Introduction
The different clinical presentations of cardiac arrhythmias reflect either reduced blood pressure or cardiac output, or both, as a direct result of the rhythm abnormality. Unlike most chronic diseases, hypertension, for example, rhythm disorders frequently behave intermittently, in an all-or-none fashion, chronic persistent or permanent atrial fibrillation being the major exception.
The impact of an arrhythmia is usually only evident during the arrhythmia. Symptoms of arrhythmia could be most pronounced at the onset or the termination of an intermittent arrhythmia. While an underlying chronic disease may be continuously present, any arrhythmia caused by it usually emerges entirely randomly and unpredictably.
Although symptoms and their severity vary considerably, the consequence of a rhythm disturbance tends to be rather binary: nuisance or death. For patient safety, our strategy should focus initially on the latter. Fortunately, the majority of arrhythmias fall within the nuisance category. Atrial fibrillation remains the exception: it can be present continuously, and significant consequences lie between nuisance and death, such as stroke and heart failure.
The approach outlined here is a suggested one. The very first encounter with an arrhythmia patient could be someone handing you a rhythm strip wherein no clinical information is available. We should keep three goals in mind while approaching a patient with suspected arrhythmia. Once completed, we can initiate a treatment strategy.
- Establish probable cause that an arrhythmia exists
- Determine whether there may be a risk of dying
- Document the arrhythmia
Establishing probable cause refers to the likelihood that an arrhythmia exists and is based mainly on symptoms. A risk assessment is undertaken if there is a reasonable concern that death may be an outcome because of a personal history of structural heart disease, a family history of sudden death, or, in the case of primary arrhythmia syndromes, unique ECG abnormalities. An asymptomatic arrhythmia should not preclude the need for a risk assessment.
Making the diagnosis means documenting the arrhythmia and, in particular, linking it to the patient's symptoms via some form of ambulatory monitoring. Alternatively, one can pursue an electrophysiology study (EPS) to elicit or provoke a sustained arrhythmia if it doesn't occur spontaneously and is felt to place the patient at risk. The EPS may be the only means to discover abnormalities in the conduction system, such as a prolonged HV interval, that would never be obvious from an ECG tracing. Such findings are usually associated with bradyarrhythmias.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Establishing Probable Cause that an Arrhythmia Exists
In the outpatient setting, this mainly derives from reported symptoms. Symptoms can be considered mild, moderate, or severe. Mild symptoms are generally well-tolerated and include palpitation, activity intolerance, dyspnea, or chest discomfort. Moderate symptoms include near-syncope and syncope. The most severe sign is the life-threatening condition of a nonfatal cardiac arrest.
Palpitation
Palpitation is a sensation of the strength of the heart's contraction. The adventitia surrounding the aorta and great arteries leading to the shoulders and neck, as well as the parietal pericardium surrounding the heart, have sensory nerve endings capable of delivering nociceptive signals to the brain.[1] Usually, we have no awareness of the heartbeat because the contractile force of each beat is modest and because the structures surrounding the heart and great vessels are not stretched enough to elicit sensory impulses.
Signals from mechanoreceptors, specifically the Ruffini corpuscles that sense stretch, travel along the general visceral afferent (GVA) axons to join with the general somatic afferent (GSA) fibers in the spinal cord. They join at cord levels belonging to similar anatomic locations, such as the neck or chest wall, to give identity to a part of the body the sensations are perceived to be coming from, similar to the referred pain concept.[2] When the left ventricle suddenly contracts more strongly, the resulting larger bolus of blood expands the great arteries or increases the physical movement of the heart within the pericardium and can be easily sensed.
How arrhythmias produce changes in the strength of contraction is related to abrupt changes in the rhythm's cycle length, the interval between beats. The Frank-Starling law of the heart or the length-tension relationship states that when the interval between two beats lengthens, the force of contraction of the second beat will be greater, leading to its higher developed pressure and stroke volume.[3] Cycle-lengthening permits greater left-ventricular filling and increased myofiber stretch, producing greater actin-myosin overlap and eventually leading to increased LV pressure and stroke volume.
An isolated pause, as seen with sinus node dysfunction or second-degree AV block, is a common clinical scenario. In addition to the "thump or thud" sensation, the patient may also have an impression of the pause.
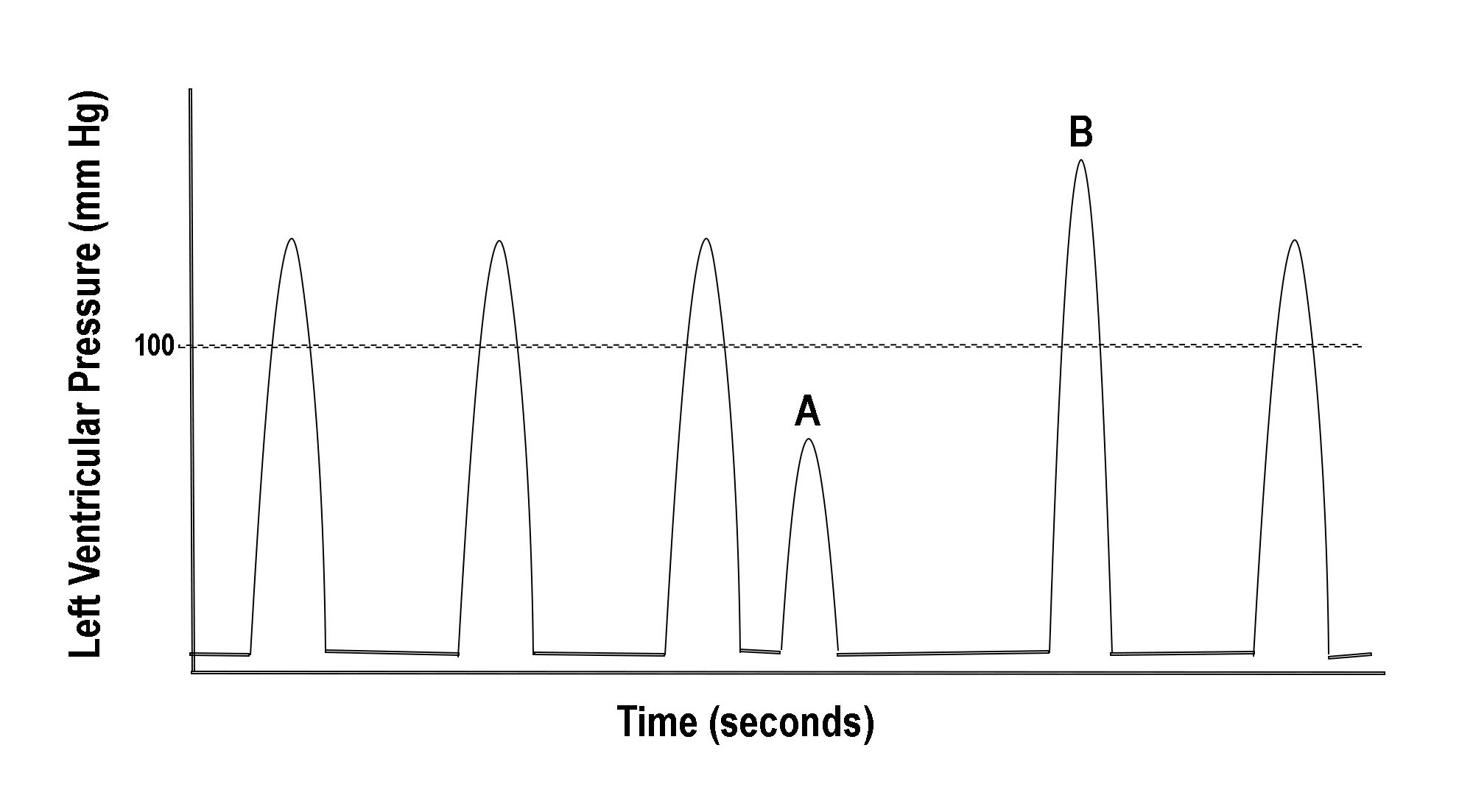
Figure 1 illustrates another example wherein changing cycle length can produce palpitation. A short cycle due to a premature complex (A) is followed by a long cycle known as a compensatory pause. The pause ends with a beat having significantly higher and more rapidly developed pressure (B). The higher pressure response is referred to as post-extrasystolic potentiation (PESP) and can be explained by two mechanisms.[4]
One is the transiently increased calcium available to the cardiac myocyte caused by a sudden cycle shortening. This additional cytosolic calcium increases the rate of pressure development of the post-extrasystolic beat (B). The second and more influential mechanism is due to the compensatory pause and the associated increase in the degree of actin-myosin filament overlap.[5]
In addition to the higher rate of pressure development, the peak developed pressure due to the long cycle is also increased. Thus, abruptly changing cycle lengths from any cause, such as that commonly seen in atrial fibrillation, will produce palpitation by one or both of these mechanisms. If cycle length changes are frequent, the patient will more likely be aware of an "irregular heartbeat."
