Introduction
Solitary fibrous tumors (SFTs) encompass the histological spectrum of rare, slow-growing fibroblastic tumors considered mesenchymal in origin and arising from mesenchymal fibroblast-like cells. This class of neoplasms includes tumors previously classified as hemangiopericytomas.[1][2] Originally described in the pleura, SFTs have subsequently been described in several extrapleural sites, including the peritoneum, liver, mediastinum, upper respiratory tract, kidney, adrenal gland, bladder, prostate, orbit, nose, paranasal structures, and other sites in the head and neck.[3] The largest published series of patients with extra-meningeal SFTs found these neoplasms widely distributed in the following sites: 31% of SFTs originated within the abdominal cavity, 29% within the extremities, 22% from the pleurae, 11% from the trunk, and 7% from other sites, including the head and neck but excluding the meninges.[4]
The orbital SFT was first described in 1994 in separate reports by Dorfman and Westra.[5] Orbital SFTs pose a diagnostic challenge; they are rare neoplasms that present similarly to other orbital lesions. Orbital SFTs rarely metastasize and are treated with complete surgical excision.[6] Patients with an orbital SFT typically present with nonspecific symptoms characterized by slowly progressive, unilateral eyelid, eye, or periorbital swelling, proptosis, edema, or a palpable mass.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
SFTs were initially thought to be mesothelial tumors as they tend to arise from the pleurae and mediastinum. Klemperer and Robin initially described an SFT in 1931 as a “localized mesothelioma.”[7] Since then, SFTs have been described in several extra-serosal tissues, including the lung, liver, thyroid, sinuses, and orbit. These extra-serosal tumors likely originate from non-mesothelial tissues, possibly fibroblast-like differentiated cells within the mesenchyme. Endoneural dendritic cells, cutaneous dendrocytes, and pericytes are all possible sources of origin.
While the exact etiology of orbital SFTs remains unclear, their electron microscopic morphology and intense staining with CD34 and vimentin are compatible with a fibroblastic differentiated cell line.[5][8][9] Current research suggests abnormal cellular differentiation and genetic mutations may contribute to developing orbital SFTs.
Epidemiology
Approximately 20% of all SFTs occur in the head and neck.[10] Orbital SFTs are rare and account for less than 1% of all orbital tumors. Orbital SFTs and SFTs of the extremities are among the most commonly reported extrapleural SFTs.[11] Orbital SFTs occur equally in males and females. While there are reports of orbital SFTs in children as young as 5 years, most cases occur in adults between the ages of 20 and 70; the median age at diagnosis is 40.[12][13][14]
Fewer than 10% of patients with pleural SFTs develop paraneoplastic disorders such as hypertrophic osteoarthropathy (HOA). HOA syndrome is more common than paraneoplastic hypoglycemia, and HOA may be underdiagnosed in SFT. Other findings associated with SFTs include low blood glucose, growth hormone, and insulin-like growth factor I or III levels.[15] However, paraneoplastic features related to orbital SFTs have not been reported in the literature.
Pathophysiology
SFTs are classified as low-aggressive, high-aggressive, or dedifferentiated.[15][16] Ultrastructural, immunohistochemical, and microscopic investigations of SFTs have revealed that neoplastic cells have mesenchymal, submesothelial, and fibroblastic origin.[7] Staining for markers CD34, bcl-2, and CD99 is helpful but nondiagnostic; morphological correlation is often required.[2][8] CD34 is expressed in most SFTs but is also common in dermatofibrosarcomas, neural tumors, epithelioid sarcomas, and Kaposi sarcomas.[2][8] CD99 and bcl-2 are also expressed by mesenchymal tumors such as rhabdomyosarcoma, Ewing sarcoma, and osteosarcoma.[17][18]
The intense expression of CD34 helps differentiate SFTs from hemangiopericytomas and fibrous histiocytomas. CD34 is fairly sensitive for SFT but relatively nonspecific.[8][17][18] Neural schwannomas exhibit CD34 expression.[19] Orbital SFTs are usually negative for biomarkers desmin, CD31, EMA, s-100 protein, and smooth muscle actin. Hence, positivity to mesenchymal markers and negativity to epithelial and muscular biomarkers justify the suggested mesenchymal origin of SFTs.[17]
STAT6 is a signal transducer and transcription activator, and strong nuclear staining of STAT6 has been identified as a hallmark of SFT by immunohistochemistry. In contrast to the protein product of NAB2, which typically operates within the nucleus as a transcriptional repressor through its interaction with EGR1, STAT6 encodes for a cytoplasmic proteinaceous transcription factor continually shuttled to the nucleus. The transcriptional activator NAB2-STAT6 promotes the expression of EGR1 target genes. STAT6 may induce fibrosis in the setting of wound healing involving macrophages, CD4 TH2 cells, and fibroblasts via IL-13 signaling.[4][20]
Histopathology
In general, SFTs vary significantly in size, with most reported lesions being less than 10 cm. While a giant orbital SFT measuring 15 cm in the greatest dimension has been reported in the literature, most orbital SFTS measure from 1 to 3 cm.[21]
