Introduction
Antiphospholipid testing is part of the diagnostic workup for possible underlying autoimmune conditions in patients presenting with arterial, venous, and microvascular thrombosis, along with gestational or obstetric complications and morbidity.[1][2][3] This testing includes 3 main antibody classifications:
- Lupus anticoagulants (IgG, IgM)
- Anticardiolipin antibodies (IgA, IgG, and IgM)
- Anti-β2-glycoprotein Ib (anti-β2GPIb) antibodies (IgA, IgG, and IgM)
These antibodies should be tested together to assess the risk profile for high-risk patients for antiphospholipid syndrome.[4][5] Incomplete antiphospholipid diagnostic testing could lead to improper diagnosis of antiphospholipid syndrome and could impose catastrophic consequences without intervention.[6][7][8]
Lupus anticoagulant refers to a diverse antibody group directed against phospholipids and phospholipid-binding proteins, which prolong certain coagulation testing in vitro and cause thrombosis in vivo. Lupus anticoagulant detection involves assessing its effect on various anticoagulation tests, and no single assay measures all the different lupus anticoagulant antibodies. Therefore, at least 2 different confirmatory tests are required to establish a positive lupus anticoagulant test. Lupus anticoagulant is considered the most predictive test for thrombosis among antibody tests when positive.[9][10]
Cardiolipin is an inner mitochondrial membrane component comprising about 20% of the total lipid composition. Anticardiolipin binding to phospholipid anions causes platelet activation. Anticardiolipin antibodies are considered more sensitive but less specific compared to lupus anticoagulants for predicting thrombosis.
Anti-β2GPIb antibodies bind to platelets at the apoER2′ and glycoprotein Ib-α receptors, activating the platelet signaling pathway involving MAPK phosphorylation, GP IIb/IIIa conformational change, P-selectin expression, and thromboxane B2 production.[11]
Etiology and Epidemiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology and Epidemiology
According to the revised Sapporo criteria, the diagnosis of persistent positive antiphospholipids is established when antiphospholipid antibodies are detected on 2 or more occasions at least 12 weeks apart.[12] This condition could indicate underlying antiphospholipid syndrome, a systemic autoimmune disease, clinically characterized by thrombotic or pregnancy or obstetric events. Antiphospholipid syndrome is classified as primary if not related to other underlying autoimmune diseases. On the other hand, secondary antiphospholipid syndrome is associated with systemic autoimmune diseases, with systemic lupus erythematosus as a common association.[6][8] In a population-based study, the estimated incidence and prevalence of antiphospholipid syndrome determined were 2.1 per 100,000 population per year and 50 per 100,000 population, respectively.[13] Triple positivity, which is defined as positivity for lupus anticoagulants, anticardiolipin antibodies, and anti-β2GPIb, is associated with greatly increased risks for thrombosis, especially with autoimmune diseases, including for a first-event thrombosis in asymptomatic carriers.[5][14]
Specimen Requirements and Procedure
The preanalytical stage for testing antiphospholipid antibodies requires careful handling to prevent sample activation, loss of coagulant proteins, and contamination.[15] Blood samples, whether in serum or citrate-anticoagulated plasma form, are required. Plasma should be platelet-poor (<1000/L), preferably using the double centrifugation technique.[16] Frozen plasma should be stored at −70 °C to maintain integrity, avoiding repeated freezing and thawing. Thawing should occur at 37 °C for 5 minutes in a water bath by total immersion and then mixed before testing. Samples can be stored at 2 to 8 °C for 2 to 3 days or at −20 °C or below for longer periods.[17] Adherence to these guidelines ensures result accuracy and minimizes pre-analytical errors.
Diagnostic Tests
Antiphospholipid antibodies are a family of autoantibodies that can interact with negatively charged phospholipids and phospholipid-binding proteins.[18][19] Specifically, these antibodies inhibit in vitro coagulation resulting from antiphospholipid binding to plasma proteins, mainly β2-glycoprotein or prothrombin, with an affinity for phospholipids. Lupus anticoagulant is a semantic paradox, as these antibodies are associated with clinical thrombosis. In contrast, an anticoagulant effect is observed in the laboratory or in vitro as a prolongation of clotting times.[5][20] Moreover, lupus anticoagulants and other antiphospholipid antibody tests are included in the Classification Criteria for Systemic Lupus Erythematosus as part of the immunologic domain but are not the sole criteria.[21] Of note, testing should be performed when a patient is clinically stable and not during an acute event, as acute thrombosis can cause inaccurate testing and can even cause transiently elevated anticardiolipin antibodies.[2] For an overview of lupus anticoagulants and other anticoagulants on various testing parameters, please see Table. Effect of Lupus Anticoagulant and Anticoagulants on Laboratory Testing.
