Introduction
Fundus fluorescein angiography (FFA) and its clinical role in ophthalmology were discovered by two medical students, Herald Novotny and David Alvis.[1] Flocks and colleagues had earlier experimented with intravenous injection of fluorescein in cats to study retinal circulation.[2] However, when the same was attempted in humans, insufficient lighting of the fundus camera and the absence of a barrier filter prevented them from getting serial images of the fundus to measure the circulation time. With time, fundus camera technology improved.
Novotny and Alvis used the new fundus camera and two different filters, blue and green, to image the fluorescence of the dye. They successfully got the first fluorescein angiogram images, which were reported in their published paper in 1961.[3] FFA allows the assessment of the anatomy, physiology, and pathology of the retinal and choroidal vasculature. Sodium fluorescein is an orange water-soluble dye. It has a low molecular weight of 376.3 Da.[4] It remains primarily bound to proteins (80%) in the blood.[5] The retinal vascular endothelium and the retinal pigment epithelium (RPE) act as barriers and do not allow the diffusion of the dye.[6] However, the choriocapillaris allows it to diffuse freely.[7] It is excreted in the urine over 24 to 36 hours and is responsible for the discoloration of urine.[5] It absorbs light in the range of 465 to 490 nm (blue light) and emits light in the range of 520 to 530 nm (green light).[4]
FFA is a diagnostic procedure where a series of photographs of both eyes are taken after injecting sodium fluorescein intravenously. Interpretation and understanding of FFA help in the accurate diagnosis and evaluation of various pathologies. This review also discusses the complications and the role of the interprofessional approach in managing patients undergoing FFA.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Both the retina and the choroid receive their blood supply from the ophthalmic artery, which is the first branch of the internal carotid artery. The ophthalmic artery divides into a proximal branch, the posterior ciliary arteries, and a distal branch, the central retinal artery.[8] To understand the blood supply of the retina, the retina can be divided into two parts. The central retinal artery supplies the inner part that extends from the internal limiting membrane to the inner nuclear layer. The outer retinal layers that extend from the outer plexiform layer to the retinal pigment epithelium (RPE) are avascular and are supplied by diffusion from the choriocapillaris.[9] The boundary between the blood supply by the central retinal artery and choriocapillaris may vary depending on the amount of light, retinal thickness, and topographical location.
The central retinal artery divides into the superior and inferior branches after entering the eye and piercing the lamina cribrosa. These further branch into nasal and temporal arcades. These arcades further bifurcate and supply the inner retinal layers. The larger retinal arteries and veins are located in the nerve fiber and ganglion cell layer. The retina may be supplied by up to 4 layers of vessels, including radial peripapillary capillary network (around the optic disc at the nerve fiber layer), superficial capillary plexus (at ganglion cell layer), and two capillary beds of deep capillary plexus (located one on either side of the inner nuclear layer). In a certain percentage of the population (6.9 to 49.5 percent, usually 20%), an additional vessel, a cilioretinal arteriole, originates from the short posterior ciliary plexus around the disc and supplies the macula.[10]
The choroid receives its blood supply via the posterior ciliary arteries. The posterior ciliary arteries (PCA) divide into two long and numerous short posterior ciliary arteries. The two long PCA enter the sclera on the medial and lateral sides and are named accordingly. These run anteriorly towards the iris to anastomose with the anterior ciliary arteries. The choroidal watershed area represents the area between the supply of each posterior ciliary artery.[11] During angiography, a slightly delayed filling may be seen in this zone.
The fovea is the central part of the retina. This area comprises a total of six layers, including the internal limiting membrane and the RPE.[12] The outer plexiform layer here lies obliquely (Henle Layer), which is the reason for the petaloid collection of fluid at the fovea on angiography.[13] The RPE cells at the fovea are longer and have a larger concentration of melanin pigment. Xanthophyll is also present in the outer plexiform layer at the fovea in high density. A capillary free zone of 400 to 500 microns in diameter is present at the fovea, called the foveal avascular zone (FAZ).[14] The fovea appears hypofluorescent because of the absence of blood vessels in the FAZ. All of these are responsible for the dark appearance of the fovea in angiography.