SFTs are usually well-circumscribed, lobulated, yellow, and solitary. True capsules and pseudo capsules are infrequently reported in the literature.[11]
Orbital SFTs demonstrate haphazardly arranged sheets of spindle cells with dull nuclei and nonapparent nucleoli.[8][22] Microscopically, patternless fibroblastic cells alternating with bundles of collagen and embedded vascular channels are surrounded by pericytes.[23][24] The arrangement of cellular and stromal components in SFT is referred to as a patternless pattern.[15] (see Image. Host-Pathological Examination, Orbital Solitary Fibrous Tumors)
Several risk categorization models for localized resected extra-meningeal SFT have been published, 2 of which have been externally verified. These verified models provide a more accurate prediction of metastatic risk, dividing patients into 3 risk categories based on patient age, mitotic count, and tumor size and necrosis.[15][4] Mitosis and necrosis are rarely reported as typical features of orbital SFTs. However, nuclear atypia, increased cellularity, necrosis, and greater than 4 mitotic figures per hpf suggest malignancy and aggressive infiltration, necessitating en bloc removal and long-term follow-up.[24]
Chung et al reported that 50% of orbital SFTs patients had positive STAT6 expression.[9] STAT6 nuclear staining, in combination with CD34 positivity, is a valuable diagnostic adjunct in distinguishing SFT from histologic imitators.[25]
History and Physical
Orbital SFTs usually present with painless and slowly progressive eye swelling over months to years. Proptosis is the most common finding in patients with an orbital SFT (see Image. Clinical Photograph of Proptosis). The optic nerve is rarely compressed or infiltrated, although vision, pupillary reaction, and visual fields may be affected.[24] Patients may report diplopia, tearing, and eyelid swelling.[9][26] The extraocular muscles may be restricted due to tumor encroachment; this is the proposed mechanism behind diplopia and is particularly common in anterior tumors.[24] Measuring proptosis with an exophthalmometer is important in identifying and diagnosing orbital SFTs.
Table 1. Underlying Mechanisms of Commonly Reported Symptoms and Signs of Orbital Solitary Fibrous Tumors
|
Sign or Symptom |
Proposed Underlying Mechanism(s) |
|
Decreased visual acuity |
Compressive optic neuropathy Dryness and ocular surface disease |
|
Orbital pain or paresthesia |
Mass effect with or without nerve compression |
|
Diplopia |
Mass effect with globe displacement or adhesion Restrictive or paralytic extraocular muscle limitation Symptomatic anisometropia |
|
Ptosis |
Mass effect with or without nerve compression |
|
Gaze-evoked amaurosis |
Gaze-dependent optic nerve compression |
|
Eyelid edema |
Inflammatory response |
|
Chemosis |
Inflammatory response Impaired venous outflow |
|
Ocular hypertension |
Increased episcleral or orbital venous pressure due to mass effect Secondary angle-closure glaucoma (rare) |
|
Proptosis |
Anterior globe displacement due to mass effect |
|
Dystopia |
Vertical or horizontal globe displacement due to mass effect |
|
Hyperopic shift |
Posterior globe compression and decreased axial length due to mass effect |
Evaluation
Imaging plays an important role in the diagnosis of orbital SFTs. Orbital SFTs may be intraconal, extraconal, or intraconal and extraconal; extraconal tumors appear to be most common.[27] SFTs of the ocular adnexa are uncommon, typically manifest as persistent orbital masses with proptosis, and usually originate within the lacrimal gland, lacrimal sac, or eyelid.[26][27] As observed in other locations, SFTs of the ocular adnexa exhibit STAT6 expression.[28]
When imaged with computed tomography (CT) or magnetic resonance imaging (MRI), orbital SFTs usually appear as well-defined soft tissue masses with occasional calcifications (see Image. Computed Tomography Image of Orbital Solitary Fibrous Tumor).[29] Bony remodeling can be observed in long-standing tumors; the bony destruction characteristic of malignant neoplasms has not been reported.[30][31] Orbital SFTs enhance with contrast in both imaging modalities.[32][26] Heterogeneity following contrast injection was more common in aggressive SFTs than indolent SFTs, at 76.5% and 40.0%, respectively.[33]
Tumor enhancement on CT and MRI is affected by the vascularity of the tumor, capillary permeability, and renal clearance.[34] Orbital SFTs are isointense on T1-weighted MRI and isointense to hypointense on T2-weighted scans, reflecting fibrous tissue with high collagen content.[27][26][30][31][35][36][37][38] However, T2-weighted images may reveal patchy hyperintense signaling consistent with internal bleeding, cystic degeneration, or very recent fibrosis.[31][38][39][34] Strong enhancement with gadolinium is typically observed with vascular tumors.[15][40]
Treatment / Management
Orbital SFTs are generally treated with en bloc, total surgical excision, given the penchant for recurrence and possible malignant degeneration in incompletely resected tumors. Exenteration may be required for infiltrating malignant tumors, as radiotherapy and chemotherapy have demonstrated limited benefit.[26][41][42][43] Recurrence is uncommon but usually occurs after an incomplete primary excision.[44][45] (B3)
