbieri M. Minoxidil (Mx) as a prophylaxis of doxorubicin--induced alopecia. Annals of oncology : official journal of the European Society for Medical Oncology. 1994 Oct:5(8):769-70 [PubMed PMID: 7826913]
Duvic M, Lemak NA, Valero V, Hymes SR, Farmer KL, Hortobagyi GN, Trancik RJ, Bandstra BA, Compton LD. A randomized trial of minoxidil in chemotherapy-induced alopecia. Journal of the American Academy of Dermatology. 1996 Jul:35(1):74-8 [PubMed PMID: 8682968]
Level 1 (high-level) evidenceKang J, Lee JW, Kwon O. Efficacy of low-dose oral minoxidil in the management of anticancer therapy-induced alopecia in patients with breast cancer: A retrospective cohort study. Journal of the American Academy of Dermatology. 2023 May:88(5):1170-1173. doi: 10.1016/j.jaad.2022.12.005. Epub 2022 Dec 13 [PubMed PMID: 36526083]
Level 2 (mid-level) evidenceGlaser DA, Hossain P, Perkins W, Griffiths T, Ahluwalia G, Weng E, Beddingfield FC. Long-term safety and efficacy of bimatoprost solution 0·03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: a randomized controlled trial. The British journal of dermatology. 2015:172(5):1384-94. doi: 10.1111/bjd.13443. Epub 2015 Mar 7 [PubMed PMID: 25296533]
Level 1 (high-level) evidenceRozner RN, Freites-Martinez A, Shapiro J, Geer EB, Goldfarb S, Lacouture ME. Safety of 5α-reductase inhibitors and spironolactone in breast cancer patients receiving endocrine therapies. Breast cancer research and treatment. 2019 Feb:174(1):15-26. doi: 10.1007/s10549-018-4996-3. Epub 2018 Nov 22 [PubMed PMID: 30467659]
Freites-Martinez A, Chan D, Sibaud V, Shapiro J, Fabbrocini G, Tosti A, Cho J, Goldfarb S, Modi S, Gajria D, Norton L, Paus R, Cigler T, Lacouture ME. Assessment of Quality of Life and Treatment Outcomes of Patients With Persistent Postchemotherapy Alopecia. JAMA dermatology. 2019 Jun 1:155(6):724-728. doi: 10.1001/jamadermatol.2018.5071. Epub [PubMed PMID: 30840033]
Level 2 (mid-level) evidenceBommareddy K, Hamade H, Lopez-Olivo MA, Wehner M, Tosh T, Barbieri JS. Association of Spironolactone Use With Risk of Cancer: A Systematic Review and Meta-analysis. JAMA dermatology. 2022 Mar 1:158(3):275-282. doi: 10.1001/jamadermatol.2021.5866. Epub [PubMed PMID: 35138351]
Level 1 (high-level) evidenceJimenez JJ, Alvarez E, Bustamante CD, Yunis AA. Pretreatment with 1,25(OH)2D3 protects from Cytoxan-induced alopecia without protecting the leukemic cells from Cytoxan. The American journal of the medical sciences. 1995 Aug:310(2):43-7 [PubMed PMID: 7631641]
Hidalgo M, Rinaldi D, Medina G, Griffin T, Turner J, Von Hoff DD. A phase I trial of topical topitriol (calcitriol, 1,25-dihydroxyvitamin D3) to prevent chemotherapy-induced alopecia. Anti-cancer drugs. 1999 Apr:10(4):393-5 [PubMed PMID: 10378674]
Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. The New England journal of medicine. 1989 Apr 13:320(15):980-91 [PubMed PMID: 2648151]
Lodewijckx J, Robijns J, Claes M, Pierson M, Lenaerts M, Mebis J. The use of photobiomodulation therapy for the management of chemotherapy-induced alopecia: a randomized, controlled trial (HAIRLASER trial). Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2023 Apr 15:31(5):269. doi: 10.1007/s00520-023-07743-1. Epub 2023 Apr 15 [PubMed PMID: 37060420]
Level 1 (high-level) evidenceFreites-Martinez A, Shapiro J, van den Hurk C, Goldfarb S, Jimenez JJ, Rossi AM, Paus R, Lacouture ME. Hair disorders in cancer survivors. Journal of the American Academy of Dermatology. 2019 May:80(5):1199-1213. doi: 10.1016/j.jaad.2018.03.056. Epub 2018 Apr 14 [PubMed PMID: 29660423]
Núñez-Torres R, Martín M, García-Sáenz JÁ, Rodrigo-Faus M, Del Monte-Millán M, Tejera-Pérez H, Pita G, de la Torre-Montero JC, Pinilla K, Herraez B, Peiró-Chova L, Bermejo B, Lluch A, González-Neira A. Association Between ABCB1 Genetic Variants and Persistent Chemotherapy-Induced Alopecia in Women With Breast Cancer. JAMA dermatology. 2020 Sep 1:156(9):987-991. doi: 10.1001/jamadermatol.2020.1867. Epub [PubMed PMID: 32756886]
Sinha P, Bhatt S, Radhakrishnan S, Neema S, Sinha A. Anagen Effluvium after Methotrexate: An Idiosyncratic Reaction. International journal of trichology. 2020 Mar-Apr:12(2):93-96. doi: 10.4103/ijt.ijt_49_20. Epub 2020 May 5 [PubMed PMID: 32684684]
Miteva M, Misciali C, Fanti PA, Vincenzi C, Romanelli P, Tosti A. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. The American Journal of dermatopathology. 2011 Jun:33(4):345-50. doi: 10.1097/DAD.0b013e3181fcfc25. Epub [PubMed PMID: 21430504]
Level 3 (low-level) evidence