Venous drainage of the inner retina occurs into the branch retinal veins, which then flow into the central retinal vein. The central retinal vein drains into the cavernous sinus. The choroid is drained through the vortex veins. These veins drain into the superior and inferior orbital veins, which drain into the cavernous sinus and pterygoid plexus, respectively.[9]
Indications
Unexplained Vision Loss
Diabetic Retinopathy
- To rule out macular ischemia in cases of diabetic retinopathy with unexplained poor vision.[15]
- To differentiate between focal or diffuse leakage in cases with macular edema. The focal leak occurs from microaneurysms, and the diffuse leak arises from the retinal capillaries. This helps to decide the target areas for the macular laser. A focal laser targets the leaking microaneurysm in focal macular edema, and a macular grid may be used to manage diffuse macular edema.[16]
- To confirm cases of proliferative diabetic retinopathy where neovascularization is not well demarcated on clinical examination.
- In cases with asymmetric diabetic retinopathy, look for signs of choroidal filling delay and rule out other differential diagnoses.
- To document and confirm findings in cases with asteroid hyalosis.[17][18] FFA gives excellent clarity of images in such cases.[17]
- To differentiate between diabetic macular edema (DME) and Irvine Gass syndrome (pseudophakic cystoid macular edema).[16] Pseudophakic cystoid macular edema (CME) shows disc leak and petaloid macular leak and may show perivascular stain or leak (signs of intraocular inflammation) on FFA. In DME, there is a preponderance of microaneurysms and other retinal vascular changes typical of diabetic retinopathy without evidence of intraocular inflammation. In many cases, changes due to both diabetes and pseudophakic CME may co-exist.
Retinal Vein Occlusions
- It may be prudent to wait for 2 or 3 months to allow for the resolution of hemorrhages before considering angiography in these cases, as the hemorrhages cause blocked fluorescence.
- To classify ischemic and nonischemic central retinal vein occlusion (CRVO). The central vein occlusion study (CVOS) defined ischemic CRVO or nonperfused CRVO as having at least 10 disc areas of nonperfusion in the central 7 field FFA. However, this definition may need re-evaluation with the advent of ultra-widefield FFA.
- It helps in cases with clinically undetectable tributary vein occlusions.
- To confirm the areas of subtle neovascularization.
- To look for areas of capillary nonperfusion.
Choroidal Neovascularization
- To identify and document the activity of choroidal neovascular membrane (CNVM) in various retinal diseases, including age-related macular degeneration (AMD), pigment epithelium detachment (PED), choroidal rupture, RPE rip, angioid streaks, serpiginous choroiditis, presumed ocular histoplasmosis syndrome, Best vitelliform macular dystrophy, choroidal osteoma, healed choroidal tuberculoma, and others.[19][20][21]
- To confirm the diagnosis in cases of myopic CNVM. Myopic CNVM may not show hyper-cyanescence and thus may not be detectable on Indocyanine green angiography (ICGA) imaging.[22] ICGA imaging is useful in cases with lacquer cracks.[23]
- To classify classic and occult CNVM.
Other Indications
- To confirm the diagnosis of ocular ischemic syndrome in cases with neovascularization of the iris, venous dilatation, and mid-peripheral retinal hemorrhages.
- To identify the type of leak in cases of central serous chorioretinopathy (CSCR). The location of the leak helps in planning focal laser photocoagulation in these cases.
- Confirmation of diagnosis of parafoveal telangiectasia and to identify and confirm CNVM in these cases.
- To confirm the diagnosis of ischemic optic neuropathy and other neuro-ophthalmic diseases.
- To confirm activity in cases of retinal vasculitis.
- To look for capillary nonperfusion areas and neovascularization in cases of resolved retinal vasculitis.