Preoperative embolization can shrink tumor size, reducing intraoperative bleeding and surgical time. Liquid, particles, or coils can be used for embolization. Liquid embolic agents are commonly used for aneurysms, arteriovenous malformations, and fistulas, while coils are preferred for occluding large vessels. There has been a case report of a large orbital SFT preoperatively embolized with 500- to 700-µm tris-acryl gelatin microspheres.[46][47](B3)
Surgical techniques for resecting orbital tumors include the fronto-orbital or pteronial approaches and lateral or medial orbitotomy. The size and location of the neoplasm dictate the method employed. The transcranial fronto-orbital approach is employed to treat intracranial lesions involving the optic canal or occurring medial to the optic nerve at the apex.[13] Intraoperative use of the ultrasonic aspirator system SONO-PET to fragment neoplastic tissue for removal while simultaneously irrigating and aspirating the operative field may be beneficial.[48](B3)
Additionally, stereotactic radiosurgery may be performed to treat benign and malignant tumors or vascular abnormalities. Although data is lacking, stereotactic radiosurgery may be a suitable alternative for adjuvant treatment following partial resection of orbital SFTs or to treat recurrence.[49][50] Tata et al reported a case of a recurrent orbital SFT treated with gamma knife radiosurgery, resulting in effective tumor control and regression.[50] Clinical monitoring with serial examinations and imaging can be cautiously performed in individuals who have undergone previous tumor resection.[9](A1)
Differential Diagnosis
The differential diagnosis of orbital SFTs includes other tumors that can cause proptosis, such as schwannomas, hemangiomas, fibrous histiocytomas, meningiomas, and hemangiopericytomas.[51] Capillary hemangiomas are the most prevalent orbital vascular tumors in infants and children, and cavernous hemangiomas are the most prevalent vascular orbital tumors in adults.
Capillary hemangiomas typically grow fastest in the first 6 months of life and then begin to involute.[52] Although enhancing characteristics demonstrated on CT and MR imaging may be similar to those of orbital SFTs, an orbital capillary hemangioma can be distinguished by its infiltrative nature, nodular and irregular margin, and hyperintensity on T2-weighted MR images.[52] Cavernous hemangiomas appear as well-defined oval masses when imaged with CT and MRI and most commonly occur within the intrazonal area of the orbit. Cavernous hemangiomas are distinguished from orbital SFTs by hyperintensity with fibrous septa on T2-weighted MR images and different enhancing properties, demonstrating delayed pooling of contrast material on dynamic investigations.[52]
It is imperative to differentiate hemangiopericytomas and orbital SFTs; the treatments differ, and hemangiopericytomas have a propensity for recurrence and metastasis. Hemangiopericytomas are somatic soft tissue neoplasms that are well-encapsulated, highly vascular, and commonly found in the extra-orbital region near the paranasal sinuses.[52][53] In imaging studies, orbital hemangiopericytomas may be the most difficult tumors to distinguish from orbital SFTs. Hemangiopericytomas may be associated with bone erosion or infiltration into neighboring tissues. On both T1 and T2-weighted MR images, hemangiopericytomas are isointense to the gray matter.[52]
Inconspicuous contrast enhancement can also be seen in orbital schwannoma, neurofibroma, fibrous histiocytoma, lymphoma, pseudotumor, and metastatic disease.[54]
Table 2. Imaging Findings of Orbital Neoplasms
|
Neoplasm |
Imaging Findings |
|
Orbital Solitary Fibrous Tumor |
Well-delineated; densely enhancing homogeneously or heterogeneously with areas of calcifications |
|
Schwannoma |
Densely enhanced homogenous extraconal mass with characteristic expansion into bone without erosions |
|
Cavernous hemangioma |
Well-circumscribed, homogenous mass slightly hyperdense to muscle, commonly located intraconally |
|
Fibrous histiocytoma |
Well-circumscribed soft tissue mass that is isointense to muscle |
|
Meningioma |
Diffuse, tubular thickening of the optic nerve sheath that enhances homogeneously, often producing a characteristic "tram track" sign on axial windows or a "doughnut" sign on coronal windows |
|
Hemangiopericytoma |
Vividly enhancing soft tissue mass on CT, while isointense on T1- and T2-weighted MR images |
Prognosis
The prognosis of orbital SFTs will vary with several factors, including the size, location, extent of invasion, and histological characteristics of the neoplasm. For wholly resected tumors, the prognosis is generally excellent. Approximately 75% of SFTs have a benign course, with the remaining 25% exhibiting invasion to surrounding tissues, recurrence, or distant metastasis. Moreover, 95% of recurrent orbital SFTs have benign histology, while up to 75% of histopathologically verified malignant tumors have nonaggressive clinical behavior.
