Introduction
Congenital femoral deficiency (CFD) is a rare inborn disability characterized by femoral underdevelopment or absence. The condition has a broad spectrum of presentations, ranging from the congenital short femur to severe proximal focal femoral deficiencies. Hip joint stability and mobility are often compromised, especially if associated with joint malorientation and soft tissue contractures. The affected limb's growth is inhibited, with severity depending on the presence of underlying conditions.[1]
CFD patients usually present with coxa vara proximally and femoral valgus distally. The condition is generally associated with other skeletal abnormalities, with fibular hemimelia being the most common association. Caudal regression syndrome, caudal dysplasia, cruciate ligament deficiency, and lumbosacral spine deformities may also occur concomitantly with CFD.[2]
Femoral Anatomy
The femur, the largest bone in the human body, is situated in the thigh and is a vital component of the lower limb's skeletal framework. The femur's proximal end articulates with the hip joint. The shaft is integral to the overall function and mobility of the lower limb, providing support, transmitting mechanical forces, protecting vital structures, and contributing to joint stability and alignment. The distal femur articulates with the tibia and patella, forming the knee joint.
The anterior compartment contains the quadriceps femoris muscle group, which includes 4 muscles: rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius. These muscles extend the knee joint. The femoral nerve, a branch of the lumbar plexus, innervates the quadriceps femoris.
The muscles in the medial compartment are primarily adductors and include the adductor longus, adductor brevis, magnus, pectineus, and gracilis. These muscles are responsible for adducting the thigh and assisting with hip flexion. Most of these muscles are innervated by the obturator nerve. The hamstring part of the adductor magnus is innervated by the tibial nerve, a branch of the sciatic nerve.
The posterior compartment contains the hamstring muscles, which include the biceps femoris, semitendinosus, and semimembranosus. These muscles flex the knee and extend the hip. The sciatic nerve innervates the muscles of the posterior compartment.
The femur receives its blood supply primarily from the femoral artery and its branches, including the deep femoral artery. The femoral vein accompanies the femoral artery.
The knee joint is typically aligned so that the femur and tibia form a straight line when viewed from the front or back. This alignment allows for efficient weight distribution and smooth knee movements during activities such as walking, running, and jumping. In CFD, the knee angulation may be affected due to femoral underdevelopment or absence.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
CFD's exact etiology is unknown. However, multiple theories are postulated, including cellular nutritional disturbance during mitosis, local vascular damage to mesenchymal tissue, and intrauterine thigh compression during femoral diaphysis ossification.[3] Hereditary transmission does not seem to play a significant role.[4]
Epidemiology
Congenital femoral deficiency has an incidence of 1.1 to 2.0 per 100,000 live births and seems to be relatively more common in female children with a male-to-female ratio of 1 to 2. Most CFD cases are unilateral (85% to 90%), though bilateral presentations may also be seen. The right femur is more commonly affected in unilateral CFD.[5]
Pathophysiology
Most congenital anomalies occur between 4 and 8 weeks postfertilization when embryogenesis takes place. A complex growth factor cascade manages fetal lower extremity growth, expressed successively at various developmental checkpoints. During limb bud formation, mesenchymal cells play a pivotal role in integrating the positional information received from the proximal-distal, anteroposterior, and dorsal-ventral axes. CFD represents a dysplastic phenomenon with variable degrees of femoral compromise, ranging from hypoplasia to aplasia. The condition results in abnormal gait, impaired childhood growth, cosmetic implications, and psychosocial and behavioral disturbances.[6]
History and Physical
CFD may be identified prenatally. Ultrasonographic femoral length measurements can detect CFD during routine antenatal check-ups. The predicted limb-length discrepancy can be estimated in such scenarios using the multiplier method at birth and maturity.[7][8][9]
If prenatal ultrasound does not clearly show the defect, CFD may also be noted at birth or soon afterward. However, mild cases may not be recognized until later in childhood. Parents may notice leg-length asymmetry or abnormalities in the affected limb's appearance, such as bowing or shortening. Individuals with CFD may experience mobility difficulties, including limping or altered gait patterns, as they grow and begin weight-bearing activities. People with severe limb-length discrepancy may also experience secondary issues such as joint contractures, back pain, or difficulties with activities of daily living.