- To evaluate cases of uveitis, retinitis, and posterior scleritis.
- To evaluate intraocular tumors.
- To evaluate various retinal vascular disorders, including retinopathy of prematurity, sickle cell retinopathy, and others.
- To evaluate the retinal dystrophies.
- To evaluate and document neovascularization and vascular status of the anterior segment, including cornea and iris.[24]
Contraindications
Fluorescein angiography is relatively contraindicated in pregnancy. It is classified as a category C drug. There is a lack of well-controlled studies assessing the safety of intravenous fluorescein in pregnancy. Therefore it is usually avoided in pregnant females, especially in the first trimester.[25] Fluorescein is also excreted in breast milk, and its excretion may be prolonged (up to 3 days). The risk of fluorescein-induced phototoxicity related to phototherapy in a neonate consuming breast milk of a mother who has undergone FFA needs evaluation.[26]
The risk of phototoxicity with the dye is due to the generation of a superoxide anion after exposure to light at a wavelength of 480 nm.[27] However, in the particular reported case of phototherapy-induced phototoxicity in a premature neonate, other factors were also held accountable.[28] Deficiency of superoxide dismutase, an antioxidant, in premature infants, reduced serum albumin leading to an increased free fraction of fluorescein in serum, increased risk of developing hyperbilirubinemia, and impaired renal clearance of fluorescein was hypothesized as additional risk factors.[28][29]
A history of severe allergic reactions is an absolute contraindication for fluorescein administration. In patients with a history of moderate allergic reactions to fluorescein, pretreatment with an antihistaminic agent or corticosteroid can be done.[25] Intradermal skin testing is often negative, even in cases with a previous history of allergic reaction, and is therefore not very helpful before performing the procedure.[25]
A history of cardiovascular or renal disease is not a contraindication for FFA. However, the healthcare team should be aware of this history, especially for managing allergic reactions in these patients.[25] Studies have evaluated the effect of intravenous fluorescein injection in patients with impaired renal function. A study reported increased serum levels of creatinine for three days after the administration of fluorescein in patients with diabetes. However, they did not observe any adverse effects related to fluorescein.[30] These studies did not find any adverse effect of fluorescein administration in these cases despite fluorescein being eliminated by excretion in urine predominantly.[25] FFA is not contraindicated even in dialysis patients, as the dye will be eliminated through hemodialysis.[25][31]
Equipment
A fundus camera equipped with an excitation and barrier filter is required for taking photographs. Cobalt blue excitation filter transmits blue light in the range of 465 to 490 nm, and this is in the range of absorption peak of fluorescein. The barrier filter transmits light in the range of 520 to 530 nm, which is the range of the emission peak of fluorescein. With the advent of digital angiography, a computer with software is required for processing the images captured.
The adult dose of sodium fluorescein is 500 mg intravenously. A vial of sodium Fluorescein dye (2 ml of 25%, 2.5 ml of 20%, 5 ml of 10 %, or 10 ml of 5 %) is required. A 23 gauge scalp vein needle set and a 5 ml syringe with a needle to draw the dye, armrest, tourniquet, and alcohol swab are also required. Standard emergency equipment to manage anaphylaxis should always be ready before starting the procedure.
Personnel
The healthcare team for carrying out the FFA procedure includes nursing staff, an anesthetist, and an ophthalmologist.