Tumor recurrence can occur as soon as 3 months or as long as 40 years after primary excision.[12] Furthermore, the involvement of certain critical structures within the orbit, such as the optic nerve or major blood vessels, can significantly impact the prognosis of orbital SFTs. Tumors located near vital structures may be more challenging to excise completely, leading to an increased risk of complications, potential functional impairment, and recurrence.
Complications
Space-occupying lesions can cause complications due to direct mass effects. Compressive optic neuropathy is the most common complication of large intraconal orbital SFTs. Chronic pain, paresthesia, and glaucoma are possible complications of infiltrating malignant orbital SFTs.[55]
Any surgical resection of an orbital mass carries the risks of retrobulbar hemorrhage, diplopia, ptosis, strabismus, reduced vision, blindness, postoperative inflammation, cellulitis, or inadequate tumor excision.[56]
Recurrence or malignant transformation may result from incomplete tumor removal. Close monitoring following orbital surgery is critical.[57]
Postoperative and Rehabilitation Care
Patients whose tumors have been completely excised may benefit from routine follow-up. In contrast, those with incomplete tumor removal should be monitored more frequently, undergoing serial imaging to monitor for tumor recurrence. Long-term surveillance can aid in detecting potential tumor regrowth or metastasis and ensure timely intervention if required.[58]
Consultations
An ophthalmologist should thoroughly evaluate any patient presenting with an orbital mass, restriction of extraocular movements, diplopia, proptosis, ptosis, or the sudden onset of periorbital swelling. Referral to an ocular oncologist or oculoplastic surgeon may be required. Further consultations with a retina or glaucoma specialist may be required if the patient develops intraocular complications.[58]
Deterrence and Patient Education
Patients with orbital SFTs must be counseled regarding the significance and importance of postoperative follow-up to monitor for disease reoccurrence. Patients with small, asymptomatic tumors may elect to be observed with serial examination and imaging studies.
Enhancing Healthcare Team Outcomes
Orbital SFTs are very rare neoplasms. Healthcare practitioners should know the nonspecific early indicators of an orbital mass, including proptosis, vision loss, limited extraocular muscle movements, ptosis, and eyelid swelling. Although the primary management of orbital SFTs is surgical excision, some patients with small tumors may elect to be observed with serial examinations and imaging studies.[59]
Media
(Click Image to Enlarge)
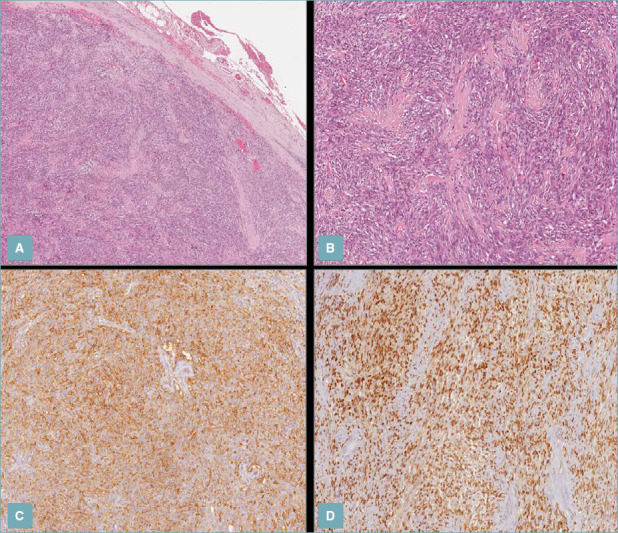
Host-Pathological Examination, Orbital Solitary Fibrous Tumors. (A) Low magnification shows a uniformly hypercellular tumor with pushing borders, circumscribed by a fibrous pseudo-capsule (H&E staining; 50x magnification). (B) Higher magnification showing moderately eosinophilic bland-looking spindle cells arranged in intersecting short fascicles with interspersed stellate-shaped, keloid-type collagen fibers (H&E staining; 100x magnification); (C,D) Neoplastic cells are positively stained with CD34 (C) and STAT-6 (D) (immunoperoxidase staining; 100x magnification).
G Broggi et al. Pathologica. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8183349/
(Click Image to Enlarge)
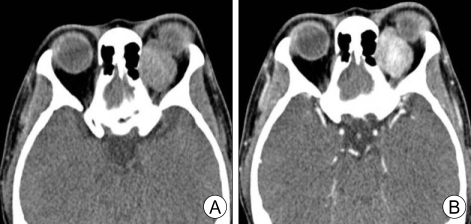
Computed Tomography Image of Orbital Solitary Fibrous Tumor. Orbital computed tomography (CT) scan revealing 25 × 20 × 20 mm-sized well-demarcated extraconal superomedial orbital mass. A) Precontrast CT scan. B) Postcontrast CT scan showing intensely enhancing mass.