The laboratory diagnosis of antiphospholipid antibodies typically employs 2 main categories of screening methods:
- Immunological assays (anticardiolipin and anti-β2GPIb)
- Clot-based assays, which include incorporating phospholipids into the reagent system and evaluating clotting time, are utilized to detect various lupus anticoagulant antibodies) [22]
Immunological Assays
Enzyme-linked immunosorbent assay (ELISA) technique is commonly employed to detect anticardiolipin and anti-β2GPIb. In this method, the solid phase is coated with cardiolipin and β2 GPI as cofactors. The ELISA method detects IgM, IgG, and IgA classes of anticardiolipin antibodies.[5] An important point is that lupus anticoagulants and anticardiolipin antibodies differ in their phospholipid-binding characteristics; hence, the detection of anticardiolipin antibodies is not specific for the presence of lupus anticoagulants, although they may be present in the same patient.[23] Anti-β2GPIb antibodies also utilize the ELISA technique. Both of these antibodies are primarily IgG and IgM; IgA antibodies have been isolated and are associated with increased thrombosis, but the IgA antibodies are typically associated with IgG and IgM.[24]
Clot-Based Assays
Lupus anticoagulant testing is a multi-step process. The initial screening test uses reagents with a low phospholipid quantity, followed by the same test with a high phospholipid quantity to show that the test prolongation is phospholipid-dependent (and not clotting factor-dependent). The dilute Russell viper venom time (dRVVT) and activated partial thromboplastin time (APTT) are the most commonly used tests.[9]
Dilute Russell viper venom time: The dRVVT is widely regarded as the preferred test for screening and confirmation of lupus anticoagulant. dRVVT is more sensitive and specific compared to other global tests. Importantly, it is also not affected by factor VIII or IX inhibitors. The dRVVT test comprises a screening reagent containing a limited amount of phospholipids with Russell viper venom and a confirmatory reagent containing additional phospholipids with the same amount of Russell viper venom to confirm the presence of phospholipid-dependent lupus anticoagulant. Russell viper venom bypasses factor VII of the extrinsic pathway and the contact and antihemophilic factors of the intrinsic pathway.[9][25]
In normal plasma, in the absence of lupus anticoagulants, factors V and X are directly activated by Russell viper venom, which in the presence of phospholipid and calcium ions, leads to clot formation. In patients with lupus anticoagulant, autoantibodies bind to the epitopes of the reagent phospholipids, thereby preventing the activation of the prothrombinase complex. As a result, the clotting time when using screening reagents in laboratory tests is prolonged.[26]
Activated partial thromboplastin time: As lupus anticoagulants bind to the phospholipid complex, they prolong phospholipid-based coagulation assays. Logically, the APTT is prolonged, and this property has been used to detect lupus anticoagulants.[27]
In the context of lupus anticoagulant detection, the APTT test has certain limitations as follows:
- An important variable related to the suitability of APTT reagents in detecting lupus anticoagulants is the composition of phospholipids used in the reagent system. Different reagents have varying sensitivity for the presence of lupus anticoagulants based on their phospholipid composition.[28]
- The APTT test is affected by the presence of factor VIII, IX, and XI inhibitors.[29] Mixing studies are performed to rule out a lack of clotting factors as a cause of APTT prolongation. A 1:1 or 1:4 (normal plasma to patient plasma) is used in mixing studies. Failure to correct the prolongation of clotting time using the mixing studies indicates the presence of lupus anticoagulants.[30]
- The APTT test is also used to monitor heparin therapy. However, the test's ability to specifically detect lupus anticoagulant is reduced.[31] The presence of heparin can be ruled out using a thrombin time test. If the thrombin time is normal, then heparin is likely not present. However, if the thrombin time is abnormal, it could be due to heparin. In this case, a heparin neutralization test can be used to confirm the presence of heparin. In this test, various concentrations of protamine sulfate are added to plasma before the addition of the thrombin reagent. When protamine sulfate neutralizes all the heparin present, the clotting time reverts to normal value.[32]
Tissue thromboplastin inhibition test: The tissue thromboplastin inhibition (TTI) test, also known as the dilute prothrombin time (dPT) test, utilizes a diluted thromboplastin reagent. In this test, the thromboplastin reagent is diluted 1:50 or 1:500 with saline, and the results are expressed as the ratio of patient values to normal control values.[33]
The TTI test is affected by numerous variables:
- Species and tissue origin of thromboplastin can affect the test results as different sources have varying sensitivity and responsiveness. Rabbit brain thromboplastin, conventionally utilized in the TTI test, is highly sensitive to lupus anticoagulant. However, it is susceptible to inter-laboratory variability due to natural discrepancies in the composition of the rabbit brain.[34]
- The choice of normal reference plasma is the most critical variable because, depending on the laboratory, the choice of reference plasma could be lyophilized plasma, a frozen plasma pool, or fresh plasma. Therefore, the patient-to-normal ratio can change according to the choice of normal plasma. To reduce variability, laboratories should develop standardized protocols for selecting and handling reference plasma.[35]
- Some IgM lupus anticoagulants do not prolong the TTI test.[36]
Kaolin clotting time: Kaolin clotting time (KCT) is similar to APTT, the difference being that the KCT reagent is devoid of phospholipids and incorporates kaolin as a contact activator through the intrinsic pathway.[37] The test is performed on a range of normal and patient plasma. Different response patterns are obtained, indicating the presence of lupus anticoagulants or the deficiency of 1 or more coagulation factors.[38]
The KCT test, although sensitive, is not specific for detecting lupus anticoagulants. In addition, it has the following limitations:
- Test automation is challenging, making it more labor-intensive and prone to variability.[39]
- The test shows prolonged results with factor VIII, IX, XI, and XII deficiency or corresponding inhibitors.[37]
- The test is also highly sensitive to the presence of heparin.[40]
Platelet neutralization procedure: The platelet neutralization procedure (PNP) is based on the ability of platelets to correct in vitro coagulation abnormalities significantly.[41] The disrupted platelet membranes in the freeze-thawed platelet suspension neutralize phospholipid antibodies in the plasma of patients with lupus anticoagulant. After the patient plasma is mixed with the freeze-thawed platelet suspension, the APTT is shortened compared to the original baseline APTT due to increased phospholipid availability and platelet absorption of lupus anticoagulant. However, failure to shorten the clotting time suggests a coagulation factor inhibitor.[42] A correction of the baseline APTT by a defined amount of time, typically 3 to 5 seconds or more, when platelet suspension is added compared to the control suggests the presence of lupus anticoagulant.[41]
The PNP test, although useful, has not gained wide usage due to the following reasons:
- Due to limited stability, the platelet preparations lose their activities on storage and hence do not show reproducible results.[19]
- They cannot differentiate between lupus anticoagulants and factor VIII inhibitors.[17]
Hexagonal phase phospholipid neutralization: The hexagonal phase neutralization assay is a comprehensive test involving the mixing of patient plasma with normal control plasma and subsequent incubation, both with and without hexagonal phase (II) phosphatidylethanolamine (HPE) phospholipid. Subsequently, an APTT analysis is conducted simultaneously on both samples using a lupus anticoagulant-sensitive reagent with a low phospholipid concentration.[43] In cases where lupus anticoagulant is present, its activity is neutralized by the HPE, leading to a shorter clotting time compared to the sample incubated without HPE. The inclusion of normal plasma in the patient mixture serves to correct for any potential factor deficiency. If there is no notable reduction in clotting time in the tube containing HPE, it suggests the presence of a specific factor inhibitor rather than a lupus anticoagulant. The test results are typically reported as the variance in clotting time between the tube lacking HPE and the one containing HPE.[44]