Information about family history, congenital conditions, and antenatal exposure to radiation or potentially teratogenic drugs or infection must be elicited. A family history of CFD is usually absent.[10]
A complete physical examination enables clinicians to identify associated deformities. Patients with CFD exhibit limb-length discrepancy on physical examination. The affected leg may also demonstrate angular deformities such as genu varum (bowed legs) or genu valgum (knock knees), particularly if associated with knee abnormalities. Patients with concomitant muscular disorders may exhibit visible differences in muscle bulk or function between the affected and unaffected limbs. In some cases, foot and ankle deformities such as talipes equinovarus (clubfoot) or pes planus (flatfoot) may also be present, further contributing to movement difficulties.
The range of motion of the hip, knee, and ankle, which may have contractures, must be examined. Patients with CFD often have increased hip external rotation or limited hip external rotation, flexion, and abduction besides femoral shortening.[11]
Beyond the musculoskeletal findings, patients with CFD may exhibit systemic abnormalities as part of a congenital syndrome. Genitourinary, cardiovascular, or other visceral anomalies may be noted. The facies, upper extremities, and spine should be examined for abnormal appearance. Overall, the clinical presentation of patients with CFD is heterogeneous and may involve a combination of musculoskeletal, functional, and visceral findings that require comprehensive evaluation and management.
Evaluation
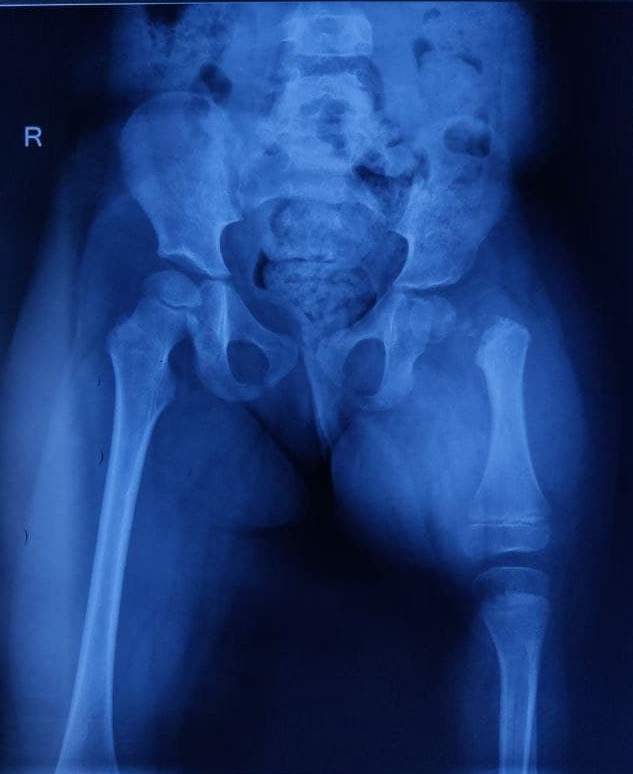
Initial radiographs help evaluate CFD, which includes a full-length standing anteroposterior (AP) view, with the patella facing forward, and the long lateral film (see Image. Congenital Femoral Deficiency Radiography). Pull-down supine long AP and lateral radiographs, including the pelvis and lower extremities, should be obtained when assessing an infant or nonambulating child. Both lower limbs are examined to ensure maximum knee extension.
An AP radiograph assesses the overall ossific anatomy of the lower extremities, classifying the CFD type, measuring femoral and tibial length, and ruling out acetabular dysplasia with the acetabular index and center-edge angle. A lateral radiograph assesses the knee's fixed flexion deformity. Other appropriate diagnostic imaging studies include magnetic resonance imaging (MRI), hip arthrography, and computed tomography scan (CT).