Palpitation is a nonspecific symptom caused by various arrhythmias, including transient sinus pauses, PACs, PVCs, intermittent second-degree AV block, and nonsustained tachyarrhythmias. Thus, palpitation provides no insight as to whether the patient is developing a tendency toward tachyarrhythmia, bradyarrhythmia, or neither.
However, patients with episodes of tachycardia often recognize they are experiencing a series of consecutive beats with short cycle lengths producing the sensation of a "racing heart." In history-taking, one should attempt to distinguish ectopy from the more significant problem of tachycardia by asking the patient about a racing heart.
A patient may complain of palpitation, but when a monitor is issued, there is no arrhythmia connected to any symptom. This is commonly referred to as cardiac awareness and is usually due to transient, adrenergically-mediated increases in sinus rate or stroke volume. It can also be due to arterial pulse pressure widening of any cause. An unexpected explanation for palpitation is skeletal muscle fasciculation in the neck or intercostal space, creating the impression that something deeper in the thorax is causing the sensation. Advise the patient to look specifically at that body part, in the mirror if necessary, the next time it happens.
The drama of palpitation is related to the mystery of the symptom's cause, which is generally unknown. Fear of the unknown produces anxiety. The more anxiety a patient has, the more likely their palpitation problem will come front and center. In the case of cardiac awareness, it is important to stress to the patient that this problem is not "in their head"; it is real. In your discussions, emphasis should be on the reassurance that they don't have an arrhythmia.
Activity or Exercise Intolerance
Arrhythmias are classified as tachyarrhythmias or bradyarrhythmias. The impact either rhythm disturbance has on blood pressure or cardiac output will explain why heart rate extremes interfere with the ability to engage in physical activity.
Low heart rate (HR) has little effect on blood pressure or may increase it, but it decreases cardiac output (CO). CO, the product of SV and HR, is the amount of blood flowing throughout the body, measured in L/minute, and is a measure of "volume flow rate." Unlike stroke volume, heart rate can enable far greater adjustments in CO when needed, such as during exercise. If SV was 80 ml and remained unchanged, an increase in HR from 50 to 150 bpm would raise CO from 4 liters/min to 12 liters/min. Stroke volume cannot provide for such wide-ranging potential.
Weakness and fatigue can occur at rest or following exercise. Low blood pressure explains such symptoms at rest, but lightheadedness would be a co-complaint. New or excessive fatigue in response to exercise may more readily suggest an abnormal cardiovascular response involving inadequate CO. Lightheadedness accompanying exercise indicates hypotension and suggests an excess drug effect or orthostatic intolerance. It may also raise the specter of left-main coronary obstruction or LV outlet obstruction in the cases of aortic stenosis and hypertrophic obstructive cardiomyopathy. Heart rate extremes can also produce weakness/fatigue at rest or during exercise.
- When the Heart Rate is Too Slow: A Problem of Inadequate Cardiac Output
Resting cardiac output is typically 4 to 5 liters/min, but one could easily be satisfied with values as low as 2 L/minute. However, with exercise, if HR did not increase to allow CO to meet the heightened skeletal muscle metabolic demands, one would feel exhausted just going up a flight of stairs. SV increases cannot match HR increases as a means of improving CO. The inability of HR to increase to a level commensurate with a given level of exercise is called chronotropic incompetence. Its most common symptoms are fatigue and weakness. Chronotropic incompetence can produce symptoms among patients with normal cardiac structure and function.
Since a reduced HR alone is unlikely to cause hypotension, near-syncope or syncope are not expected manifestations of pure bradycardia. However, two conditions associated with bradycardia always result in hypotension. One is neurally-mediated syncope (NMS), in which reduced systemic vascular resistance (SVR) accompanies the bradycardia, and the other is a long pause. Both are discussed under the topic of near-syncope and syncope.
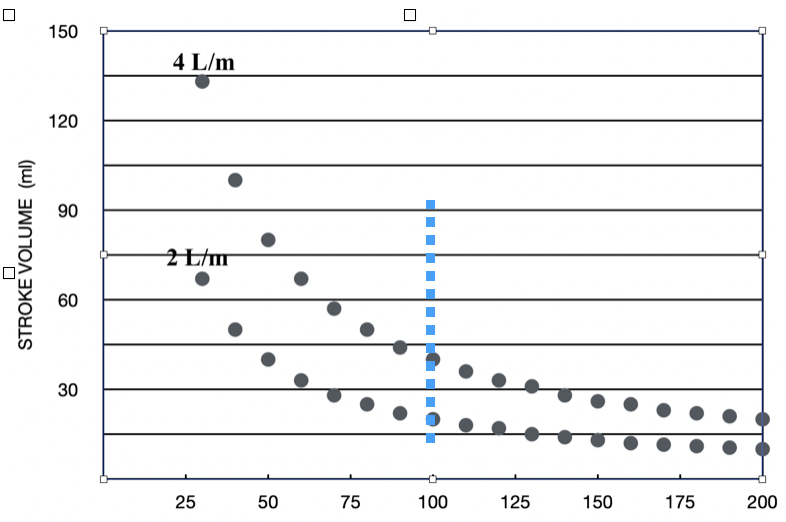
At low heart rates, the length-tension relationship will ensure good pressure development as long as circulating blood volume and SVR remains normal. Figure 2 helps to clarify this. At rates below 100 bpm (left of dashed line), Frank-Starling exhibits substantial latitude: small increases in cycle length produce significant increases in SV. Increases in SV raise will raise blood pressure.