J Kyung et al. J Korean Neurosurg Soc. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2729832/
(Click Image to Enlarge)
References
Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006 Jan:48(1):63-74 [PubMed PMID: 16359538]
Olson NJ, Linos K. Dedifferentiated Solitary Fibrous Tumor: A Concise Review. Archives of pathology & laboratory medicine. 2018 Jun:142(6):761-766. doi: 10.5858/arpa.2016-0570-RS. Epub [PubMed PMID: 29848035]
el-Naggar AK, Ro JY, Ayala AG, Ward R, Ordóñez NG. Localized fibrous tumor of the serosal cavities. Immunohistochemical, electron-microscopic, and flow-cytometric DNA study. American journal of clinical pathology. 1989 Nov:92(5):561-5 [PubMed PMID: 2479254]
Salas S, Resseguier N, Blay JY, Le Cesne A, Italiano A, Chevreau C, Rosset P, Isambert N, Soulie P, Cupissol D, Delcambre C, Bay JO, Dubray-Longeras P, Krengli M, De Bari B, Villa S, Kaanders JHAM, Torrente S, Pasquier D, Thariat JO, Myroslav L, Sole CV, Dincbas HF, Habboush JY, Zilli T, Dragan T, Khan R K, Ugurluer G, Cena T, Duffaud F, Penel N, Bertucci F, Ranchere-Vince D, Terrier P, Bonvalot S, Macagno N, Lemoine C, Lae M, Coindre JM, Bouvier C. Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Annals of oncology : official journal of the European Society for Medical Oncology. 2017 Aug 1:28(8):1979-1987. doi: 10.1093/annonc/mdx250. Epub [PubMed PMID: 28838212]
Dorfman DM, To K, Dickersin GR, Rosenberg AE, Pilch BZ. Solitary fibrous tumor of the orbit. The American journal of surgical pathology. 1994 Mar:18(3):281-7 [PubMed PMID: 8116796]
Goodlad JR, Fletcher CD. Solitary fibrous tumour arising at unusual sites: analysis of a series. Histopathology. 1991 Dec:19(6):515-22 [PubMed PMID: 1786936]
Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. American journal of industrial medicine. 1992:22(1):1-31 [PubMed PMID: 1415270]
Level 3 (low-level) evidenceWestra WH, Gerald WL, Rosai J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. The American journal of surgical pathology. 1994 Oct:18(10):992-8 [PubMed PMID: 7522416]
Tam ES, Chen EC, Nijhawan N, Harvey JT, Howarth D, Oestreicher JH. Solitary fibrous tumor of the orbit: a case series. Orbit (Amsterdam, Netherlands). 2008:27(6):426-31. doi: 10.1080/01676830802344508. Epub [PubMed PMID: 19085297]
Level 3 (low-level) evidenceChung HR, Tam K, Han AY, Obeidin F, Nakasaki M, Chhetri DK, St John MA, Kita AE. Solitary Fibrous Tumors of the Head and Neck: A Single-Institution Study of 52 Patients. OTO open. 2022 Jul-Sep:6(3):2473974X221098709. doi: 10.1177/2473974X221098709. Epub 2022 Jul 12 [PubMed PMID: 35845143]
Musyoki FN, Nahal A, Powell TI. Solitary fibrous tumor: an update on the spectrum of extrapleural manifestations. Skeletal radiology. 2012 Jan:41(1):5-13. doi: 10.1007/s00256-010-1032-z. Epub 2010 Oct 16 [PubMed PMID: 20953607]
Le CP, Jones S, Valenzuela AA. Orbital solitary fibrous tumor: a case series with review of the literature. Orbit (Amsterdam, Netherlands). 2014 Apr:33(2):145-51. doi: 10.3109/01676830.2013.853806. Epub 2013 Dec 2 [PubMed PMID: 24295271]
Level 3 (low-level) evidenceGiuffrè I, Faiola A, Bonanno E, Liccardo G. Solitary fibrous tumor of the orbit. Case report and review of the literature. Surgical neurology. 2001 Oct:56(4):242-6 [PubMed PMID: 11738672]
Level 3 (low-level) evidenceBlandamura S, Alaggio R, Bettini G, Guzzardo V, Valentini E, Bedogni A. Four cases of solitary fibrous tumour of the eye and orbit: one with sarcomatous transformation after radiotherapy and one in a 5-year-old child's eyelid. Journal of clinical pathology. 2014 Mar:67(3):263-7. doi: 10.1136/jclinpath-2013-201820. Epub 2013 Oct 9 [PubMed PMID: 24108432]
