Introduction
Improving diagnostic techniques has lead to the increase of incidental and non-incidental adrenal tumor detection. When incidentally discovered, adrenal tumors necessitate thorough work-up to determine if they are hormonally functional, malignant, or metastatic. Patients with hormonally functional adrenal tumors should have targeted preoperative optimization, in addition to routine age-appropriate optimization.[1][2]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
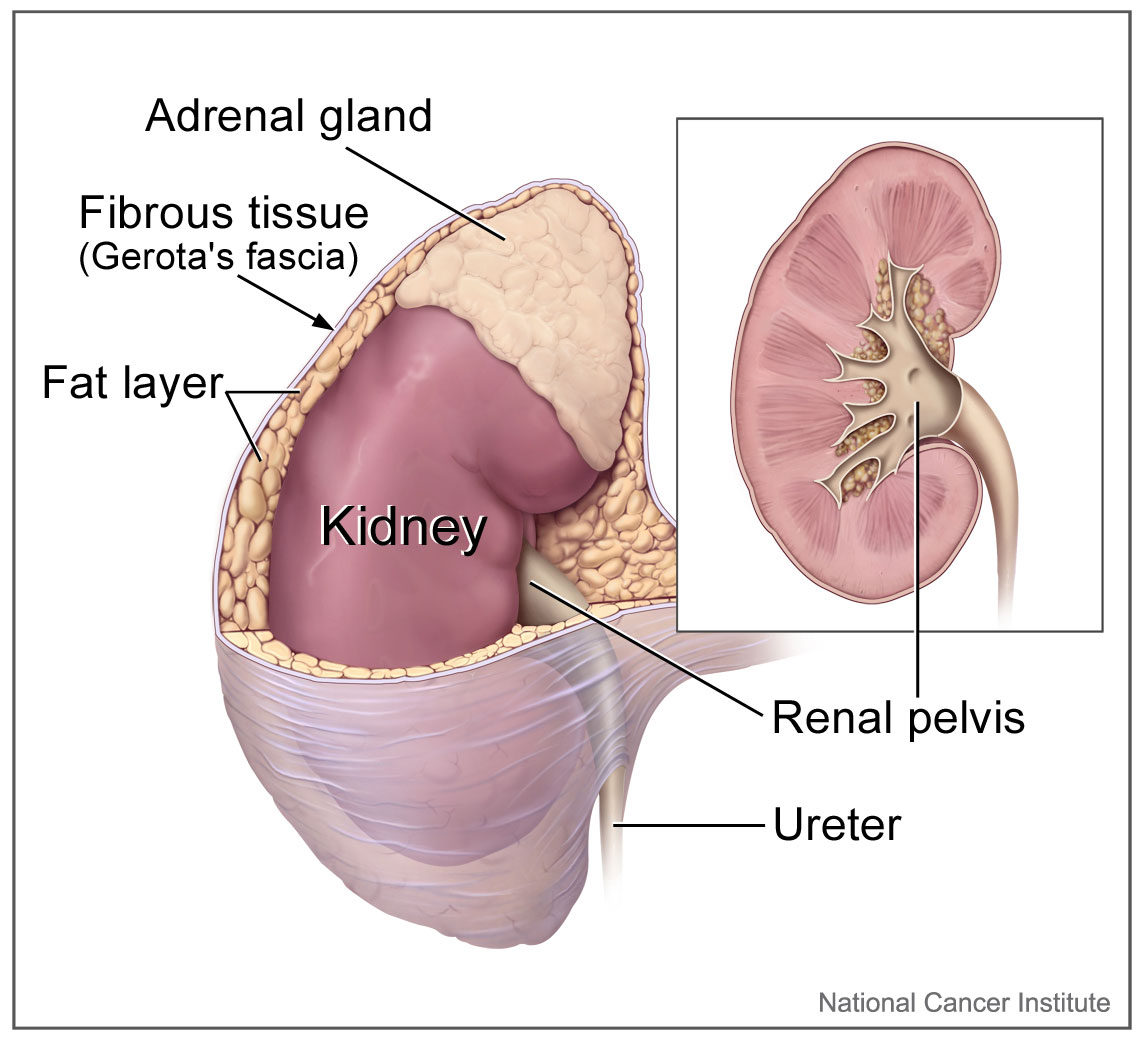
The right and left adrenal glands are located within Gerota fascia adjacent to the kidneys. The arterial supply of the adrenal glands consists of small arterial branches arising from the inferior phrenic artery, the aorta, and the renal arteries. Venous drainage of the left adrenal gland is to the left renal vein through the left adrenal vein, while the right adrenal vein drains directly into the inferior vena cava.