Above 100 bpm, the corresponding SV values decline, eventually translating into lower blood pressure, but not until a critically short cycle length is reached, as is discussed below. The reciprocal relationship between SV and HR is shown for two levels of CO, 4L/min or 2 L/min.
- When The Heart Rate is Too Fast: A Problem of Hypotension
The explanation for activity intolerance when HR is inappropriately high also relates to the length-tension relationship. Any activity above resting is accompanied by increased adrenergic tone: epinephrine and norepinephrine are summoned to increase HR, SV, and SVR, raising both CO and BP to support the higher tissue perfusion demands. In contrast, catecholamine release is minimal or absent during a tachyarrhythmia, at least during its initial period. Without it, pressure rapidly declines: shorter cycle lengths produce less LV filling, lower SV, and lower arterial pressure.
In addition to causing fatigue, low blood pressure introduces the symptom of near-syncope. Depending on how low BP falls, syncope can become a problem. The degree of hypotension is related to how high HR increased, tachycardia duration, and the patient's circulating blood volume and SVR. Prolonged episodes of PSVT, such as AV nodal reentry or atrioventricular reentry, may produce profound fatigue for several hours after normal sinus rhythm is restored. However, this may be more related to lactic acid accumulation.
In order of importance, the determinants of blood pressure include SVR, circulating blood volume, and SV. In the case of tachyarrhythmia, the variables that matter are Frank-Starling, blood volume, and posture.
Inappropriately high HR coexisting with impaired cardiac structure/function will only worsen matters. For example, patients with a normal ejection fraction (EF) but in whom there is diastolic dysfunction (HFpEF) can be remarkably intolerant of high HR. Similarly, those afflicted with heart failure and reduced EF (HFrEF) will not tolerate either HR extreme.
Heart rate is a significant determinant of myocardial oxygen consumption. Among patients with significant CAD, ischemic consequences may quickly become evident among patients with significant CAD who develop tachyarrhythmias.[6]
Dyspnea
Airflow through the lungs is called minute ventilation (VE) and represents the respiratory system's equivalent to cardiac output. Respiratory rate (RR) and tidal volume (TV) are analogous to HR and SV, respectively. In the case of the lungs, however, TV increases contribute substantially more to VE than RR -just the opposite of the heart.
Dyspnea is often misunderstood, except for the person who can't breathe. It refers to a reduced capacity to breathe but also involves sensory receptors within the lung parenchyma, which are stimulated under certain conditions. Dyspnea involves a heightened awareness of one's respiratory status and has a strong emotional component.[7]
Hypoxia doesn't cause dyspnea in everyone.[8] It causes hyperpnea (increased TV) and tachypnea (increased RR) to raise VE and is usually accomplished without feeling air starved. However, a limit can be reached in anyone's lungs wherein that increase in minute ventilation cannot meet the metabolic demands of the person at any given moment, regardless of the level of activity. That will activate the sensory receptors in the lungs to produce a subjective sensation approximating suffocation.
Supplemental oxygen may relieve dyspnea even when one is not hypoxic by reducing chemoreceptor activation and the accompanying reduced minute ventilation.[9] Additional factors can embellish the dyspnea response, such as the subjective "context" in which it occurs and other conditions, such as fluid within the alveolar spaces during LV failure.
Dyspnea can be a symptom of tachyarrhythmia in someone with normal or abnormal pulmonary function, but it's more likely in those with coexisting obstructive or restrictive lung disease. The usual cause is elevated LV filling pressure.[10] As cycle length shortens, LV filling and stroke volume decrease. A reduced SV leaves more volume in the LV at the end of systole (higher ESV).
Climbing LVESV can restore the appropriate LV end-diastolic pressure (LVEDP) among patients with normal cardiac function. However, in the presence of coexisting LV dysfunction, the rise in LV filling volume may produce a disproportionate increase in LV end-diastolic pressure (LVEDP) due to poor LV compliance. The increased LVEDP will lead to increased pulmonary venous pressure to the extent of edema formation within the lung parenchyma.
Sudden onset or resolution of dyspnea can signal tachyarrhythmia. In contrast, the more chronically elevated LVEDP accompanying structural heart disease will produce dyspnea with a more insidious onset and offset. If a tachyarrhythmia is causing dyspnea, coexisting structural heart disease is likely, and an echocardiogram should be considered.
Chest Discomfort
Chest discomfort is the least likely symptom associated with an ectopic arrhythmia. Its description is often characterized as "pulsatile" since the discomfort is brief, coinciding with single heartbeats. A specific quality (sharp, dull, burning, pressure) may be difficult to declare, but ectopy can be very uncomfortable.
While angina may be an unlikely manifestation of arrhythmias, it shouldn't be ignored, given our population's high prevalence of coronary artery disease (CAD). Heart rate is a major determinant of myocardial oxygen consumption, so anyone having a history of CAD, particularly with >75% coronary stenoses, can experience angina during tachycardia.
When an arrhythmia does cause angina, it is more likely a sustained one because it requires time to manifest. During history taking, one should always elicit the pattern and qualities consistent with angina. If angina is being described, one should refocus the conversation in that direction and inquire about a family history of CAD and the usual risk factors. In general, arrhythmia is rarely the initial manifestation of CAD.
Nelson et al. have stressed that >1 mm ST-segment depression is commonly seen on the ECG during PSVT, regardless of any chest discomfort.[11] Because the horizontal or downsloping ST change resembles a subendocardial injury pattern, one might recommend cardiac catheterization. How quickly the ST segment change normalizes upon restoration of sinus rhythm places the ECG finding in proper context: prompt normalization implies normal coronaries.
Aberrant intraventricular conduction of any degree during PSVT will also produce suspicious ST segment and T wave abnormalities. These changes reflect the abnormal pattern of repolarization attributed to the pattern of abnormal depolarization. Nelson et al. also reported that the occurrence of chest discomfort during tachycardia is not helpful. Although most of the PSVT patients in the published series did experience some chest discomfort, none had CAD by cardiac catheterization.
A positive troponin is equally uninformative: 50% of PSVT patients with normal coronary arteries developed a positive troponin, so neither it nor chest discomfort predicts underlying CAD.[12]
Near-Syncope and Syncope
Lightheadedness and fainting are the consequences of a decline in blood pressure to such an extent that perfusion to the entire cerebral cortex is critically reduced. Near-syncope and syncope are not due to a reduction in cardiac output, per se, unless the heartbeat stops altogether. Cardiac output can be reduced if blood pressure is reduced, but one should not conflate the two. Septic shock is a condition emphasizing the difference. In this disorder, blood pressure is very low due to reduced SVR and may be associated with near-syncope. Simultaneously, CO is very high, at times exceeding 8-10 L/minute. Stroke volume and circulating blood volume are not low. Thus, hypotension can coexist with a very high CO.