MRI helps assess the proximal femur's integrity and differentiates pseudoarthrosis from an intact cartilaginous femoral neck. This modality can also help evaluate the knee joint for any associated ligament deficiency.
Hip arthrography is performed under general anesthesia. This imaging study differentiates between delayed femoral head ossification and pseudoarthrosis. After injecting the dye, the lower extremity is manipulated to visualize the proximal femur. Conjoined femoral head and proximal femur movement indicate the presence of a cartilaginous connection between these structures, classifying the condition as type 1b. Hip arthrography can also differentiate between Paley types 2a and 2b. The femoral head is present in both types, but femoral head and acetabular fusion, indicating a more severe defect, signifies a type 2b CFD.
CT scan is preferred in older children, as acetabular and proximal femoral ossification is already complete in these patients. Three-dimensional CT reconstruction may be used to compare the dysplastic acetabulum with the contralateral side.
Treatment / Management
Nonsurgical CFD Treatment Approach
Conservative management of ambulatory difficulties includes prostheses, orthoses, and shoe lifts to treat limb length discrepancy. Children younger than 6 can be followed biannually. Annual assessment by limb-length radiographs is indicated in children older than 6.[12]
Surgical CFD Management
The anticipated development of both extremities should be estimated, and the end limb-length difference should be clearly defined before choosing a procedure. Surgery may improve prosthesis-fitting for a child with a more than 20 cm projected gap or greater than 50% shortening of the affected limb. Limb lengthening techniques may be considered if the projected difference is less than 20 cm. The hip and knee must first be stabilized before limb lengthening.
Lengthening construction is preferred over prosthetic reconstruction surgery in patients with CFD type 1a, 1b, 2a, or 2b. Before choosing any procedure. Preliminary operations are often performed between ages 2 and 3 in proximal focal femoral deficiency (PFFD) cases because the proximal femur typically undergoes delayed ossification, especially in the femoral neck and subtrochanteric regions.[13]
Type 1
This condition has the best chance of reconstruction among PFFD cases. Hip stability using the center-edge angle should be assessed radiographically before deciding on a lengthening procedure. Dega osteotomy should be performed before lengthening if the acetabular index is less than 30° and the center-edge angle is less than 20°.[14] However, the typical abnormalities seen in developmental dysplasia of the hip (DDH) differ from those observed in PFFD hips. While Salter and Dega pelvic osteotomies can address DDH cases with an anteriorly positioned acetabular deficit, the deficit in PFFD hips is typically located posteriorly. Some experts advocate for allowing natural growth of the hip in PFFD cases.[15](B2)
Coxa vara correction is the priority before a lengthening procedure if the neck-shaft angle is less than 120°. The superhip procedure should be considered in cases where coxa vara and hip dysplasia are present. The superhip operation releases soft tissue around the pelvis and proximal femur to fix flexion, abduction, and external rotation contractures. A proximal femoral osteotomy with either a rush rod or side plate combined with compression screw fixation provides stability, correcting varus deformity. Any remaining acetabular dysplasia can be treated with a Dega pelvic osteotomy. The joint abductor-quadriceps tendon and tensor fascial lata are sutured to the cartilaginous greater trochanter to balance the soft tissues and hip abductor function. The osteotomies, including pelvic and femoral, should be performed at least 12 months before the first lengthening. The condition can be reclassified from type 1b to type 1a after a successful superhip procedure followed by femoral neck ossification.[16]
Lengthening is performed a year after the preoperative surgery for abnormally short or incompletely ossified femurs. The procedure must be restricted to a maximum of 5 to 8 cm or 20% of the original femur length in one treatment to reduce complications. Lengthening is completed every 4 years according to a rule of thumb: the rule of 4 years. Consequently, the 2nd and 3rd lengthening will occur at ages 8 and 12. Three lengthenings will result in a gain of 24 cm at 12 years, as each lengthening adds 8 cm. Contralateral distal femoral growth plate physiodesis may result in an additional gain of 5 cm, bringing the total gain to 30 cm if the difference in limb length is greater than 25 cm. [17][18](B3)
Type 2
A mobile femoral head can be reattached to the existing part of the femur using a superhip 2 procedure by reconstructing the greater trochanter in the femoral neck. In contrast, an immobile femoral head may be detached from the acetabulum, transitioning it to type 2a. A superhip 2 procedure can be conducted afterward. Alternatively, the femoral head may be removed from the acetabulum, paving the way for a superhip 3 procedure, which involves trochanteric arthroplasty. Initially, a soft tissue release may be scheduled, followed by femur lengthening. A pelvic support osteotomy can be performed at skeletal maturity.