The adrenal gland is comprised of two distinct layers, the outer adrenal cortex, and the inner adrenal medulla, which have distinct hormonal production.
The adrenal medulla is made up of ectodermal cells of neural crest origin and is the site of catecholamine production, which includes dopamine, norepinephrine, and epinephrine.
The adrenal cortex is divided into three zones, from superficial to deep being the zona glomerulosa, zona fasiculata, and the zona reticularis. The zona glomerulosa is the site of mineralocorticoid (aldosterone) production. The zona fasciculata is the site of glucocorticoid (cortisol) production. The zona reticularis is the site of androgen production (DHEA-S, DHEA, and androstenedione).
Indications
Clinical Indications for Adrenalectomy include[3][2]:
- Functional adrenal mass identified on preoperative work-up:
- Primary aldosteronism
- Pheochromocytoma
- Adrenal Cushing syndrome
- Cushing disease refractory to pituitary targeted therapy
- Ectopic Cushing syndrome with an unknown or inoperable primary site
- Adrenal mass >4cm in size on preoperative imaging
- Adrenocortical carcinoma or metastatic tumor
Contraindications
Severe coagulopathy and poor cardiopulmonary performance status are absolute contraindications adrenalectomy by any technique. Minimally-invasive contraindications included: large tumor size (>6cm) and adrenocortical carcinoma.
Equipment
Laparoscopic equipment includes the following:
- Arterial line and central line for right-sided adrenalectomy, pheochromocytoma, or those with increased cardiovascular risk.
- Cloward prone positioner or gel rolls for retroperitoneal approach. Surgical bean bag positioner for a transabdominal approach.
Personnel
Collaboration between an experienced adrenal surgeon and an anesthesiologist is important in adrenal surgery, given the potential for abrupt changes in hemodynamics throughout the case.[4]
Preparation
Preoperative preparation for the patients undergoing adrenalectomy is of the utmost importance. Workup should include biochemical workup to determine if a nodule or mass secreting aldosterone, cortisol, or catecholamines is present.
In cases of increased catecholamine secretion (pheochromocytoma), patients should be initialed on alpha-blockade until orthostasis is achieved, with the addition of beta-blockade as needed. Preoperative hydration and electrolyte repletion should also be done through a high-sodium diet and increase fluid intake the week prior to surgery. Patients with hyperaldosteronism on biochemical workup should undergo adrenal venous sampling to confirm lateralization of the affected adrenal gland as preoperative imaging can be misleading in many cases.[5][6][7]
Technique or Treatment
Laparoscopic Transabdominal Adrenalectomy[8]
Positioning:
Lateral decubitus with affected gland upward, flex bed at the lower chest. The surgeon and assistant stand facing the front of the patient. No need for foley or preoperative antibiotics.
Anesthesia:
- General anesthesia with endotracheal intubation.
- Central venous catheter and arterial line for right-sided adrenalectomy, pheochromocytoma, or cardiovascular risk.
Operative steps:
- The abdomen is entered at Palmar's point using a Veress needle to technique.
- CO2 insufflation increased to the pressure of 15mmHg and a 5mm port placed using an optical trocar.
- A 30° endoscope placed, and three additional ports are placed, two 5mm trocars, and one 12mm trocar.