Similarly, the patient presenting to the emergency room with third-degree AV block and a ventricular rate of 25-30 bpm typically has normal blood pressure. While his CO is significantly reduced, his level of consciousness is not because cerebral perfusion pressure is being maintained despite the low HR; again, due to the cardiac muscle length-tension relationship, a normal SVR, and a normal blood volume. In order of importance, the determinants of arterial pressure are SVR, blood volume, and SV.
The important distinction between sustained bradycardia and pauses is highlighted in the circumstances of near-syncope and syncope. What symptoms would a patient report in whom third-degree AV block suddenly occurred? The sudden cessation of impulse traffic through the AV node will unmask the phenomenon of overdrive suppression of a subsidiary pacemaker site, resulting in a pause. The pause may be brief or quite long, partly depending on both the age of the patient and whether any offending rate-slowing drug is present.
The pause duration will determine symptoms: none, near-syncope, or syncope. Inevitably, an escape rhythm, either junctional bradycardia or an idioventricular rhythm, establishes itself, enabling the return of blood pressure. Thus, a long pause followed by sustained bradycardia will produce a symptom complex that includes transient syncope followed by unexplained fatigue. On the other hand, a combination of bradycardia and low blood pressure brings to mind the very different situations of a vagal reaction, NMS, or drug toxicity involving a beta-blocker or calcium channel blocker.
How long of a pause is necessary to produce loss of consciousness was determined by a unique clinical study conducted in 1943 and published in the Archives of Neurology and Psychiatry (50:510). The authors, Rossen, Kabat, and Anderson, questioned what minimum time interval of absent cerebral blood flow a fighter pilot could tolerate before losing consciousness. The rapid ascension immediately following a dive-bombing was known to cause syncope, and there was a need to determine how to minimize or prevent it. It was a matter of defining a time limit.
Unwitting subjects underwent transient bilateral carotid artery occlusion using a leather collar containing an inflatable rubber bladder. It was inflated to 600 psi within 0.12 seconds and kept inflated until an observer noted the subject's "loss of visual tracking," typically occurring 1 to 2 seconds before the onset of syncope. 85% of the subjects lost consciousness no sooner than 6-7 seconds of bilateral carotid occlusion. An additional 7% required >8 seconds to faint.
Considerable variation in the pause duration capable of producing syncope is seen clinically. In some patients whose heartbeat ceases, either by a long pause or an episode of ventricular tachycardia/fibrillation, consciousness and postural tone can be maintained for remarkably long periods. Syncope is unlikely when that duration is <6 seconds.
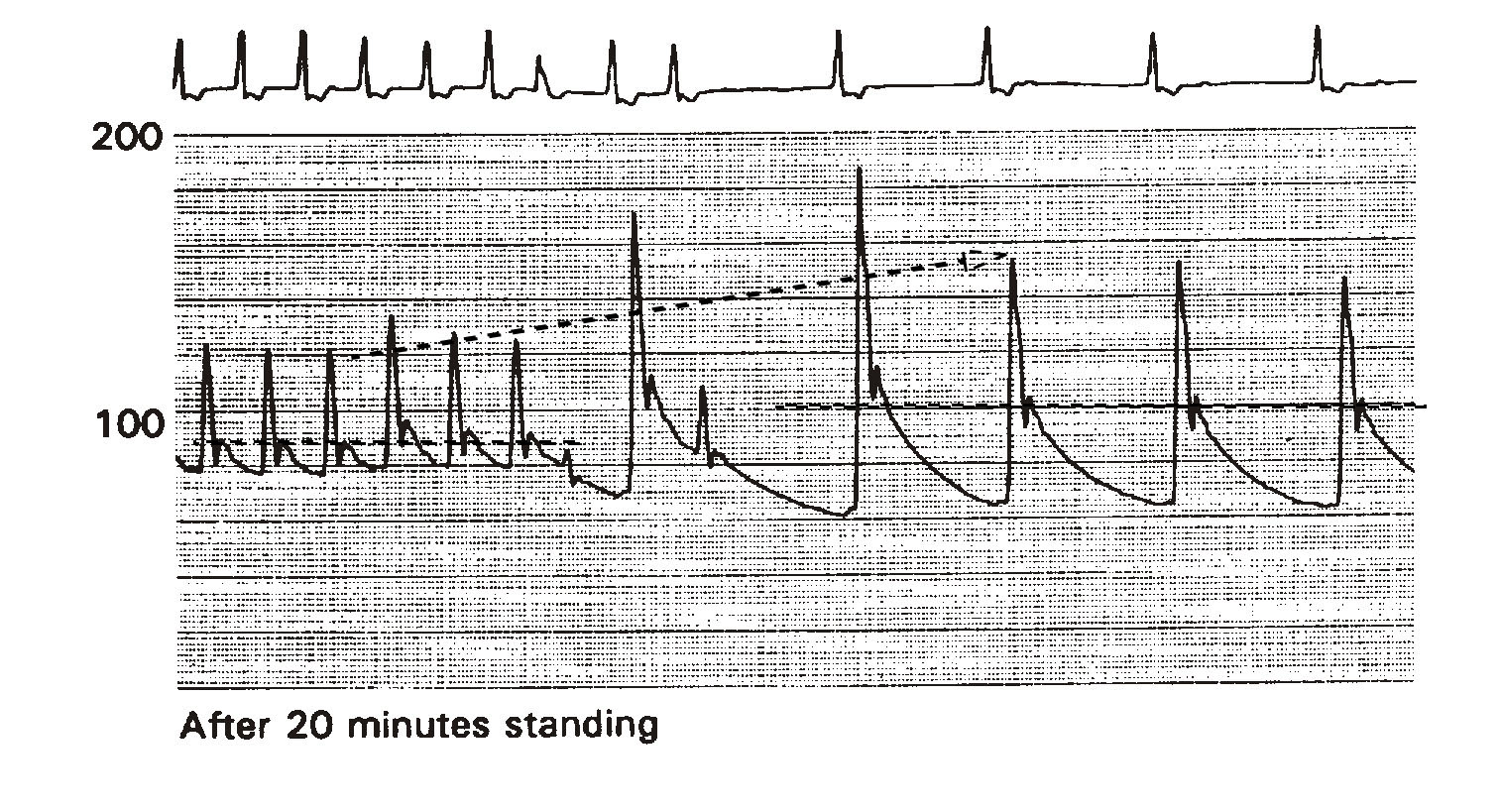
Figure 3 illustrates the effect of bradycardia on arterial pressure. The example is from a head-up tilt test (HUTT) performed on a 65-year-old patient presenting with recurrent syncope. He has a history of chronic permanent atrial fibrillation previously treated with combined AV junctional ablation/VVI pacemaker implantation. His EF is 45%. Shown are the ECG and phasic intraarterial pressure tracings. The pacemaker's lower rate limit was abruptly reprogrammed from 80 to 30 bpm -low enough to replicate symptoms of severe bradycardia. Since this patient has been standing on the tilt table for 20 minutes, he is volume challenged, representing an additional hemodynamic disadvantage during sudden bradycardia development.