Type 3
The most reliable and predictable management option for type 3 CFD is prosthetic reconstruction surgery with either a Syme amputation or rotationplasty (turn-about technique). Initially, the Van Nes rotationplasty was popularized by Torode, later modified by Brown, and then Paley.
Iliofemoral fusion has been suggested as a treatment option for severe PFFD (Aitken class C or D). Meanwhile, Brown suggested combining rotationplasty with iliofemoral arthrodesis to treat unstable PFFD hips. The Paley-Winkelmann rotationplasty can be used to manage type 3c CFD. Rotationplasty requires foot flexibility and sensitivity, ankle mobility, and adequate plantarflexion strength. Patients with severe deformity may undergo rotationplasty to align the ankle of the affected side with the knee of the unaffected side. The knee is turned into a hip joint by bending it 90°. The femur is fused to the pelvis, and the foot is converted into a knee joint. A leg prosthesis is then attached to the short limb.[19]
Differential Diagnosis
Conditions that may be included in the differential diagnosis of CFD include the following:
- DDH is characterized by abnormal hip joint development, which can lead to hip instability, subluxation, or dislocation. While DDH primarily affects the hip joint, severe cases may involve femoral abnormalities.
- Legg-Calvé-Perthes disease is a childhood condition affecting the hip joint, leading to femoral head avascular necrosis. While this condition primarily involves the hip joint, femoral head ossification abnormalities may be present, causing limping or hip pain.
- Various skeletal dysplasias, such as multiple hereditary exostoses or diastrophic dysplasia, can affect the development of the femur and other long bones. These conditions may present with bone growth, shape, or density abnormalities.
- Conditions that produce unequal limb lengths, such as congenital limb length discrepancy or acquired conditions like trauma or infection, may mimic some features of CFD. However, these disorders typically do not involve femoral developmental abnormalities.
- Genetic syndromes such as Holt-Oram or TAR syndrome may be associated with skeletal abnormalities, including those affecting the femur. These conditions often present with additional features beyond isolated femoral deficiency. Campomelic and femoral hypoplasia with unusual facies syndromes present with bilateral CFD.
The differential diagnosis of CFD requires a comprehensive evaluation, including clinical examination, imaging studies, and consideration of the patient's medical and family history. Collaboration with specialists in orthopedics, genetics, and other relevant fields may be necessary to reach an accurate diagnosis and formulate an appropriate management plan.
Staging
The Paley classification system for congenital femoral deficiency (CFD) categorizes the condition into several types based on the severity and characteristics of the femoral deficiency.[20] The classification scheme is as follows:
- Type 1: Intact femur with mobile hip and knee
- 1a: Normal ossification
- 1b: Delayed ossification – subtrochanteric type
- 1c: Delayed ossification – neck type
- Type 2: Mobile pseudarthrosis with mobile knee
- 2a: Femoral head mobile in the acetabulum
- 2b: Femoral head absent or stiff in the acetabulum
- Type 3: Diaphyseal deficiency of femur
- 3a: Knee motion more than 45°
- 3b: Knee motion less than 45°
- 3c: Complete absence of femur
- Type 4: Distal deficiency of femur (at the knee joint)
Each type represents a different level of femoral deficiency severity, guiding treatment decisions and prognostic considerations. The Paley classification system describes the anatomical variations seen in CFD and may not encompass all possible presentation variations. Additionally, treatment approaches may vary based on individual patient characteristics and specific clinical considerations.