- Dissection is dependent on the side of the adrenal gland.
- LEFT:
- Mobilize the splenic flexure of the colon.
- Mobilize the spleen, taking down the splenocolic and splenorenal ligaments.
- Dissect the avascular plane between the anterior surface of Gerota's fascia and the posterior surface of the spleen and pancreas.
- The left adrenal vein can be found draining into the left renal vein.
- RIGHT
- Incise the peritoneal reflection of the triangular ligament of the liver
- Retract the liver and
- Dissection of the adrenal gland begins medially along with the IVC, identifying the right adrenal vein draining into the IVC.
- LEFT:
- Once identified, the adrenal vein is transected using clips, bipolar clamp, or harmonic dissector.
- Dissection is then continued, freeing the adrenal gland from the surrounding tissue, taking small arterial branches throughout the dissection.
- When the adrenal gland has been completely dissected, it is placed in a retrieval bag and removed through the 12mm port incision.
- The wound bed is irrigated and inspected for hemostasis.
- The fascia of the 12mm port site is closed with 2-0 absorbable suture.
- Skin incisions are closed using 4-0 absorbable sutures.
Posterior Retroperitoneoscopic Adrenalectomy[8]
Positioning:
Prone, gel rolls to lift the pelvis and at the chest to allow the abdomen to hang freely if Cloward Prone Positioner is not available. Hip and knees flexed at 110°. No need for foley or preoperative antibiotics.
Anesthesia:
- General anesthesia with endotracheal intubation.
- Central venous catheter and arterial line for right-sided adrenalectomy, pheochromocytoma, or cardiovascular risk.
Operative steps:
- Transverse incision 1.5cm in length just below the tip of the 12th rib and deepened to the retroperitoneal space with blunt and sharp dissection of the posterior abdominal wall.
- The retroperitoneal space is developed bluntly with digital dissection for placement of a 5mm trocar 6-8cm laterally, just below the 11 The port is placed under finger guidance.
- A 12mm balloon port is placed in the first incision.
- CO2 insufflation is used to create the retroperitoneal space with a pressure of 20-30mmHg.
- A 30° endoscope is inserted, and Gerota's fascia visualized.
- Gerota's fascia is opened using a grasper in the lateral trocar, and the fatty tissue of the abdominal wall is pushed down to create the retroperitoneal space.
- A third trocar is then placed, making a 10mm incision 4-5cm medial to the initial incision 4cm below the tip of the 12th rib, parallel to the spine, under direct visualization. The camera is then placed in this port.
- The upper third of the kidney is then freed of perirenal tissue, allowing it to be turned upward and retracted.
- The lower pole of the adrenal gland is mobilized, identifying the Gerota's anteriorly through the dissection of fatty tissue.
- Dissection is dependent on the side of the adrenal gland.
- Right: Dissection of the adrenal gland begins lateral and posterior to the right lobe of the liver and continued medially until the lateral IVC is observed. Dissection is then continued cephalad, taking small arterial branches throughout the dissection. The gland is dissected bluntly from Gerota's laterally.
- Left: Dissection of the adrenal gland begins lateral, between the kidney and the adrenal gland, behind the spleen, and continued medially. The inferior part of the adrenal gland is identified and dissected free until the adrenal vein is identified medially. Small arterial branches are coagulated throughout this dissection.
- The adrenal vein is identified and transected using a bipolar clamp or harmonic dissector. Clips are not routinely used but may be employed for a large right adrenal vein.
- The upper pole of the adrenal gland is dissected free from its attachments.
- Once the adrenal gland has been completely dissected, it is placed in a retrieval bag and removed through the 12mm port incision.
- The bed of the adrenal gland is irrigated and inspected for hemostasis.
- Skin incisions are closed using 4-0 absorbable sutures.