Immediately upon the development of marked bradycardia, mean arterial pressure rose from about 85 mmHg to 100 mmHg (dashed horizontal lines). Systolic pressure increased from 125 to 155 mmHg (dashed arrow). Palpitation was the only symptom. In the absence of a long pause, bradycardia alone does not cause hypotension and, therefore, alone cannot cause syncope or near-syncope. If his CO measured 4 L/min at a heart rate of 80 bpm, it was reduced to 1.5 L/min at 30 bpm.
On the other hand, tachyarrhythmias cause near-syncope or syncope as determined by the length-tension relationship. Sudden cycle shortening causes reduced LV filling, leading to reduced contractile force, hence, lower LV and arterial pressure. A coincidental rise in SVR or blood volume would avert hypotension, but neither will occur.
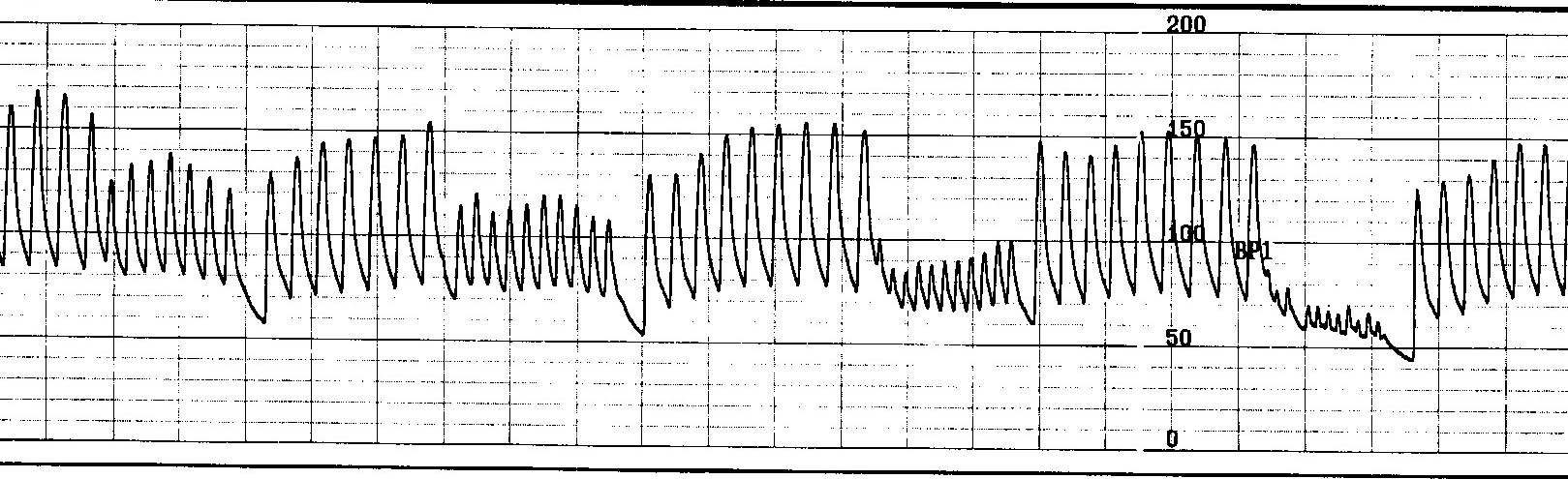
Figure 4 shows the effect of the sudden development of nonsustained atrial tachycardia on arterial pressure. Burst atrial pacing is performed at incremental rates to attempt tachycardia induction.
Unlike the patient in figure 4, this patient is supine and, therefore, not volume challenged, so his blood volume status won't be a liability regarding his blood pressure response to a heart rate change. Each train is associated with an abrupt drop in pressure. During the last train, systolic pressure fell below 65 mmHg, causing near-syncope. Had the 200 bpm train lasted for >7 seconds, he would likely be on his way to unconsciousness.
Blood pressure must fall below a critical level, usually around 60 to 80 mm Hg. and remain there for a minimum period of at least 7 seconds. A heart rate extreme causing near-syncope or syncope can be implicated when it is suddenly very fast, but not if slow.
In the majority of patients that present with syncope, the ultimate etiology is not due to an arrhythmia regardless of whether there is known underlying heart disease; instead, it is frequently caused by a transient disorder in blood pressure regulation known as neurally mediated syncope (NMS) in which a sudden drop in SVR occurs. As part of the reflex vagal response, there is also a coincidental decline in heart rate, which might lead you to believe the bradycardia caused the fainting if only rhythm were being monitored. Such patients often complain of lightheadedness upon standing from a seated or lying position.[13]
The "suddenness" of collapse or the associated occurrence of injury cannot distinguish ventricular tachycardia from NMS because the onset and loss of postural tone in both can be abrupt. The recovery from NMS and VT is also prompt. In contrast, lengthy post-ictal states always follow seizures. Near-syncope can be confused with vertigo. In history-taking, one should seek the complaint of a "room spinning" sensation, invariably precipitated by head or body movement, including standing from a lying position. While these patients do not lose consciousness, they may fall due to the vestibular system-mediated gait/stance instability.
Carotid vascular ultrasound studies are rarely recommended as part of a syncope evaluation since cerebrovascular embolic events rarely ever cause syncope. Embolic events can cause "lateralizing" signs such as sensorimotor deficits affecting only one side of the body. If the vertebrobasilar artery delivers the embolism, the brainstem's reticular formation may be a target and produce impaired wakefulness. While one may appear to be losing consciousness, death is more likely because of the collateral damage involving such vital areas as the respiratory center.
Nonfatal Cardiac Arrest
A nonfatal cardiac arrest is the most severe manifestation of an arrhythmia. It is invariably due to either ventricular tachycardia (VT) or ventricular fibrillation (VF), and structural heart disease is more often present than not. CAD is most commonly found among the elderly, and hypertrophic cardiomyopathy can be found among the younger. There is overlap. Included among the younger population are the genetically-determined primary arrhythmia syndromes in which no other cardiac structure/function abnormality exists. All of these conditions cause non-sustained or sustained VT/VF. Again, the non-sustained episodes must last >7 seconds to cause syncope. Recovery of consciousness is prompt if the VT/VF is nonsustained, defined as a tachycardia lasting <30 beats or 15 seconds.
These patients require a workup that would include an electrocardiogram, echocardiography, cardiac catheterization, likely electrophysiologic testing, and possibly a cardiac MRI. The workup's objective is to diagnose and determine if recurrent cardiac arrest is expected and whether the underlying condition can be intervened upon and possibly resolved. An ICD is nearly always indicated, regardless of the findings.
Unique Clinical Situations
- Asymptomatic Atrial Fibrillation Causing Nonischemic Cardiomyopathy
Severe reductions in EF can occur following only a few months of continuous high ventricular rates, setting the stage for sudden cardiac death. This condition is usually reversible by restoring and maintaining sinus rhythm as long as there is no other condition responsible for LV dysfunction. These patients may undergo ICD implantation but may want it removed later once LV function normalizes. Because the cardiomyopathy developed without symptoms and warning, the entire tachyarrhythmia process causing redevelopment of the dilated cardiomyopathy could repeat itself, so an ICD system extraction could be ill-advised just because LV function transiently normalizes.