Prognosis
The prognosis of a patient with CFD varies with disease severity and management timeliness. Early treatment is generally associated with better outcomes. Abnormal gait with cosmetic implications may develop over time if CFD is untreated.
Complications
Complications associated with CFD vary depending on the condition's severity and the specific treatment approach employed, including the following:
-
Limb length inequality
-
Joint contractures
-
Hip instability
-
Degenerative joint disease
-
Psychosocial challenges
-
Surgical complications, such as infection, nerve injury, vascular injury, or failure of fixation devices
-
Functional limitations
Associated bony deformities such as fibular hemimelia, caudal regression syndrome, caudal dysplasia, cruciate ligament deficiency, and lumbosacral spine deformities can also cause mobility and cosmetic issues.
Postoperative and Rehabilitation Care
Gluteal sets, hip adduction with a towel, abdominal brace placement, and lying hamstring stretch are recommended within 6 weeks of surgery. Shoulder push-ups, hip extensions on the stomach, calf stretches with towels, hip abductions with therabands, and hip extensions on the hands and knees may be performed after 6 weeks. The patient and family are offered stretches and strengthening exercises following rotationplasty to improve active and passive range of motion. Strength training is subsequently introduced. The child and family are first instructed on hip, ankle, foot, and toe stretching exercises. Wheelchairs and crutches provide mobility and transportation. The physiotherapist teaches the patient and family members how to wrap the limb in compression stockings to reduce swelling. A prosthetic fitting is often recommended when bone union is achieved.[21][22]
Deterrence and Patient Education
Patients with CFD should be promptly evaluated and managed appropriately to prevent persistent gait abnormalities and cosmetic, behavioral, and psychosocial changes. Proper rehabilitation, physical therapy, and appropriate orthoses are paramount after surgical correction for the best functional outcomes.
Pearls and Other Issues
CFD is a rare congenital orthopedic condition characterized by abnormal fetal femoral development. The affected femur is often shorter than normal and may have various structural abnormalities, such as the absence or underdevelopment of the femoral head, neck, or shaft. This condition typically affects one leg and may result in limb length discrepancy, hip joint instability, and gait abnormalities.
Early diagnosis of CFD allows for timely intervention. Promptly identifying and managing the condition by closely monitoring infants and children with suspected limb abnormalities optimizes outcomes. CFD treatment plans are individualized to the patient's needs, considering factors such as the condition's severity, age, functional goals, and patient preferences. Options may include limb reconstruction surgeries, limb lengthening procedures, prosthetic fitting, or a combination of approaches. Optimizing limb alignment and function is essential in CFD management, with surgical interventions aiming to correct limb deformities, achieve limb length equality, and improve joint stability to enhance mobility and function.
Long-term follow-up is required to monitor for complications, assess functional outcomes, and address evolving needs. Regular evaluations by the interprofessional team allow for timely intervention and adjustments to treatment plans as needed. Psychosocial support is vital, as living with a visible physical difference can impact a child's well-being. Resources and support provided to patients and families should address concerns related to self-esteem, body image, social interactions, and emotional well-being. Educating patients and families empowers them to understand CFD and its treatment options, potential complications, and long-term expectations, enabling active participation in decision-making and treatment adherence.