Preparation
Before starting the procedure, informed consent from the patient should be obtained. The benefits and risks involved with the procedure should be explained to the patient. The healthcare team should know about the patient's history of allergies. In cases with a history of hypersensitivity to fluorescein, premedication with antihistamines or corticosteroids should be considered. Fasting of a minimum duration of 2 to 4 hours on the day of the procedure is recommended. There are no reports suggestive of a difference between the volume of fluorescein injected and the rate of adverse reactions.[32] However, studies have documented superior-quality images with 25% fluorescein solutions.[33]
For children, based on age and weight, 7.7 mg/kg of fluorescein can be injected up to a maximum dose of 500 mg. Studies using FFA for retina imaging in preterm neonates report an injection of 20% sodium fluorescein as a bolus dose of 0.04 mL/kg (8 mg/kg) followed by a saline flush.[34][35]
The pupil should be well dilated before starting the procedure. The patient should be primed about the risk of nausea so that initial images are properly acquired. The emergency cart with the equipment should be available. The lens of the camera should be checked for clarity, and alignment and focusing should be cross-verified. A few control images should be taken to check for pseudo-fluorescence and autofluorescence. The patient's arm should be placed comfortably on the armrest, and the patient should be comfortably seated. The chin should be placed on the chin rest. Explaining to the patient that the images will be taken in primary gaze and then in different gazes (for a usual fundus camera) makes the procedure swift. The scalp vein set is to be placed in the patient's arm, and some blood is drawn into the tubing to confirm the position of the needle.
The eye of concern and the exact location of the lesion should be confirmed before starting to capture the images. The color photographs are taken initially, then the dye should be injected, and the timer should be started simultaneously. When initiating the injection of dye, any extravasation of the dye should be carefully monitored. While taking the photographs, special consideration should be given to any specific area of pathology if marked by the ophthalmologist. The healthcare team should be alert to notice early signs of syncope or anaphylaxis in the patient. It is better to reassure the patient throughout the procedure and keep them informed about its progress. After the initial few images of one eye, the camera is moved to the other eye to capture images.
Oral FFA can be done after off-label oral administration of sodium fluorescein.[36] The confocal scanning laser ophthalmoscope-based systems may provide better image quality than conventional digital fundus camera-based FFA.[36]
Technique or Treatment
The fundus camera is equipped with two filters. An excitation filter (465 to 490 nm) allows only blue light to enter the eye to excite the fluorescein molecules of the dye. A barrier filter (520 to 530 nm) allows only the green light emitted by the dye to pass through.[4] It blocks the light of any other range that is reflected from the eye. Injection of the dye is timely coordinated with the process of taking photographs. Earlier images are taken every 1 to 2 seconds, followed by images of the other eye. Later photographs can be taken at a slower pace depending on the pathology.
Normal FFA Phases
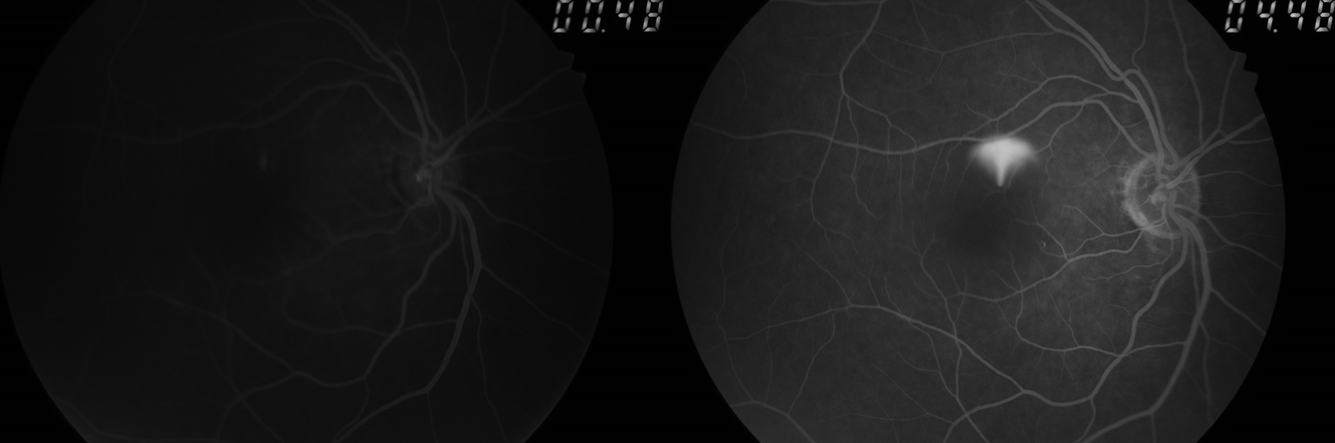
- Choroidal phase: The dye takes 10 to 15 seconds to reach the retina after being injected into the arm. It first enters the posterior ciliary arteries, and thus choroidal filling is seen first. A few islands may show delayed filling in healthy individuals also. The cilioretinal artery, when present, fills in this phase because of its origin from short posterior ciliary arteries.