Mixing tests: The mixing study is a crucial test used to distinguish between factor deficiencies and inhibitors, such as lupus anticoagulant, in cases of unexplained prolonged clotting times. The test involves mixing equal parts of patient plasma with normal pooled plasma and then evaluating the clotting time of the mixture. If the clotting time corrects, it suggests a factor deficiency; if it does not correct, it suggests the presence of a circulating inhibitor, such as lupus anticoagulant or specific factor inhibitors.[45]
Confirmatory tests: The principle behind employing confirmatory tests for lupus anticoagulants is based on their ability to counteract the impact of lupus anticoagulant by elevating the phospholipid concentration in the test environment. This counteraction reduces the prolonged coagulation time if attributable to lupus anticoagulant. In contrast, if the prolonged coagulation time results from the presence of an inhibitor targeting one of the coagulation factors, the coagulation time remains prolonged despite the increased phospholipid concentration.[26][46]
The results, expressed as a ratio, are further useful in classifying the patient as having normal, moderate, high, and very high levels of lupus anticoagulant. Once the diagnosis of the antiphospholipid syndrome is confirmed, repeat testing is not required, although some consider retesting with changes in clinical symptoms. If the results of the lupus anticoagulant are borderline, retesting after at least 1 week is suggested.[47]
Testing Procedures
Many guidelines for lupus anticoagulant detection exist, including those from the International Society on Thrombosis and Haemostasis Scientific and Standardization Subcommittee (ISTH-SSC) in 2009. These guidelines are essential for ensuring consistent testing and interpretation of lupus anticoagulant results, particularly in less-than-ideal situations such as during new thrombotic events, including stroke, myocardial infarction, or deep venous thrombosis.[6] Lupus anticoagulant testing could be part of a thrombophilia workup or suspected catastrophic antiphospholipid syndrome, where the clinical decision to start anticoagulation is imperative. The guidelines also recommended testing of prothrombin time, APTT, and thrombin time before lupus anticoagulant testing in cases of unknown clinical and pharmacological history.[3]
British Committee for Standards in Haematology (BCSH) guidelines are less stringent compared to those of the ISTH. The Clinical and Laboratory Standards Institute (CLSI) also has established guidelines.[47]
A prolonged thrombin time may indicate the presence of dabigatran or unfractionated heparin, which can affect lupus anticoagulant testing. Hence, the patient's report should include information about anticoagulation status. Low-molecular-weight heparin bridging is recommended if vitamin K antagonist anticoagulation cannot be withheld for 1 to 2 weeks before lupus anticoagulant testing. Once on low-molecular-weight heparin, lupus anticoagulant testing is performed at nadir levels of anticoagulation, at least 12 hours after the last dose or near the next dose, along with anti-Xa activity levels. In addition, lupus anticoagulant testing is performed after the incubation of plasma with heparinase.[6]
On the other hand, direct oral anticoagulation should be held for at least 48 hours before lupus anticoagulant testing with longer patients with kidney disease. Direct oral anticoagulants can prolong partial thromboplastin time. More specifically, dabigatran has a greater effect on APTT compared to rivaroxaban, whereas apixaban demonstrates the least effect.[3][6] Anticoagulant neutralizers are also employed in some instances.[3][4][12] Antiphospholipid antibody testing, including lupus anticoagulant, also varies in pregnant women; therefore, negative testing does not exclude a diagnosis of antiphospholipid syndrome, especially with high clinical suspicion. Lupus anticoagulant testing is performed more reliably during the first trimester of pregnancy and should be repeated postpartum after at least 6 weeks, and preferably after 3 months postpartum.[6]
In addition to medications and pregnancy, acute thrombotic events can alter lupus anticoagulant testing. Acute thrombotic events can cause increased or decreased APTT with increased levels of factor VIII; therefore, patients should ideally be tested or retested when clinically stable and not in the setting of an acute event.[2][3]
Interfering Factors
Considering the variability of interpreting results and cutoff values, studies have assessed the practices of multiple clinical laboratories regarding lupus anticoagulant testing to ensure the accuracy of results, such as utilizing lyophilized plasmas containing anti-β2GPIb and anticoagulated, lupus anticoagulant-negative plasma.[7] Reliability and uniformity in lupus anticoagulant testing between different laboratories must be evaluated consistently. As noted above, antiphospholipid syndrome–related thrombotic complications are most highly associated with the presence of lupus anticoagulant, even in isolated lupus anticoagulant positivity; hence, accurate measurements are crucial.[2][8]
Results, Reporting, and Critical Findings
Guidelines for reporting and interpreting lupus anticoagulant testing results emphasize the importance of a sequential, multistep testing process, close collaboration between the laboratory and the clinician, and relating the results to the patient's clinical context and ongoing treatment. Results of lupus anticoagulant testing should be reported as positive or negative, and comments such as borderline or dubious lupus anticoagulant are discouraged. Instead, in such cases, the recommendation should be to suggest retesting after 1 week or more without implying a positive or negative lupus anticoagulant. In addition, the results should always be in conjunction with the results of anticardiolipin and anti-β2GPIb to assess the risk profile, and local cutoff values must be reported along with an explanation of the results.[46]
The ISTH-SSC 2009 guidelines suggest using the 99th percentile for cutoff values, with a minimum of 40 donors required. However, accurately estimating the 99th percentile necessitates at least 120 healthy donors, and ideally 300 for statistical robustness. In-house cutoff values may significantly differ from manufacturer recommendations due to various factors.[48] Recent research advocates for nonparametric methods based on centiles for cutoff determination, as clotting times in lupus anticoagulant testing, do not follow a normal distribution. A study found a higher detection rate using the 95th centile compared to the 99th. However, removing outliers had minimal effects on inter-laboratory variability, suggesting that they are not the primary cause of variability.[47] Furthermore, the patient's anticoagulation status should be incorporated into the report, and a close interaction between the laboratory and the clinician is essential.[3]
Clinical Significance
Triple positivity, indicating positive results for lupus anticoagulants, anticardiolipin antibodies, and anti-β2GPIb, is linked to elevated risks of both initial and recurrent thrombotic events. This association is particularly notable in the presence of autoimmune diseases and when the first thrombotic event occurs in asymptomatic carriers. Insufficient diagnostic testing for antiphospholipid syndrome may result in an inaccurate diagnosis, potentially leading to serious consequences if timely interventions are not undertaken.[49] Several recent studies showed that rivaroxaban is ineffective for thrombosis prevention, leading to a recommendation for vitamin K antagonists over direct oral anticoagulants in the setting of triple positivity.[4][15][50]
Clinically, antiphospholipid syndrome is identified by thrombotic events or pregnancy-related complications. Antiphospholipid syndrome is categorized as primary when not linked to other autoimmune diseases and as secondary antiphospholipid syndrome when associated with systemic autoimmune conditions such as systemic lupus erythematosus, rheumatoid arthritis, dermatomyositis, systemic sclerosis, and Sjögren's syndrome.[51]
Antiphospholipid syndrome is associated with high morbidity rates, including cardiovascular events, such as myocardial infarction or cardiac emboli; recurrent miscarriages; intrauterine growth restriction; and venous thrombosis. Other multiorgan systemic effects can be cutaneous, renal, neurologic, and hematologic involvement, including skin ulcers, epilepsy, or autoimmune hemolytic anemia. See StatPearls' companion reference, "Antiphospholipid Syndrome," for further information.