Enhancing Healthcare Team Outcomes
CFD management requires interprofessional team collaboration between orthopedic surgeons, pediatricians, physical therapists, and prosthetists to provide comprehensive care. Orthopedic surgeons lead the team, performing surgical interventions such as limb reconstruction surgeries and joint stabilizations. Pediatricians contribute by addressing medical comorbidities and ensuring overall health monitoring. Physical therapists design customized rehabilitation programs to optimize mobility and strength, while prosthetists specialize in fitting and adjusting prosthetic devices to enhance functional independence for patients with limb length discrepancies or amputations associated with CFD.
The team ensures continuity of care, promotes patient engagement, and addresses psychosocial concerns through coordinated communication and collaboration. This patient-centered approach empowers patients and their families with knowledge and support throughout the treatment journey, improving outcomes and enhancing quality of life for individuals with CFD.
Media
(Click Image to Enlarge)
References
Doğer E, Köpük SY, Cakıroğlu Y, Cakır O, Yücesoy G. Unilateral isolated proximal femoral focal deficiency. Case reports in obstetrics and gynecology. 2013:2013():637904. doi: 10.1155/2013/637904. Epub 2013 Jul 28 [PubMed PMID: 23984135]
Level 3 (low-level) evidenceGill KG. Congenital musculoskeletal anomalies - key radiographic findings. Pediatric radiology. 2022 Apr:52(4):777-785. doi: 10.1007/s00247-021-05200-x. Epub 2021 Nov 3 [PubMed PMID: 34731287]
Uduma FU, Dim EM, Njeze NR. Proximal femoral focal deficiency - a rare congenital entity: two case reports and a review of the literature. Journal of medical case reports. 2020 Feb 5:14(1):27. doi: 10.1186/s13256-020-2350-y. Epub 2020 Feb 5 [PubMed PMID: 32019581]
Level 3 (low-level) evidenceKayser R, Mahlfeld K, Grasshoff H, Merk HR. Proximal focal femoral deficiency -- a rare entity in the sonographic differential diagnosis of developmental dysplasia of the hip, Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2005 Oct:26(5):379-84 [PubMed PMID: 16240250]
Özdemir M, Kavak RP, Ceylan AH, Cevval ZK. Isolated unilateral proximal focal femoral deficiency presenting in a young woman. BMJ case reports. 2020 Jan 21:13(1):. doi: 10.1136/bcr-2019-232714. Epub 2020 Jan 21 [PubMed PMID: 31969407]
Level 3 (low-level) evidenceKalaycioglu A, Aynaci O. Proximal focal femoral deficiency, contralateral hip dysplasia in association with contralateral ulnar hypoplasia and clefthand: a case report and review of literatures of PFFD and/or FFU. Okajimas folia anatomica Japonica. 2001 Aug:78(2-3):83-9 [PubMed PMID: 11732209]
Level 3 (low-level) evidenceGerscovich EO, Sekhon S, Loehfelm TW, Greenspan A. Fetal ultrasound: Early diagnosis and natural evolution of proximal femoral focal deficiency. Journal of ultrasonography. 2017 Dec:17(71):294-298. doi: 10.15557/JoU.2017.0043. Epub 2017 Dec 29 [PubMed PMID: 29375906]
Kudla MJ, Beczkowska-Kielek A, Kutta K, Partyka-Lasota J. Proximal femoral focal deficiency of the fetus - early 3D/4D prenatal ultrasound diagnosis. Medical ultrasonography. 2016 Sep:18(3):397-9. doi: 10.11152/mu.2013.2066.183.kud. Epub [PubMed PMID: 27622419]
D'Ambrosio V, Pasquali G, Squarcella A, Marcoccia E, De Filippis A, Gatto S, Camilla A, Pizzuti A, La Torre R, Giancotti A. Prenatal diagnosis of proximal focal femoral deficiency: Literature review of prenatal sonographic findings. Journal of clinical ultrasound : JCU. 2016 May:44(4):252-9. doi: 10.1002/jcu.22306. Epub 2015 Sep 26 [PubMed PMID: 26408260]