Level 3 (low-level) evidenceMartin-Broto J, Mondaza-Hernandez JL, Moura DS, Hindi N. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers. 2021 Jun 10:13(12):. doi: 10.3390/cancers13122913. Epub 2021 Jun 10 [PubMed PMID: 34200924]
Martin-Broto J, Cruz J, Penel N, Le Cesne A, Hindi N, Luna P, Moura DS, Bernabeu D, de Alava E, Lopez-Guerrero JA, Dopazo J, Peña-Chilet M, Gutierrez A, Collini P, Karanian M, Redondo A, Lopez-Pousa A, Grignani G, Diaz-Martin J, Marcilla D, Fernandez-Serra A, Gonzalez-Aguilera C, Casali PG, Blay JY, Stacchiotti S. Pazopanib for treatment of typical solitary fibrous tumours: a multicentre, single-arm, phase 2 trial. The Lancet. Oncology. 2020 Mar:21(3):456-466. doi: 10.1016/S1470-2045(19)30826-5. Epub 2020 Feb 14 [PubMed PMID: 32066540]
Ing EB, Kennerdell JS, Olson PR, Ogino S, Rothfus WE. Solitary fibrous tumor of the orbit. Ophthalmic plastic and reconstructive surgery. 1998 Jan:14(1):57-61 [PubMed PMID: 9513245]
Level 3 (low-level) evidenceAlexandrakis G, Johnson TE. Recurrent orbital solitary fibrous tumor in a 14-year-old girl. American journal of ophthalmology. 2000 Sep:130(3):373-6 [PubMed PMID: 11020428]
Level 3 (low-level) evidenceHeathcote JG. Pathology update: solitary fibrous tumour of the orbit. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 1997 Dec:32(7):432-5 [PubMed PMID: 9435973]
Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 Mar:27(3):390-5. doi: 10.1038/modpathol.2013.164. Epub 2013 Sep 13 [PubMed PMID: 24030747]
Zhou Q, Liu Y, Wang F, Cao Y, Lv H, Zhang X. A giant orbital solitary fibrous tumor treated by surgical excision: a case report and literature review. Diagnostic pathology. 2023 May 5:18(1):59. doi: 10.1186/s13000-023-01350-8. Epub 2023 May 5 [PubMed PMID: 37147709]
Level 3 (low-level) evidenceChen H, Xiao CW, Wang T, Wu JS, Jiang CC, Qian J, Wei CH, Wang XQ. Orbital solitary fibrous tumor: a clinicopathologic study of ten cases with long-term follow-up. Acta neurochirurgica. 2012 Feb:154(2):249-55; discussion 255. doi: 10.1007/s00701-011-1254-4. Epub 2011 Dec 28 [PubMed PMID: 22203231]
Level 3 (low-level) evidenceCranshaw IM, Gikas PD, Fisher C, Thway K, Thomas JM, Hayes AJ. Clinical outcomes of extra-thoracic solitary fibrous tumours. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009 Sep:35(9):994-8. doi: 10.1016/j.ejso.2009.02.015. Epub 2009 Apr 3 [PubMed PMID: 19345055]
Level 2 (mid-level) evidenceGupta S, Verma R, Sen R, Singh I, Marwah N, Kohli R. Solitary fibrous tumor of the orbit. Asian journal of neurosurgery. 2016 Jan-Mar:11(1):78. doi: 10.4103/1793-5482.165804. Epub [PubMed PMID: 26889300]
Blessing NW, Bermudez-Magner JA, Fernandez MP, Rosenberg AE, Dubovy SR, Johnson TE. Solitary Fibrous Tumor of the Orbit: A Case Series With Clinicopathologic Correlation and Evaluation of STAT6 as a Diagnostic Marker. Ophthalmic plastic and reconstructive surgery. 2020 Mar/Apr:36(2):164-171. doi: 10.1097/IOP.0000000000001504. Epub [PubMed PMID: 31876648]
Level 2 (mid-level) evidenceRomer M, Bode B, Schuknecht B, Schmid S, Holzmann D. Solitary fibrous tumor of the orbit--two cases and a review of the literature. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2005 Feb:262(2):81-8 [PubMed PMID: 15316820]
Level 3 (low-level) evidenceKrishnakumar S, Subramanian N, Mohan ER, Mahesh L, Biswas J, Rao NA. Solitary fibrous tumor of the orbit: a clinicopathologic study of six cases with review of the literature. Survey of ophthalmology. 2003 Sep-Oct:48(5):544-54 [PubMed PMID: 14499820]
Level 3 (low-level) evidencePetrovic A, Obéric A, Moulin A, Hamedani M. Ocular adnexal (orbital) solitary fibrous tumor: nuclear STAT6 expression and literature review. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2015 Sep:253(9):1609-17. doi: 10.1007/s00417-015-2975-5. Epub 2015 Mar 13 [PubMed PMID: 25761539]