A rare but dreaded life-threatening form of antiphospholipid syndrome is called catastrophic antiphospholipid syndrome.[52] This syndrome is diagnosed by acute clinical manifestations of widespread multiorgan thrombosis developing in less than 1 week, is evidenced by the presence of antiphospholipid antibodies, at least 3-organ involvement, and confirmed by histopathologic occlusion of small vessels. Catastrophic antiphospholipid syndrome is associated with a high mortality rate of 30% to 50% regardless of treatment.[53]
In terms of other clinical scenarios involving lupus anticoagulant, a retrospective analysis studied the possible confounding effects of lupus anticoagulants in patients who received extracorporeal membrane oxygenation while on anticoagulation.[54] The analysis found that lupus anticoagulant-positive patients did not exhibit a greater risk for developing thromboembolic complications compared to lupus anticoagulant-negative patients, but patients with lupus anticoagulants required a longer duration for extracorporeal membrane oxygenation treatment. Nevertheless, the correlation of this outcome cannot be confirmed due to the direct effect of lupus anticoagulant or merely the severity of the course of disease among these patients. Interestingly, lupus anticoagulants were also positive for patients with viral respiratory infections in this study. However, retesting after 1 to 6 months showed negative lupus anticoagulant, implying a transient presence in viral respiratory infections.[55]
In another study, lupus anticoagulant was observed to be positive among patients with SARS-CoV-2 with prolonged APTT. About 90% were positive for lupus anticoagulant, and most of these patients were also associated with factor XII deficiency. However, it is unclear if any association occurs between the presence of lupus anticoagulant and COVID-19 thrombosis.[56][57]
Quality Control and Lab Safety
A quality management system in a clinical laboratory is essential for ensuring the accuracy and reliability of test results, which directly impact patient care and diagnosis. This system involves established procedures for every testing step, from sample collection to result interpretation, to minimize the risk of errors and inconsistencies. Regular calibration and maintenance of equipment are also crucial, as they produce reliable results and reduce the chance of technical errors.[58] Key strategies for establishing a quality management system in a laboratory setting encompass organizational structuring, personnel training, equipment calibration and maintenance, method validation, quality control (QC) measures, and maintaining satisfactory customer communication.[59]
QC is an integral part of a quality management system in a clinical laboratory. QC includes both internal and external components. Internal QC includes control procedures performed within a laboratory using surrogate samples intended to simulate clinical samples from patients to calibrate the machinery.[60] The QC samples are measured at intervals along with patient samples. Correlation with expected target values for the QC samples allows the laboratory to verify that a measurement procedure is working correctly and that the results for patient samples are reliable.[61][62]
External QC, also called external quality assessment (EQA) or proficiency testing (PT), is a process that allows laboratories to assess the reliability and accuracy of their testing. EQA/PT providers circulate a set of test samples among a group of laboratories. Each laboratory measures the EQA/PT samples as patient samples and reports the results to the EQA/PT provider for evaluation.[63] The EQA/PT provider assigns a target value to determine whether the results obtained by an individual laboratory consistently agree with the target values for acceptable measurement procedure performance.[64]
All personnel must undergo comprehensive training in safety protocols, hazard awareness, and emergency procedures. The use of appropriate personal protective equipment, such as gloves, lab coats, eye protection, and respiratory masks, is essential based on the specific hazards involved. Hazardous waste must be segregated, labeled, and disposed of in compliance with regulations. A well-defined emergency plan, coupled with easily accessible safety equipment, is critical for handling accidents or spills. Prompt reporting of safety incidents ensures thorough investigation and corrective actions. In a clinical laboratory, safety is a collective responsibility. Adhering to protocols and fostering a safety culture protect staff well-being and enhance the precision and reliability of results, contributing to improved patient care.[65][66]
Enhancing Healthcare Team Outcomes
The importance of diagnostic testing for antiphospholipid antibodies requires multi-specialty participation and interprofessional management, starting with primary care providers and moving to inpatient and emergency settings. Appropriate referrals to rheumatologists and hematologists in the setting of connective tissue disease and thrombotic events should be performed promptly.
Depending on the affected organs, specialists such as neurologists, nephrologists, obstetricians, gynecologists, and cardiologists are crucial to ensure a comprehensive approach to patient care and mitigate the risk of potential catastrophic events associated with the syndrome. The accuracy of laboratory technician reports depends on appropriate, close communication between healthcare professionals, nurses, pharmacists, and laboratory staff in the presence of ongoing anticoagulation and other external factors. An interdisciplinary approach is essential to properly interpret results for overall management and determine the patient's clinical treatment and prognosis.