Severe reductions in EF can occur following only a few months of continuous high ventricular rates, setting the stage for sudden cardiac death. This condition is nearly always reversible by restoring and maintaining sinus rhythm as long as there is no other condition responsible for LV dysfunction. These patients may undergo ICD implantation but want it removed later once LV function normalizes. Because the cardiomyopathy developed without symptoms and warning, the entire process could repeat itself, so ICD system extraction is ill-advised just because LV function is normalized.
- "Man down" with no Vital Signs
A patient who experiences NMS and loses consciousness in public will often be given CPR by bystanders if it's not otherwise evident that they are already waking up. While most would not expect this to lead to an eventual ICD implantation, it does happen. One thing is sure, CPR will not terminate an episode of sustained VT/VF. Only an AED shock will generally terminate VT/VF.
Patients who lose consciousness from NMS are arousable during CPR because CPR is a form of noxious stimuli. It can be very painful. The "man down" scenario is why the first step in CPR, "Annie, Annie, are you OK?" should never be overlooked. CPR may well be the noxious stimuli of choice in this situation because, if the patient is truly in the thick of a cardiac arrest, the appropriate therapy is already in progress.
Estimating the Risk of Dying
Patient History
In general, high-risk groups merit additional evaluation. They include those with a history of recurrent syncope, known structural heart disease including congenital heart disease, and a family history of premature (age <50) death. Also included are special interest groups such as competitive athletes, those involved in public transportation, or who drive for a living. Pursuing additional evaluation may sometimes be necessary even when there is no suggestion of a risk of dying: any patient or their family member may have significant anxiety over the symptoms and their cause to the extent that they will want you to continue an investigation, no matter how evidence-based and optimistic your explanations are.
The 12-lead ECG
After a good history, the next step is the 12-lead ECG. In the context of an arrhythmia evaluation, its primary purpose is to uncover evidence for structural heart disease, as they can be associated with ventricular tachyarrhythmias. Findings such as Q waves (old MI), primary repolarization abnormalities (recent MI), left ventricular hypertrophy (longstanding hypertension), and complete left bundle branch block (hypertension or dilated cardiomyopathy) should prompt formal cardiology referral.
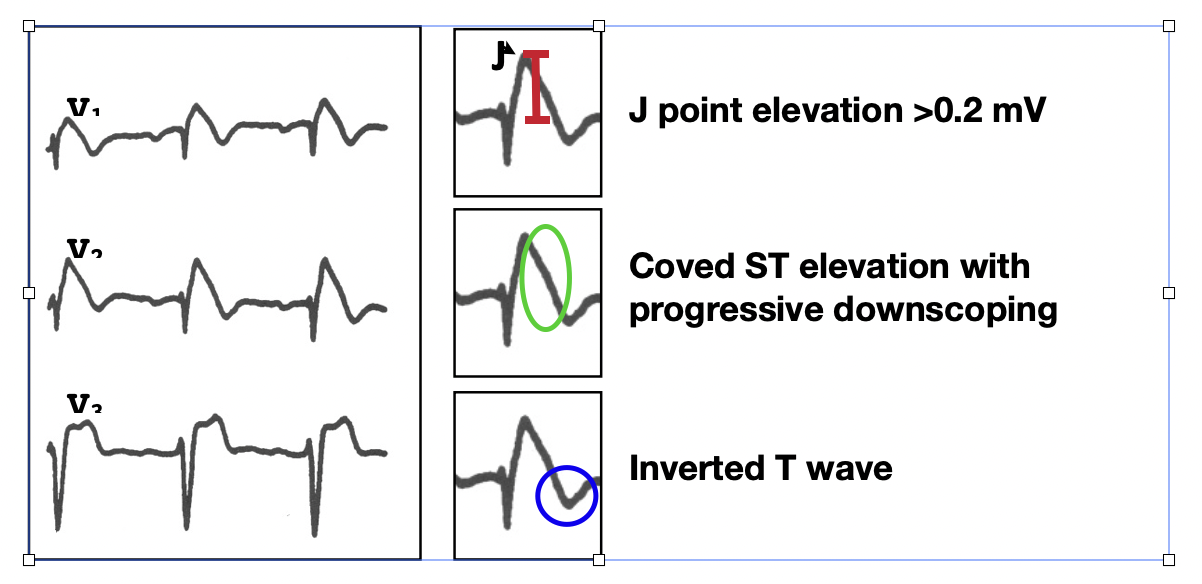
Genetic disorders affecting cardiac cell membrane function and causing conduction or repolarization disorders leading to sudden cardiac death (SCD) can exist without structural heart disease. These generally have their own unique and readily recognizable ECG features and include the Long QT and Brugada syndromes. The ECG may provide the ONLY evidence for probable cause. A "loss-of-function" of the late sodium channel (iNa+late) defect leads to shortening of repolarization principally in the right ventricular epicardium and the characteristic ST-elevation seen in the Brugada syndrome.[14]
The diagnostic ECG findings in the Brugada syndrome are shown in figure 5. When present in at least 2 of the three precordial leads, V1, V2, and V3, the type I pattern necessary for the diagnosis is satisfied. The most common misdiagnosis of a type I Brugada ECG is that of an acute anterior myocardial infarction.
The long QT syndrome is due to a genetic defect involving the two voltage-gated potassium channels (iKs or iKr), or it involves the late sodium inward current, iNa+late. These abnormalities produce the three most common forms of the syndrome, LQT1, LQT2, and LQT3. They are differentiated by the T wave and ST interval appearance. In all three, the QTc is prolonged (>440 msec in men, >460 msec in women), but in LQT1, the T wave is symmetric; in LQT2, the T waves tend to be lower amplitude and notched, as is illustrated below. [see Figure 6] In LQT3, the ST segment is the only prolonged portion.
Not until the QTc exceeds 500 msec is there a significant risk of developing syncope due to polymorphic VT, and it is usually nonsustained.[15] Drugs are the most common cause of acquired long QT syndrome. In addition to the class III antiarrhythmic drugs, there are numerous non-cardiac drugs, including some antipsychotics and the macrolide family of antibiotics.
Echocardiography
Echocardiography will provide an estimate of the EF, whether cardiac hypertrophy/enlargement is present, and will disclose regional wall motion abnormalities suggesting prior infarction. These three features imply a potential structural basis for lethal ventricular arrhythmias.
Exercise Stress Testing
In general cardiology, exercise stress testing is used to diagnose or exclude CAD, particularly when combined with nuclear imaging. However, in searching for an arrhythmia problem, the standard regular Bruce exercise protocol can disclose a tendency for adrenergically-mediated arrhythmias, both benign and malignant. Benign ventricular arrhythmias typically arise from the right ventricular outflow tract. The more malignant arrhythmias involve genetically-determined channelopathies, including catecholaminergic polymorphic ventricular tachycardia (CPVT) and long QT syndromes. Chronotropic incompetence can be diagnosed using the exercise stress test to justify pursuing permanent pacing.