Complications
Primary adrenal insufficiency occurs after bilateral adrenalectomy. Signs and symptoms are volume depletion, hypotension, hyponatremia, hyperkalemia, fever, abdominal pain. Patients are managed by replacement therapy based on glucocorticoids (hydrocortisone or cortisone), mineralocorticoids (fludrocortisone) in cases of confirmed corticoids or aldosterone deficiency, respectively.
Only women with androgens deficiency, which presents low libido, depressive symptoms, and/or low energy levels despite otherwise optimized glucocorticoid and mineralocorticoid replacement, are candidates for dehydroepiandrosterone (DHEA) replacement. Other consequences of bilateral adrenalectomy include hypercortisolism due to excess ACTH stimulation of residual adrenal tissue, adrenal crisis, and the development of an aggressive corticotropic tumor called Nelson’s syndrome. Nelson’s syndrome is less common and is typically associated with skin hyperpigmentation due to hypersecretion of proopiomelanocortin (POMC) products.[9][6][7]
Diagnosis requires one of the following: 1) expanding pituitary mass lesion compared with pre-bilateral adrenalectomy imaging or 2) plasma level of ACTH more than 200 ng/mL in addition to progressive elevations of ACTH (an increase of >30%) on at least three consecutive occasions. Adrenal crisis is a life-threatening complication of adrenal insufficiency. Patients in the adrenal crisis typically present with profoundly impaired well-being, hypotension, nausea and vomiting, and fever responding well to parenteral hydrocortisone administration. Infections are the major precipitating causes of adrenal crisis.[10][11][7]
Clinical Significance
Adrenal causes of hypertension, diabetes, hyperlipidemia, and obesity are potentially underdiagnosed and may go untreated.[12] Additionally, there has been an increase in the number of incidentally found adrenal masses with improvements in axial imaging over time.
The clinician must be able to work-up and manage patients with adrenal masses, both functional and non-functional, to treat these patients with minimal morbidity. When planning for adrenalectomy, considerations of hormonal changes and preoperative preparation for these changes is as important and demands as much of the surgeon's attention as the technical aspects of the case.
Enhancing Healthcare Team Outcomes
Adrenalectomy has been shown to have a relatively low risk of postoperative complications, with an overall rate of 3.6%.[13]. Improved patient outcomes and decreased hospital costs have been demonstrated when adrenalectomy is performed by a high-volume adrenal surgeon (>/=6 adrenalectomies/year).[14]
An interprofessional approach to adrenalectomy aids in the prevention and avoidance of intraoperative and postoperative complications, and in some cases, allows for same-day discharge.[15] These improvements are only achieved with close communication across the care team-which includes the patient's endocrinologist, surgeon, anesthesiologist, nurses, and all additional health professionals that may be involved in the patient's care.
Media
(Click Image to Enlarge)
References
Willatt J, Chong S, Ruma JA, Kuriakose J. Incidental Adrenal Nodules and Masses: The Imaging Approach. International journal of endocrinology. 2015:2015():410185. doi: 10.1155/2015/410185. Epub 2015 Apr 29 [PubMed PMID: 26064109]
Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J, American Association of Clinical Endocrinologists, American Association of Endocrine Surgeons. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2009 Jul-Aug:15(5):450-3 [PubMed PMID: 19632968]