Media
(Click Image to Enlarge)
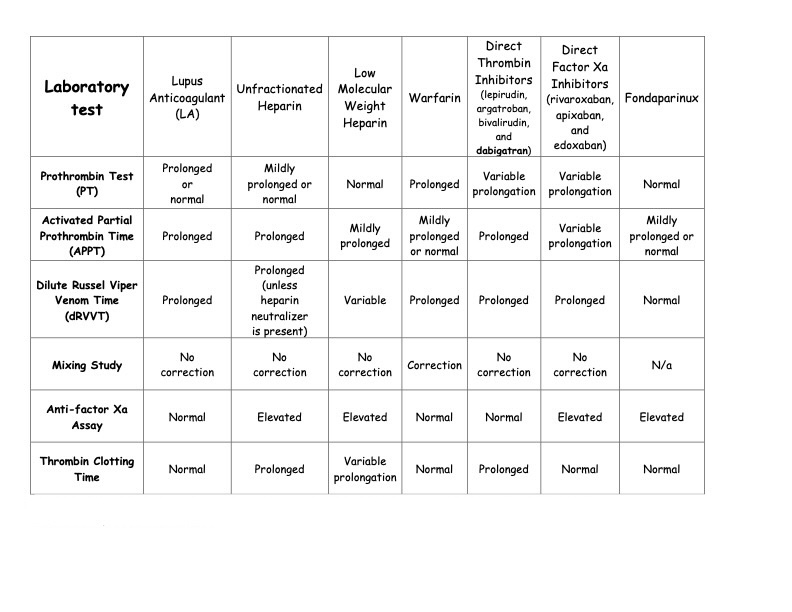
The effect of lupus anticoagulant and various anticoagulation medications, including warfarin, dabigatran, and other direct oral anticoagulants, on various clotting and anticoagulation tests.
Table: Effect of Lupus Anticoagulant and Anticoagulants on Laboratory Testing. Adapted from Ortel TL. Laboratory diagnosis of the lupus anticoagulant. Curr Rheumatol Rep. 2012;14(1):64-70. doi: 10.1007/1 1926-011-0225-3.
References
Cohen H, Mackie IJ, Devreese KMJ, International Society for Thrombosis and Haemostasis Scientific and Standardization Committee for Lupus Anticoagulant/Antiphospholipid Antibodies. Clinical and laboratory practice for lupus anticoagulant testing: An International Society of Thrombosis and Haemostasis Scientific and Standardization Committee survey. Journal of thrombosis and haemostasis : JTH. 2019 Oct:17(10):1715-1732. doi: 10.1111/jth.14560. Epub 2019 Aug 1 [PubMed PMID: 31271706]
Level 3 (low-level) evidenceOrtel TL. Antiphospholipid syndrome: laboratory testing and diagnostic strategies. American journal of hematology. 2012 May:87 Suppl 1(Suppl 1):S75-81. doi: 10.1002/ajh.23196. Epub 2012 Mar 31 [PubMed PMID: 22473619]
Tripodi A, Cohen H, Devreese KMJ. Lupus anticoagulant detection in anticoagulated patients. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis. Journal of thrombosis and haemostasis : JTH. 2020 Jul:18(7):1569-1575. doi: 10.1111/jth.14846. Epub [PubMed PMID: 32619349]
Tripodi A, Scalambrino E, Clerici M, Peyvandi F. Laboratory Diagnosis of Antiphospholipid Syndrome in Anticoagulated Patients. Biomedicines. 2023 Jun 19:11(6):. doi: 10.3390/biomedicines11061760. Epub 2023 Jun 19 [PubMed PMID: 37371855]
Devreese KMJ, Zuily S, Meroni PL. Role of antiphospholipid antibodies in the diagnosis of antiphospholipid syndrome. Journal of translational autoimmunity. 2021:4():100134. doi: 10.1016/j.jtauto.2021.100134. Epub 2021 Nov 6 [PubMed PMID: 34816113]
Favaloro EJ, Pasalic L. Lupus anticoagulant testing during anticoagulation, including direct oral anticoagulants. Research and practice in thrombosis and haemostasis. 2022 Feb:6(2):e12676. doi: 10.1002/rth2.12676. Epub 2022 Mar 15 [PubMed PMID: 35316943]
Tripodi A, Biasiolo A, Chantarangkul V, Pengo V. Lupus anticoagulant (LA) testing: performance of clinical laboratories assessed by a national survey using lyophilized affinity-purified immunoglobulin with LA activity. Clinical chemistry. 2003 Oct:49(10):1608-14 [PubMed PMID: 14500585]
Level 3 (low-level) evidenceHeikal N, Martins TB, White SK, Willis R, Ware Branch D, Schmidt RL, Tebo AE. Laboratory Evaluation of Antiphospholipid Syndrome. American journal of clinical pathology. 2019 Oct 7:152(5):638-646. doi: 10.1093/ajcp/aqz085. Epub [PubMed PMID: 31305881]
Apipongrat D, Lamool R, Arnutti P, Ruangpratheep C, Chantkran W. Comparison of different algorithms for lupus anticoagulant detection: a single-center experience. Research and practice in thrombosis and haemostasis. 2024 Jan:8(1):102333. doi: 10.1016/j.rpth.2024.102333. Epub 2024 Jan 30 [PubMed PMID: 38404944]
Petri MA, Avci M, Magder LS. Evaluation of different ways to identify persistent positivity of lupus anticoagulant in systemic lupus erythematosus. Lupus science & medicine. 2020 Nov:7(1):. doi: 10.1136/lupus-2020-000406. Epub [PubMed PMID: 33139453]
Capozzi A, Manganelli V, Riitano G, Recalchi S, Truglia S, Alessandri C, Longo A, Garofalo T, Misasi R, Valesini G, Conti F, Sorice M. Tissue factor over-expression in platelets of patients with anti-phospholipid syndrome: induction role of anti-β2-GPI antibodies. Clinical and experimental immunology. 2019 Apr:196(1):59-66. doi: 10.1111/cei.13248. Epub 2019 Jan 15 [PubMed PMID: 30549270]
Park SH, Jang S, Park CJ, Chi HS. Clinical Application of Revised Laboratory Classification Criteria for Antiphospholipid Antibody Syndrome: Is the Follow-Up Interval of 12 Weeks Instead of 6 Weeks Significantly Useful? BioMed research international. 2016:2016():2641526. doi: 10.1155/2016/2641526. Epub 2016 Aug 17 [PubMed PMID: 27610369]
Kempers EK, Dalm VASH, van Rijn MJE, Mulders AGMGJ, Leebeek FWG, de Maat MPM, Jansen AJG. Indication and outcome of lupus anticoagulant and antiphospholipid antibodies testing in routine clinical practice. Rheumatology advances in practice. 2021:5(3):rkab093. doi: 10.1093/rap/rkab093. Epub 2021 Nov 27 [PubMed PMID: 34917873]
Level 3 (low-level) evidenceTripodi A. Laboratory testing for lupus anticoagulants: a review of issues affecting results. Clinical chemistry. 2007 Sep:53(9):1629-35 [PubMed PMID: 17712001]
Magnette A, Chatelain M, Chatelain B, Ten Cate H, Mullier F. Pre-analytical issues in the haemostasis laboratory: guidance for the clinical laboratories. Thrombosis journal. 2016:14():49. doi: 10.1186/s12959-016-0123-z. Epub 2016 Dec 12 [PubMed PMID: 27999475]