The following is a list of potentially lethal conditions that can be diagnosed relatively easily:
| Condition | Test |
| VT associated with CAD | EPS |
| AV Block | Rhythm Strip |
| Cardiomyopathy | ECHO |
| Long or Short QTS | EKG |
| RV Dysplasia | EKG/ECHO |
| Brugada Syndrome | EKG |
| Catecholaminergic PMVT | Exercise stress test |
| Anomalous Coronary | CT ANGIO |
Documenting the Arrhythmia
This last step in the evaluation of the patient with suspected cardiac arrhythmia involves documentation of the arrhythmia. Doing so requires matching symptoms with arrhythmia.
The 24-hour Holter and External Event Monitors
Ambulatory monitoring is the most effective means of documenting any arrhythmia. The classic 24-hour Holter monitor and the external event monitor or recorder are included. The 24-hour Holter is only useful to detect arrhythmias associated with symptoms that occur AT LEAST DAILY. Holter monitors also provide "full disclosure," as every heartbeat is kept for review after the monitoring period concludes. A 24-hour Holter is instrumental in diagnosing postural orthostatic tachycardia syndrome (POTS) by noting the pattern of the hourly average heart rates: 20 to 30% higher during the day while the patient is upright compared to supine nocturnal rates.
External event monitors are even more helpful because the longer monitoring period provides better sampling, increasing the likelihood of documentation. Event monitors were initially called loop recorders because the actual recording lasted for only a few minutes before re-recording itself. In response to a symptom, the patient activates the device by pressing a button that stops recording once it reaches the end of the loop. In this manner, the device discloses the patient's rhythm before, during, and following the symptom occurrence.
Some external loop recorders provide full disclosure. Most include either a patient-activated feature or enable auto-triggering to document when a heart rate or rhythm irregularity threshold is exceeded. An advantage to full disclosure is the ability to quantify arrhythmia "burden" rather than addressing the symptom-arrhythmia connection. Burden refers to how much arrhythmia is present and is defined either as a percent of the total number of beats in the case of ectopy or as a percent of the total recording time in the case of sustained arrhythmias like atrial fibrillation.
Typically, a 1 to 4-week monitoring period is offered. Given the potential for detecting an unexpected but potentially serious arrhythmia, an arrangement must be made to have someone be available to receive the tracing promptly and act on the information. Who will be responsible for addressing that potentially deadly arrhythmia that occurred in the early morning? In some instances, the information provided by the event monitor is not made available until after the end of the recording period, which would be inadvisable for the patient undergoing a syncope evaluation.
Both 24-hour Holter and event monitors provide "summary" statements and trend analyses in graphs and tables to give you a birdseye view of the information. How well this information is portrayed on the summary sheet varies from vendor to vendor. In any monitoring situation, an asymptomatic mon-sustained arrhythmia is discoverable but needs to be interpreted with caution as it usually does not necessarily provide a substantial degree of certainty regarding the existence of a more high-risk sustained arrhythmia.[16]
The failure of an event monitor to provide an answer is related to several circumstances. For example, after a 30-day monitoring period, one may find the patient had no arrhythmia and no symptoms; they may have had symptoms that did not correlate with any rhythm disturbance, or the arrhythmia that is documented is unlikely to have caused the symptom; arrhythmias may occur without any symptoms at all, so-called asymptomatic arrhythmias. The physician may have issued the event monitor to clarify the nature of one particular symptom, but the patient triggered the monitor for a host of other symptoms. The patient may have removed the monitor and wasn't wearing it at the time of a symptom. The physician may have inadequately informed the patient about the use of the device, resulting in a loss of information. Finally, one may have simply needed more monitoring time for documentation.
Ambulatory monitoring is suboptimal in cases of syncope because arrhythmia is not the most common cause of impaired consciousness. As mentioned above, near-syncope or syncope is usually due to NMS. NMS involves both a cardioinhibitory component (the bradycardia) and a vasodepressor component (the fall in SVR). The fall in SVR leads to hypotension and, therefore, fainting. If diagnostic information is limited to an ECG tracing, as in the case of an event monitor, the recording of asymptomatic sustained bradycardia or a pause lasting <5 seconds may be quickly taken out of context and interpreted as a pacemaker indication. Pacemakers cannot increase SVR. Nocturnal bradyarrhythmias and pauses are nearly always benign, given their vagal basis.
In a study involving 101 patients presenting with syncope or palpitation, event monitors with or without auto-triggering were issued with the intent to compare the diagnostic yield between the two different monitoring strategies. A total of 196 relevant arrhythmias were documented, including 42 episodes of bradycardia. Strikingly, none of the bradycardia episodes were among the patient-activated group meaning that none were symptomatic and, therefore, could not be interpreted as anything other than an incidental finding.[17] The fundamental indication for a pacemaker is documented symptomatic bradycardia.[18]
In evaluating a patient with suspected cardiac arrhythmia, the usual intent of a monitor is to provide a match between symptoms and an arrhythmia. While one should not be discouraged from ordering a 3 or 4-week external event monitor in a patient with recurrent syncope, one should be prepared for incomplete information.
One should always explain the purpose of the event monitor to the patient so they understand why they are doing what they are doing. Someone must be in charge of providing instructions and making sure the patient understands. Diary entries should include symptoms, activity at the time of symptoms, and the time of day.
The Implantable Loop Recorder
Implantable loop recorders (ILR) are miniature recording devices inserted beneath the skin through a small incision overlying the third or fourth intercostal space just to the left of the sternum. The ILR can be quickly and easily implanted in the office setting using only local anesthesia. The battery lasts up to 6 years for monitoring rhythm. Currently, ILRs are most helpful in determining atrial fibrillation burden. Atrial fibrillation burden is of interest during the initial evaluation or to verify treatment success following catheter ablation, especially among asymptomatic patients. A second indication for the ILR is to establish whether atrial fibrillation may have been the cause of a stroke.
At the time of this writing, we do not know two fundamental characteristics of atrial fibrillation enabling meaningful use of the ILR in that setting: what is the minimum duration of an episode of AF to cause a stroke, and what would be a reasonable minimum burden of AF to declare ablation failure?
As a rule, it is initially impractical to use an ILR simply to diagnose an arrhythmia problem because external event monitors usually suffice. In the cases of syncope, the ISSUE-3 trial disclosed that fewer than 20% of patients given ILRs for a syncope indication would have confirmation of it within a year.[19]
To conclude, I would recommend referral to an electrophysiologist to evaluate the following problems:
- Recurrent syncope or near-syncope, and it is uncertain if it was neurally mediated
- Curable arrhythmias, including:
- AVNRT
- AVRT (WPW)
- Ectopic atrial tachycardia
- Atrial flutter
- Atrial fibrillation
- Right ventricular outflow tract (RVOT) PVCs
- Idiopathic ventricular tachycardia
- Symptomatic bradyarrhythmias
- Nonsustained ventricular tachycardia
- Wide complex tachycardia
- Nonfatal cardiac arrest
- Symptoms suggesting tachycardia but unsuccessful documentation to date
The following section, Selected Rhythm Disorders, highlights specific arrhythmias and their management. These include:
- Atrial and ventricular ectopy
- Sinus tachycardia
- Paroxysmal supraventricular tachycardia
- Atrial flutter
- Atrial fibrillation
- Non-sustained ventricular tachycardia
- Atrioventricular block
Clinical Significance
It is important to have a systematic approach to the problem of suspected cardiac arrhythmia. Understanding the significance of specific symptoms and the awareness of certain risk factors in each case enables the physician to prioritize the approach.