Graviss ER, Monteleone PA, Wampler LR, Silberstein MJ, Brodeur AE. Proximal femoral focal deficiency associated with the Robin anomalad. Journal of medical genetics. 1980 Oct:17(5):390-2 [PubMed PMID: 7218279]
Level 3 (low-level) evidenceMehta S, Rajaram S, Bhaskaran S, Goel N. Proximal femoral focal deficiency revisited: a case report. Journal of the Indian Medical Association. 2014 Feb:112(2):121, 130 [PubMed PMID: 25935972]
Level 3 (low-level) evidenceWestberry DE, Davids JR. Proximal focal femoral deficiency (PFFD): management options and controversies. Hip international : the journal of clinical and experimental research on hip pathology and therapy. 2009 Jan-Mar:19 Suppl 6():S18-25 [PubMed PMID: 19306244]
Gupta SK, Alassaf N, Harrop AR, Kiefer GN. Principles of rotationplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2012 Oct:20(10):657-67. doi: 10.5435/JAAOS-20-10-657. Epub [PubMed PMID: 23027695]
Bryant DD 3rd, Epps CH Jr. Proximal femoral focal deficiency: evaluation and management. Orthopedics. 1991 Jul:14(7):775-84 [PubMed PMID: 1871029]
Hamanishi C. Congenital short femur. Clinical, genetic and epidemiological comparison of the naturally occurring condition with that caused by thalidomide. The Journal of bone and joint surgery. British volume. 1980 Aug:62(3):307-20 [PubMed PMID: 7410462]
Level 2 (mid-level) evidenceFuller CB, Lichtblau CH, Paley D. Rotationplasty for Severe Congenital Femoral Deficiency. Children (Basel, Switzerland). 2021 Jun 1:8(6):. doi: 10.3390/children8060462. Epub 2021 Jun 1 [PubMed PMID: 34205839]
Lamberg EM, Collins CK, Hanks JE, Alaniz MB. Management of proximal femoral focal deficiency with limited resources in Haiti. Prosthetics and orthotics international. 2013 Feb:37(1):58-64. doi: 10.1177/0309364612447095. Epub 2012 Jun 1 [PubMed PMID: 22661340]
Level 3 (low-level) evidenceBedoya MA, Chauvin NA, Jaramillo D, Davidson R, Horn BD, Ho-Fung V. Common Patterns of Congenital Lower Extremity Shortening: Diagnosis, Classification, and Follow-up. Radiographics : a review publication of the Radiological Society of North America, Inc. 2015 Jul-Aug:35(4):1191-207. doi: 10.1148/rg.2015140196. Epub [PubMed PMID: 26172360]
Alman BA, Krajbich JI, Hubbard S. Proximal femoral focal deficiency: results of rotationplasty and Syme amputation. The Journal of bone and joint surgery. American volume. 1995 Dec:77(12):1876-82 [PubMed PMID: 8550656]
Prince DE, Herzenberg JE, Standard SC, Paley D. Lengthening With External Fixation Is Effective in Congenital Femoral Deficiency. Clinical orthopaedics and related research. 2015 Oct:473(10):3261-71. doi: 10.1007/s11999-015-4461-0. Epub 2015 Jul 21 [PubMed PMID: 26194561]
Mohanty RK, Sahoo S, Dey M, Milan A, Das SP. Efficacy of prosthetic rehabilitation in rotationplasty following Ewing's sarcoma: A case study. Journal of pediatric rehabilitation medicine. 2022:15(2):359-368. doi: 10.3233/PRM-210060. Epub [PubMed PMID: 35095000]
Level 3 (low-level) evidenceSo NF, Andrews KL, Anderson K, Gozola MA, Shives TC, Rose PS, Shaughnessy WJ, Sim FH. Prosthetic fitting after rotationplasty of the knee. American journal of physical medicine & rehabilitation. 2014 Apr:93(4):328-34. doi: 10.1097/PHM.0000000000000044. Epub [PubMed PMID: 24398578]
Level 2 (mid-level) evidence