Pappas AA, Palmer SK, Meece D, Fink LM. Rapid preparation of plasma for coagulation testing. Archives of pathology & laboratory medicine. 1991 Aug:115(8):816-7 [PubMed PMID: 1863193]
Molinari AC, Martini T, Banov L, Ierardi A, Leotta M, Strangio A, Santoro RC. Lupus Anticoagulant Detection under the Magnifying Glass. Journal of clinical medicine. 2023 Oct 20:12(20):. doi: 10.3390/jcm12206654. Epub 2023 Oct 20 [PubMed PMID: 37892792]
Knight JS, Branch DW, Ortel TL. Antiphospholipid syndrome: advances in diagnosis, pathogenesis, and management. BMJ (Clinical research ed.). 2023 Feb 27:380():e069717. doi: 10.1136/bmj-2021-069717. Epub 2023 Feb 27 [PubMed PMID: 36849186]
Level 3 (low-level) evidenceTripodi A. Diagnostic Challenges on the Laboratory Detection of Lupus Anticoagulant. Biomedicines. 2021 Jul 20:9(7):. doi: 10.3390/biomedicines9070844. Epub 2021 Jul 20 [PubMed PMID: 34356908]
Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, MacDonald V, Green L, Sivapalaratnam S, Pasi KJ, MacCallum P. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. The New England journal of medicine. 2020 Jul 16:383(3):288-290. doi: 10.1056/NEJMc2013656. Epub 2020 May 5 [PubMed PMID: 32369280]
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, Diamond B, Gladman DD, Hahn B, Hiepe F, Jacobsen S, Khanna D, Lerstrøm K, Massarotti E, McCune J, Ruiz-Irastorza G, Sanchez-Guerrero J, Schneider M, Urowitz M, Bertsias G, Hoyer BF, Leuchten N, Tani C, Tedeschi SK, Touma Z, Schmajuk G, Anic B, Assan F, Chan TM, Clarke AE, Crow MK, Czirják L, Doria A, Graninger W, Halda-Kiss B, Hasni S, Izmirly PM, Jung M, Kumánovics G, Mariette X, Padjen I, Pego-Reigosa JM, Romero-Diaz J, Rúa-Figueroa Fernández Í, Seror R, Stummvoll GH, Tanaka Y, Tektonidou MG, Vasconcelos C, Vital EM, Wallace DJ, Yavuz S, Meroni PL, Fritzler MJ, Naden R, Dörner T, Johnson SR. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis & rheumatology (Hoboken, N.J.). 2019 Sep:71(9):1400-1412. doi: 10.1002/art.40930. Epub 2019 Aug 6 [PubMed PMID: 31385462]
Quehenberger P, Wagner O. [Laboratory diagnosis of the lupus anticoagulants]. Hamostaseologie. 2005 Feb:25(1):50-4 [PubMed PMID: 15711720]
Sheng Y, Hanly JG, Reddel SW, Kouts S, Guerin J, Koike T, Ichikawa K, Sturgess A, Krilis SA. Detection of 'antiphospholipid' antibodies: a single chromogenic assay of thrombin generation sensitively detects lupus anticoagulants, anticardiolipin antibodies, plus antibodies binding beta(2)-glycoprotein I and prothrombin. Clinical and experimental immunology. 2001 Jun:124(3):502-8 [PubMed PMID: 11472415]
Reshetnyak T, Cheldieva F, Cherkasova M, Lila A, Nasonov E. IgA Antiphospholipid Antibodies in Antiphospholipid Syndrome and Systemic Lupus Erythematosus. International journal of molecular sciences. 2022 Aug 21:23(16):. doi: 10.3390/ijms23169432. Epub 2022 Aug 21 [PubMed PMID: 36012697]
Pengo V, Bison E, Banzato A, Zoppellaro G, Jose SP, Denas G. Lupus Anticoagulant Testing: Diluted Russell Viper Venom Time (dRVVT). Methods in molecular biology (Clifton, N.J.). 2017:1646():169-176. doi: 10.1007/978-1-4939-7196-1_14. Epub [PubMed PMID: 28804828]
Triplett DA. Use of the dilute Russell viper venom time (dRVVT): its importance and pitfalls. Journal of autoimmunity. 2000 Sep:15(2):173-8 [PubMed PMID: 10968905]
Smit B, Hudig F, Venhuizen JH, Haitjema S, Limper M, Urbanus R, Huisman A. Routine Lupus Anticoagulant Sensitive aPTT Testing Can Prevent Unnecessary LA Testing. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2023 Jan-Dec:29():10760296231183427. doi: 10.1177/10760296231183427. Epub [PubMed PMID: 37322895]
Sun H, Yang S, Li P, Shang X, Wang P, Zhang J, Yuan L, Yin R, Gao N, Zhao J. Comparative Assessment of APTT Reagents for Evaluating Anticoagulant Sensitivity of Fucosylated Glycosaminoglycans (FGs) Derived from Sea Cucumbers. Marine drugs. 2023 Oct 29:21(11):. doi: 10.3390/md21110568. Epub 2023 Oct 29 [PubMed PMID: 37999392]
Level 2 (mid-level) evidenceSantoro RC, Molinari AC, Leotta M, Martini T. Isolated Prolongation of Activated Partial Thromboplastin Time: Not Just Bleeding Risk! Medicina (Kaunas, Lithuania). 2023 Jun 17:59(6):. doi: 10.3390/medicina59061169. Epub 2023 Jun 17 [PubMed PMID: 37374373]
Moore GW, Savidge GF. The dilution effect of equal volume mixing studies compromises confirmation of inhibition by lupus anticoagulants even when mixture specific reference ranges are applied. Thrombosis research. 2006:118(4):523-8 [PubMed PMID: 16263154]
Eikelboom JW, Hirsh J. Monitoring unfractionated heparin with the aPTT: time for a fresh look. Thrombosis and haemostasis. 2006 Nov:96(5):547-52 [PubMed PMID: 17080209]
Balandina AN, Serebriyskiy II, Poletaev AV, Polokhov DM, Gracheva MA, Koltsova EM, Vardanyan DM, Taranenko IA, Krylov AY, Urnova ES, Lobastov KV, Chernyakov AV, Shulutko EM, Momot AP, Shulutko AM, Ataullakhanov FI. Thrombodynamics-A new global hemostasis assay for heparin monitoring in patients under the anticoagulant treatment. PloS one. 2018:13(6):e0199900. doi: 10.1371/journal.pone.0199900. Epub 2018 Jun 28 [PubMed PMID: 29953528]