Diseases that are present continuously, such as hypertension, provide us with an opportunity to monitor our treatment success or at least to confirm ongoing control. On the other hand, arrhythmias usually behave intermittently and in an all-or-none fashion. The impact of the arrhythmia occurs in the midst of the arrhythmia. When not occurring, there is nothing to treat. Despite coexisting with an underlying chronic disease, an arrhythmia will emerge entirely randomly and unpredictably. We have no simple or reliable means of verifying ongoing control of arrhythmias.
Another major difference is that, while symptom severity may vary considerably, the consequence of rhythm disturbances tends to be rather binary: nuisance or death. Our strategy should be focused on the latter. Luckily, the vast majority of arrhythmias fall within the nuisance category. Atrial fibrillation is the biggest exception. It can be present continuously, and significant consequences lying somewhere between nuisance and death do exist, such as stroke, heart failure, and dementia.
Once the approach is completed, a treatment strategy can be initiated. The approach outlined here is a suggestion, as the very first contact with a patient's arrhythmia may be the documented arrhythmia itself. Even if the patient is asymptomatic, the second step is still necessary because an action plan must be formulated if there is a risk.
Enhancing Healthcare Team Outcomes
One of the more relevant team members working toward a common goal is the technician responsible for providing the ambulatory monitor. This individual has the important responsibility of educating the patient about the operation of the recorder and providing assistance when needed to ensure that the information provided by the device is complete.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
WHITE JC. Cardiac pain: anatomic pathways and physiologic mechanisms. Circulation. 1957 Oct:16(4):644-55 [PubMed PMID: 13461273]
Arendt-Nielsen L, Svensson P. Referred muscle pain: basic and clinical findings. The Clinical journal of pain. 2001 Mar:17(1):11-9 [PubMed PMID: 11289083]
Level 3 (low-level) evidenceAllen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. Journal of molecular and cellular cardiology. 1985 Sep:17(9):821-40 [PubMed PMID: 3900426]
Level 3 (low-level) evidenceArentzen CE, Rankin JS, Anderson PA, Feezor MD, Anderson RW. Force-frequency characteristics of the left ventricle in the conscious dog. Circulation research. 1978 Jan:42(1):64-71 [PubMed PMID: 145327]
Level 3 (low-level) evidenceCooper MW, Lutherer LO, Lust RM. Postextrasystolic potentiation and echocardiography: the effect of varying basic heart rate, extrasystolic coupling interval and postextrasystolic interval. Circulation. 1982 Oct:66(4):771-6 [PubMed PMID: 6180844]
Level 3 (low-level) evidenceHoffman JI, Buckberg GD. The myocardial oxygen supply:demand index revisited. Journal of the American Heart Association. 2014 Jan 21:3(1):e000285. doi: 10.1161/JAHA.113.000285. Epub 2014 Jan 21 [PubMed PMID: 24449802]
Level 3 (low-level) evidenceGigliotti F. Mechanisms of dyspnea in healthy subjects. Multidisciplinary respiratory medicine. 2010 Jun 30:5(3):195-201. doi: 10.1186/2049-6958-5-3-195. Epub 2010 Jun 30 [PubMed PMID: 22958405]
. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. American journal of respiratory and critical care medicine. 1999 Jan:159(1):321-40 [PubMed PMID: 9872857]
Level 1 (high-level) evidenceParshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O'Donnell DE, American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. American journal of respiratory and critical care medicine. 2012 Feb 15:185(4):435-52. doi: 10.1164/rccm.201111-2042ST. Epub [PubMed PMID: 22336677]
Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002 Nov 6:288(17):2144-50 [PubMed PMID: 12413374]
Nelson SD, Kou WH, Annesley T, de Buitleir M, Morady F. Significance of ST segment depression during paroxysmal supraventricular tachycardia. Journal of the American College of Cardiology. 1988 Aug:12(2):383-7 [PubMed PMID: 3392331]
Chow GV, Hirsch GA, Spragg DD, Cai JX, Cheng A, Ziegelstein RC, Marine JE. Prognostic significance of cardiac troponin I levels in hospitalized patients presenting with supraventricular tachycardia. Medicine. 2010 May:89(3):141-148. doi: 10.1097/MD.0b013e3181dddb3b. Epub [PubMed PMID: 20453600]
Level 2 (mid-level) evidenceAlboni P, Brignole M, Menozzi C, Raviele A, Del Rosso A, Dinelli M, Solano A, Bottoni N. Diagnostic value of history in patients with syncope with or without heart disease. Journal of the American College of Cardiology. 2001 Jun 1:37(7):1921-8 [PubMed PMID: 11401133]
Antzelevitch C. Cellular basis and mechanism underlying normal and abnormal myocardial repolarization and arrhythmogenesis. Annals of medicine. 2004:36 Suppl 1():5-14 [PubMed PMID: 15176418]
Obeyesekere MN, Antzelevitch C, Krahn AD. Management of ventricular arrhythmias in suspected channelopathies. Circulation. Arrhythmia and electrophysiology. 2015 Feb:8(1):221-31. doi: 10.1161/CIRCEP.114.002321. Epub [PubMed PMID: 25691556]
Level 3 (low-level) evidenceArnar DO, Mairesse GH, Boriani G, Calkins H, Chin A, Coats A, Deharo JC, Svendsen JH, Heidbüchel H, Isa R, Kalman JM, Lane DA, Louw R, Lip GYH, Maury P, Potpara T, Sacher F, Sanders P, Varma N, Fauchier L, ESC Scientific Document Group, EHRA Scientific Documents Committee. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2019 Mar 18:21(6):844–845. doi: 10.1093/europace/euz046. Epub [PubMed PMID: 30882141]
Level 3 (low-level) evidenceBalmelli N, Naegeli B, Bertel O. Diagnostic yield of automatic and patient-triggered ambulatory cardiac event recording in the evaluation of patients with palpitations, dizziness, or syncope. Clinical cardiology. 2003 Apr:26(4):173-6 [PubMed PMID: 12708623]
Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM, ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. European heart journal. 2021 Sep 14:42(35):3427-3520. doi: 10.1093/eurheartj/ehab364. Epub [PubMed PMID: 34455430]
Brignole M, Deharo JC, Menozzi C, Moya A, Sutton R, Tomaino M, Ungar A. The benefit of pacemaker therapy in patients with neurally mediated syncope and documented asystole: a meta-analysis of implantable loop recorder studies. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2018 Aug 1:20(8):1362-1366. doi: 10.1093/europace/eux321. Epub [PubMed PMID: 29267867]
Level 1 (high-level) evidence