Level 3 (low-level) evidenceFesta S, Lee HJ, Langer P, Klein KM. Solitary fibrous tumor of the orbit: CT and pathologic correlation. Neuroradiology. 1999 Jan:41(1):52-4 [PubMed PMID: 9987770]
Level 3 (low-level) evidenceKim TA, Brunberg JA, Pearson JP, Ross DA. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR. American journal of neuroradiology. 1996 Oct:17(9):1767-72 [PubMed PMID: 8896635]
Level 3 (low-level) evidenceGanly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, Edgar M, Shah JP. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Archives of otolaryngology--head & neck surgery. 2006 May:132(5):517-25 [PubMed PMID: 16702568]
Leoncini G, Maio V, Puccioni M, Franchi A, De Giorgi V, Ucci F, Santucci M, Massi D. Orbital solitary fibrous tumor: a case report and review of the literature. Pathology oncology research : POR. 2008 Jun:14(2):213-7. doi: 10.1007/s12253-008-9055-7. Epub 2008 May 21 [PubMed PMID: 18493869]
Level 3 (low-level) evidenceHélage S, Revel MP, Chabi ML, Audureau É, Ferretti G, Laurent F, Alifano M, Mansuet-Lupo A, Buy JN, Vadrot D. Solitary fibrous tumor of the pleura: Can computed tomography features help predict malignancy? A series of 56 patients with histopathological correlates. Diagnostic and interventional imaging. 2016 Mar:97(3):347-53. doi: 10.1016/j.diii.2015.04.013. Epub 2015 Nov 2 [PubMed PMID: 26542536]
Kim HJ, Kim HJ, Kim YD, Yim YJ, Kim ST, Jeon P, Kim KH, Byun HS, Song HJ. Solitary fibrous tumor of the orbit: CT and MR imaging findings. AJNR. American journal of neuroradiology. 2008 May:29(5):857-62. doi: 10.3174/ajnr.A0961. Epub 2008 Feb 13 [PubMed PMID: 18272558]
Level 2 (mid-level) evidenceHayashi S, Kurihara H, Hirato J, Sasaki T. Solitary fibrous tumor of the orbit with extraorbital extension: case report. Neurosurgery. 2001 Nov:49(5):1241-5 [PubMed PMID: 11846919]
Level 3 (low-level) evidenceJohnson TE, Onofrey CB, Ehlies FJ. Echography as a useful adjunct in the diagnosis of orbital solitary fibrous tumor. Ophthalmic plastic and reconstructive surgery. 2003 Jan:19(1):68-74 [PubMed PMID: 12544795]
Level 3 (low-level) evidenceKim HY, Lee SY, Kang SJ, Kim HJ. Solitary fibrous tumor of the orbit: a poorly-recognized orbital lesion. Acta ophthalmologica Scandinavica. 1999 Dec:77(6):704-8 [PubMed PMID: 10634569]
Level 3 (low-level) evidenceDunfee BL, Sakai O, Spiegel JH, Pistey R. Solitary fibrous tumor of the buccal space. AJNR. American journal of neuroradiology. 2005 Sep:26(8):2114-6 [PubMed PMID: 16155167]
Level 3 (low-level) evidenceKim HJ, Lee HK, Seo JJ, Kim HJ, Shin JH, Jeong AK, Lee JH, Cho KJ. MR imaging of solitary fibrous tumors in the head and neck. Korean journal of radiology. 2005 Jul-Sep:6(3):136-42 [PubMed PMID: 16145288]
Level 2 (mid-level) evidenceGinat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR. American journal of roentgenology. 2011 Mar:196(3):487-95. doi: 10.2214/AJR.10.4948. Epub [PubMed PMID: 21343490]
Clayton AC, Salomão DR, Keeney GL, Nascimento AG. Solitary fibrous tumor: a study of cytologic features of six cases diagnosed by fine-needle aspiration. Diagnostic cytopathology. 2001 Sep:25(3):172-6 [PubMed PMID: 11536441]
Level 3 (low-level) evidenceDietrich CG, Roeb E, Breuer E, Matern S. [Solitary fibrous thoracic wall tumor. Progression with percutaneous radiotherapy]. Deutsche medizinische Wochenschrift (1946). 2001 Jan 5:126(1-2):12-5 [PubMed PMID: 11200659]
Level 3 (low-level) evidencePolito E, Tosi GM, Toti P, Schürfeld K, Caporossi A. Orbital solitary fibrous tumor with aggressive behaviorThree cases and review of the literature. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2002 Jul:240(7):570-4 [PubMed PMID: 12136289]
Level 3 (low-level) evidenceCarrera M, Prat J, Quintana M. Malignant solitary fibrous tumour of the orbit: report of a case with 8 years follow-up. Eye (London, England). 2001 Feb:15(Pt 1):102-4 [PubMed PMID: 11318269]