Arnout J, Vanrusselt M, Huybrechts E, Vermylen J. Optimization of the dilute prothrombin time for the detection of the lupus anticoagulant by use of a recombinant tissue thromboplastin. British journal of haematology. 1994 May:87(1):94-9 [PubMed PMID: 7947261]
Eschwège V, Seddiki S, Robert A. The tissue thromboplastin inhibition test in the detection of lupus anticoagulants: importance of a correction factor eliminating the influence of fibrinogen level. Thrombosis and haemostasis. 1996 Jul:76(1):65-8 [PubMed PMID: 8819253]
Gerbutavicius R, Fareed J, Messmore HL Jr, Iqbal O, Hoppensteadt DA, Wehrmacher WH, Demir M, Piccolo P, Ahmad S, Ma Q, Griniute R. Reference intervals of the dilute tissue thromboplastin inhibition and dilute Russell's viper venom tests revisited. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2002 Apr:8(2):115-24 [PubMed PMID: 12121051]
Sciascia S, Baldovino S, Schreiber K, Solfietti L, Radin M, Cuadrado MJ, Menegatti E, Erkan D, Roccatello D. Thrombotic risk assessment in antiphospholipid syndrome: the role of new antibody specificities and thrombin generation assay. Clinical and molecular allergy : CMA. 2016:14():6. doi: 10.1186/s12948-016-0043-2. Epub 2016 Jul 15 [PubMed PMID: 27429595]
Radhakrishnan K. Kaolin clotting time. Methods in molecular biology (Clifton, N.J.). 2013:992():335-9. doi: 10.1007/978-1-62703-339-8_25. Epub [PubMed PMID: 23546725]
Rosove MH, Ismail M, Koziol BJ, Runge A, Kasper CK. Lupus anticoagulants: improved diagnosis with a kaolin clotting time using rabbit brain phospholipid in standard and high concentrations. Blood. 1986 Aug:68(2):472-8 [PubMed PMID: 3730611]
Dragoni F, Minotti C, Palumbo G, Faillace F, Redi R, Bongarzoni V, Avvisati G. As compared to kaolin clotting time, silica clotting time is a specific and sensitive automated method for detecting lupus anticoagulant. Thrombosis research. 2001 Jan 15:101(2):45-51 [PubMed PMID: 11342205]
Despotis GJ, Alsoufiev AL, Spitznagel E, Goodnough LT, Lappas DG. Response of kaolin ACT to heparin: evaluation with an automated assay and higher heparin doses. The Annals of thoracic surgery. 1996 Mar:61(3):795-9 [PubMed PMID: 8619695]
Radhakrishnan K. Platelet neutralization test. Methods in molecular biology (Clifton, N.J.). 2013:992():349-51. doi: 10.1007/978-1-62703-339-8_27. Epub [PubMed PMID: 23546727]
Triplett DA, Brandt JT, Kaczor D, Schaeffer J. Laboratory diagnosis of lupus inhibitors: a comparison of the tissue thromboplastin inhibition procedure with a new platelet neutralization procedure. American journal of clinical pathology. 1983 Jun:79(6):678-82 [PubMed PMID: 6846258]
Triplett DA, Barna LK, Unger GA. A hexagonal (II) phase phospholipid neutralization assay for lupus anticoagulant identification. Thrombosis and haemostasis. 1993 Nov 15:70(5):787-93 [PubMed PMID: 8128436]
Chandler JB, Torres R, Rinder HM, Tormey CA. Lupus anticoagulant testing and anticoagulation do not mix: quantitation of discrepant results and potential approaches to reduce false positives. British journal of haematology. 2014 Dec:167(5):704-7. doi: 10.1111/bjh.13030. Epub 2014 Jul 18 [PubMed PMID: 25041401]
Moore GW. Mixing studies for lupus anticoagulant: mostly no, sometimes yes. Clinical chemistry and laboratory medicine. 2020 Mar 26:58(4):492-495. doi: 10.1515/cclm-2019-1248. Epub [PubMed PMID: 31874095]
Devreese KMJ, de Groot PG, de Laat B, Erkan D, Favaloro EJ, Mackie I, Martinuzzo M, Ortel TL, Pengo V, Rand JH, Tripodi A, Wahl D, Cohen H. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. Journal of thrombosis and haemostasis : JTH. 2020 Nov:18(11):2828-2839. doi: 10.1111/jth.15047. Epub [PubMed PMID: 33462974]
Moore GW. Testing for Lupus Anticoagulants. Seminars in thrombosis and hemostasis. 2022 Sep:48(6):643-660. doi: 10.1055/s-0042-1744363. Epub 2022 Jun 1 [PubMed PMID: 35649428]
Talon L, Fourneyron V, Senectaire S, Tardieu M, Tillier M, Trapani A, Trayaud A, Vaissade A, Sapin AF, Lebreton A, Sinegre T. Lupus anticoagulant laboratory diagnosis by applying the 2020 ISTH-SSC guidelines. Thrombosis research. 2023 Apr:224():38-45. doi: 10.1016/j.thromres.2023.02.009. Epub 2023 Feb 18 [PubMed PMID: 36827954]
Chayoua W, Kelchtermans H, Moore GW, Musiał J, Wahl D, de Laat B, Devreese KMJ. Identification of high thrombotic risk triple-positive antiphospholipid syndrome patients is dependent on anti-cardiolipin and anti-β2glycoprotein I antibody detection assays. Journal of thrombosis and haemostasis : JTH. 2018 Oct:16(10):2016-2023. doi: 10.1111/jth.14261. Epub 2018 Aug 24 [PubMed PMID: 30079628]