Zeiger MA, Siegelman SS, Hamrahian AH. Medical and surgical evaluation and treatment of adrenal incidentalomas. The Journal of clinical endocrinology and metabolism. 2011 Jul:96(7):2004-15. doi: 10.1210/jc.2011-0085. Epub 2011 Jun 1 [PubMed PMID: 21632813]
Al-Qurayshi Z, Robins R, Buell J, Kandil E. Surgeon volume impact on outcomes and cost of adrenal surgeries. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2016 Oct:42(10):1483-90. doi: 10.1016/j.ejso.2016.06.392. Epub 2016 Jun 23 [PubMed PMID: 27378161]
Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2016 Feb:101(2):364-89. doi: 10.1210/jc.2015-1710. Epub 2016 Jan 13 [PubMed PMID: 26760044]
Level 1 (high-level) evidenceFunder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2016 May:101(5):1889-916. doi: 10.1210/jc.2015-4061. Epub 2016 Mar 2 [PubMed PMID: 26934393]
Level 3 (low-level) evidenceRomero DG, Yanes Cardozo LL. Clinical Practice Guideline for Management of Primary Aldosteronism: What is New in the 2016 Update? International journal of endocrinology and metabolic disorders. 2016:2(3):. doi: 10.16966/2380-548X.129. Epub 2016 Jul 11 [PubMed PMID: 28018978]
Level 1 (high-level) evidenceMadani A, Lee JA. Surgical Approaches to the Adrenal Gland. The Surgical clinics of North America. 2019 Aug:99(4):773-791. doi: 10.1016/j.suc.2019.04.013. Epub 2019 May 27 [PubMed PMID: 31255206]
Ji C, Lu Q, Chen W, Zhang F, Ji H, Zhang S, Zhao X, Li X, Zhang G, Guo H. Retrospective comparison of three minimally invasive approaches for adrenal tumors: perioperative outcomes of transperitoneal laparoscopic, retroperitoneal laparoscopic and robot-assisted laparoscopic adrenalectomy. BMC urology. 2020 Jun 9:20(1):66. doi: 10.1186/s12894-020-00637-y. Epub 2020 Jun 9 [PubMed PMID: 32517679]
Level 2 (mid-level) evidenceRochon RM, Gimon T, Buie WD, Brar MS, Dixon E, MacLean AR. Expedited discharge in uncomplicated acute appendicitis: Decreasing the length of stay while maintaining quality. American journal of surgery. 2019 May:217(5):830-833. doi: 10.1016/j.amjsurg.2019.03.007. Epub 2019 Mar 13 [PubMed PMID: 30890264]
Level 2 (mid-level) evidenceOrtiz DI, Findling JW, Carroll TB, Javorsky BR, Carr AA, Evans DB, Yen TW, Wang TS. Cosyntropin stimulation testing on postoperative day 1 allows for selective glucocorticoid replacement therapy after adrenalectomy for hypercortisolism: Results of a novel, multidisciplinary institutional protocol. Surgery. 2016 Jan:159(1):259-65. doi: 10.1016/j.surg.2015.05.034. Epub 2015 Sep 28 [PubMed PMID: 26422766]
Käyser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, Hermus AR, Lenders JW, Deinum J. Study Heterogeneity and Estimation of Prevalence of Primary Aldosteronism: A Systematic Review and Meta-Regression Analysis. The Journal of clinical endocrinology and metabolism. 2016 Jul:101(7):2826-35. doi: 10.1210/jc.2016-1472. Epub 2016 May 12 [PubMed PMID: 27172433]
Level 1 (high-level) evidenceLimberg J, Ullmann TM, Gray KD, Stefanova D, Zarnegar R, Li J, Fahey TJ 3rd, Beninato T. Laparoscopic Adrenalectomy Has the Same Operative Risk as Routine Laparoscopic Cholecystectomy. The Journal of surgical research. 2019 Sep:241():228-234. doi: 10.1016/j.jss.2019.03.042. Epub 2019 Apr 28 [PubMed PMID: 31029933]
Anderson KL Jr, Thomas SM, Adam MA, Pontius LN, Stang MT, Scheri RP, Roman SA, Sosa JA. Each procedure matters: threshold for surgeon volume to minimize complications and decrease cost associated with adrenalectomy. Surgery. 2018 Jan:163(1):157-164. doi: 10.1016/j.surg.2017.04.028. Epub 2017 Nov 6 [PubMed PMID: 29122321]
Moughnyeh M, Lindeman B, Porterfield JR, Dream S. Outpatient robot-assisted adrenalectomy: Is it safe? American journal of surgery. 2020 Aug:220(2):296-297. doi: 10.1016/j.amjsurg.2020.04.037. Epub 2020 May 5 [PubMed PMID: 32402438]