Level 3 (low-level) evidenceSayit AT, Elmali M, Gul A, Sullu Y. Solitary fibrous tumor of the orbit: Computed tomography and histopathological findings. Journal of cancer research and therapeutics. 2019 Jul-Sep:15(3):719-721. doi: 10.4103/jcrt.JCRT_1194_16. Epub [PubMed PMID: 31169250]
Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Seminars in interventional radiology. 2008 Sep:25(3):204-15. doi: 10.1055/s-0028-1085930. Epub [PubMed PMID: 21326511]
Level 3 (low-level) evidenceYazıcı B, Hakyemez B, Muz ÖE, Yazıcı Z, Yalçınkaya U. Preoperative Endovascular Embolization of Orbital Solitary Fibrous Tumor With 500-700 Micron Tris-Acryl Gelatin Microspheres. Turkish journal of ophthalmology. 2022 Oct 28:52(5):356-359. doi: 10.4274/tjo.galenos.2022.79438. Epub [PubMed PMID: 36317827]
Navarro-Perea C, Calleja-García C, Bengoa-González Á, Garrido MC, Mencía-Gutiérrez E, Pérez-Trigo S. Orbital Solitary Fibrous Tumor: Four Case Reports-Clinical and Histopathological Features. Case reports in ophthalmological medicine. 2021:2021():5822859. doi: 10.1155/2021/5822859. Epub 2021 Jun 9 [PubMed PMID: 34211794]
Level 3 (low-level) evidenceNakahara K, Yamada M, Shimizu S, Fujii K. Stereotactic radiosurgery as adjuvant treatment for residual solitary fibrous tumor. Case report. Journal of neurosurgery. 2006 Nov:105(5):775-6 [PubMed PMID: 17121144]
Level 3 (low-level) evidenceTata A, Cohen-Inbar O, Sheehan JP. Treatment of orbital solitary fibrous tumour with gamma knife radiosurgery and systematic review of literature. BMJ case reports. 2016 Oct 7:2016():. doi: 10.1136/bcr-2016-217114. Epub 2016 Oct 7 [PubMed PMID: 27758816]
Level 1 (high-level) evidenceHa JK, Park BJ, Kim YH, Lim YJ. Orbital solitary fibrous tumor : a case report and diagnostic clues. Journal of Korean Neurosurgical Society. 2009 Jul:46(1):77-80. doi: 10.3340/jkns.2009.46.1.77. Epub 2009 Jul 31 [PubMed PMID: 19707501]
Level 3 (low-level) evidenceBilaniuk LT. Orbital vascular lesions. Role of imaging. Radiologic clinics of North America. 1999 Jan:37(1):169-83, xi [PubMed PMID: 10026736]
Tailor TD, Gupta D, Dalley RW, Keene CD, Anzai Y. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 Oct:33(6):1739-58. doi: 10.1148/rg.336135502. Epub [PubMed PMID: 24108560]
Dalley RW. Fibrous histiocytoma and fibrous tissue tumors of the orbit. Radiologic clinics of North America. 1999 Jan:37(1):185-94 [PubMed PMID: 10026737]
Williams M, Ahmad T, Chin LS, Richardson TE, Mangla R, Zain SM, Mirchia K. Clinical, Pathologic, and Radiologic Features of Orbital Solitary Fibrous Tumors and Meningiomas. Cureus. 2021 Nov:13(11):e19678. doi: 10.7759/cureus.19678. Epub 2021 Nov 17 [PubMed PMID: 34976466]
Topilow NJ, Tran AQ, Koo EB, Alabiad CR. Etiologies of Proptosis: A review. Internal medicine review (Washington, D.C. : Online). 2020 Mar:6(3):. doi: 10.18103/imr.v6i3.852. Epub [PubMed PMID: 32382689]
Laplant J, Cockerham K. Primary Malignant Orbital Tumors. Journal of neurological surgery. Part B, Skull base. 2021 Feb:82(1):81-90. doi: 10.1055/s-0040-1722635. Epub 2021 Feb 18 [PubMed PMID: 33777620]
Ajouz H, Sohail AH, Hashmi H, Martinez Aguilar M, Daoui S, Tembelis M, Aziz M, Zohourian T, Brathwaite CEM, Cerfolio RJ. Surgical considerations in the resection of solitary fibrous tumors of the pleura. Journal of cardiothoracic surgery. 2023 Feb 24:18(1):79. doi: 10.1186/s13019-023-02168-7. Epub 2023 Feb 24 [PubMed PMID: 36823638]
Ali MJ, Honavar SG, Naik MN, Vemuganti GK. Orbital solitary fibrous tumor: A clinicopathologic correlation and review of literature. Oman journal of ophthalmology. 2011 Sep:4(3):147-9. doi: 10.4103/0974-620X.91274. Epub [PubMed PMID: 22279406]
Level 3 (low-level) evidence