Capecchi M, Abbattista M, Ciavarella A, Uhr M, Novembrino C, Martinelli I. Anticoagulant Therapy in Patients with Antiphospholipid Syndrome. Journal of clinical medicine. 2022 Nov 26:11(23):. doi: 10.3390/jcm11236984. Epub 2022 Nov 26 [PubMed PMID: 36498557]
Gardiner C, Hills J, Machin SJ, Cohen H. Diagnosis of antiphospholipid syndrome in routine clinical practice. Lupus. 2013 Jan:22(1):18-25. doi: 10.1177/0961203312460722. Epub 2012 Sep 17 [PubMed PMID: 22988029]
Nayer A, Ortega LM. Catastrophic antiphospholipid syndrome: a clinical review. Journal of nephropathology. 2014 Jan:3(1):9-17. doi: 10.12860/jnp.2014.03. Epub 2014 Jan 1 [PubMed PMID: 24644537]
Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo MC, Amoura Z, Andrade D, Andreoli L, Artim-Esen B, Atsumi T, Avcin T, Belmont HM, Bertolaccini ML, Branch DW, Carvalheiras G, Casini A, Cervera R, Cohen H, Costedoat-Chalumeau N, Crowther M, de Jesus G, Delluc A, Desai S, De Sancho M, Devreese KM, Diz-Kucukkaya R, Duarte-Garcia A, Frances C, Garcia D, Gris JC, Jordan N, Leaf RK, Kello N, Knight JS, Laskin C, Lee AI, Legault K, Levine SR, Levy RA, Limper M, Lockshin MD, Mayer-Pickel K, Musial J, Meroni PL, Orsolini G, Ortel TL, Pengo V, Petri M, Pons-Estel G, Gomez-Puerta JA, Raimboug Q, Roubey R, Sanna G, Seshan SV, Sciascia S, Tektonidou MG, Tincani A, Wahl D, Willis R, Yelnik C, Zuily C, Guillemin F, Costenbader K, Erkan D, ACR/EULAR APS Classification Criteria Collaborators. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis & rheumatology (Hoboken, N.J.). 2023 Oct:75(10):1687-1702. doi: 10.1002/art.42624. Epub 2023 Aug 28 [PubMed PMID: 37635643]
Kornfehl A, Brock R, Staudinger T, Schellongowski P, Nagler B, Hermann A, Robak O, Schwameis M, Quehenberger P, Buchtele N. Prevalence and Impact of Lupus Anticoagulant in Patients Receiving Extracorporeal Membrane Oxygenation. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2023 Jan-Dec:29():10760296231207062. doi: 10.1177/10760296231207062. Epub [PubMed PMID: 37853541]
Woo S, Kim B, Heo NH, Kim MS, Yoon YA, Choi YJ. Association of Lupus Anticoagulant status with Disease Course in SARS-CoV-2 (COVID-19) Infection. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2022 Jan-Dec:28():10760296221127276. doi: 10.1177/10760296221127276. Epub [PubMed PMID: 36172745]
Favaloro EJ, Henry BM, Lippi G. Is Lupus Anticoagulant a Significant Feature of COVID-19? A Critical Appraisal of the Literature. Seminars in thrombosis and hemostasis. 2022 Feb:48(1):55-71. doi: 10.1055/s-0041-1729856. Epub 2021 Jun 15 [PubMed PMID: 34130341]
Al-Ahmad M, Al Rasheed M, Altourah L, Rodriguez-Bouza T, Shalaby N. Lupus anticoagulant activity and thrombosis post COVID-19 vaccination. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2023 Jan 1:34(1):75-78. doi: 10.1097/MBC.0000000000001161. Epub 2022 Aug 9 [PubMed PMID: 35946452]
Pillai S, Calvert J, Fox E. Practical considerations for laboratories: Implementing a holistic quality management system. Frontiers in bioengineering and biotechnology. 2022:10():1040103. doi: 10.3389/fbioe.2022.1040103. Epub 2022 Nov 3 [PubMed PMID: 36406233]
Level 2 (mid-level) evidenceCarey RB, Bhattacharyya S, Kehl SC, Matukas LM, Pentella MA, Salfinger M, Schuetz AN. Practical Guidance for Clinical Microbiology Laboratories: Implementing a Quality Management System in the Medical Microbiology Laboratory. Clinical microbiology reviews. 2018 Jul:31(3):. doi: 10.1128/CMR.00062-17. Epub 2018 May 2 [PubMed PMID: 29720490]
Level 2 (mid-level) evidenceKinns H, Pitkin S, Housley D, Freedman DB. Internal quality control: best practice. Journal of clinical pathology. 2013 Dec:66(12):1027-32. doi: 10.1136/jclinpath-2013-201661. Epub 2013 Sep 26 [PubMed PMID: 24072731]
Level 2 (mid-level) evidenceWestgard JO. Internal quality control: planning and implementation strategies. Annals of clinical biochemistry. 2003 Nov:40(Pt 6):593-611 [PubMed PMID: 14629798]
Level 2 (mid-level) evidenceKearney E. Internal quality control. Methods in molecular biology (Clifton, N.J.). 2013:1065():277-89. doi: 10.1007/978-1-62703-616-0_18. Epub [PubMed PMID: 23996371]
Level 2 (mid-level) evidenceJames D, Ames D, Lopez B, Still R, Simpson W, Twomey P. External quality assessment: best practice. Journal of clinical pathology. 2014 Aug:67(8):651-5. doi: 10.1136/jclinpath-2013-201621. Epub 2014 Mar 12 [PubMed PMID: 24621574]
Level 2 (mid-level) evidenceKristensen GB, Meijer P. Interpretation of EQA results and EQA-based trouble shooting. Biochemia medica. 2017 Feb 15:27(1):49-62. doi: 10.11613/BM.2017.007. Epub [PubMed PMID: 28392726]
Meisenhelder J, Bursik S, Lunn G, Strober W. Laboratory safety. Current protocols in human genetics. 2008 Apr:Appendix 2():Appendix 2A. doi: 10.1002/0471142905.hga02as57. Epub [PubMed PMID: 18428418]
Asiry S, Ang LC. Laboratory Safety: Chemical and Physical Hazards. Methods in molecular biology (Clifton, N.J.). 2019:1897():243-252. doi: 10.1007/978-1-4939-8935-5_21. Epub [PubMed PMID: 30539449]