- Arterial phase: The dye enters the arteries usually 1 to 2 seconds after the choroidal phase.
- Arteriovenous: This is seen 1 to 2 seconds after the arterial phase. The dye flows into the precapillary arterioles, capillaries, and postcapillary venules. A laminar flow can be seen in this phase when the dye enters the large veins. The laminar flow is seen because the protein-bound fluorescein is pushed to the sides by the free-flowing plasma and red bleed cells, which flow in the center.
- Venous: This has three phases early, mid, and late depending on the filling of the dye. The early phase shows a laminar flow with complete venous filling in the mid-phase, followed by a reduced dye concentration in the arteries in the late phase. A peak phase of the angiogram is seen when the perifoveal capillary network is well-defined.
- Recirculation: After the venous phase, the dye concentration starts reducing in the vessels, with complete emptying at around 10 minutes. In this phase, staining of the optic disc, Bruch’s membrane, choroid, and sclera are seen.
Complications
Extravasation of the dye under the skin is a severe side effect and should be avoided. It leads to severe pain and may cause necrosis of the skin. Applying an icepack to the area of extravasation helps relieve pain. Nausea is the most frequent side effect (0.06 to 15.29 %).[25] However, it is transient. Vomiting does not occur frequently. In patients with a previous history of nausea and vomiting, treatment with oral promethazine hydrochloride (25 to 50 mg) one hour before the procedure helps prevent the episode. Vasovagal syncope occurs in apprehensive patients. It may occur even at the sight of the needle. It may cause the patient to lose consciousness for a few minutes, but it is reversible.
A more severe reaction in the form of bradycardia and hypotension may occur. In this case, the patient is made to lie down with their feet elevated. Blood pressure is monitored. The most frequent allergic reactions are mild. This includes hives or itching. Antihistamines are useful in these cases. Severe allergic reactions like bronchospasm, laryngeal edema, and acute myocardial infarction are life-threatening and need urgent care. A few cases of death have been documented with intravenous administration of fluorescein. The emergency equipment and drugs cart should always be available. Epinephrine, steroids, and bronchodilators like aminophylline help treat bronchospasm and severe anaphylactic reaction that may occur. The overall incidence of death has been reported as 1 in 2,22,000.[37]
Clinical Significance
To interpret the fluorescein angiography images, knowledge of the following terms is important:
Hyperfluorescence
- Preinjection Fluorescence
- Autofluorescence: It is the property of certain biological structures to emit fluorescent light when they absorb light in the absence of fluorescein dye. This occurs due to the presence of fluorophores like lipofuscins or porphyrins. A normal optic disc does not have any fluorophores. Optic disc drusen show excessive accumulation of mitochondria. Porphyrins are present in mitochondria. Optic disc-drusen superficial or buried show autofluorescence due to porphyrins in these dysfunctional mitochondria.[38][39] The role of calcium as a cause for autofluorescence of optic disc drusen needs further research.[40] Retinal astrocytic hamartomas also show autofluorescence.[41]
- Pseudofluorescence: This occurs due to the presence of old filters or allowing transmission of wavelength beyond blue and green light. This nonfluorescent light is reflected from various lesions, including exudates, sclera, and scar, and is perceived as a bright spot on the preinjection angiogram images. Dehemoglobinised blood also reflects blue light that passes through the barrier filters causing it to cause hyperfluorescence on preinjection images. This may happen even if the filters are not worn out or old if there are overlapping wavelengths of the barrier and excitation filters.
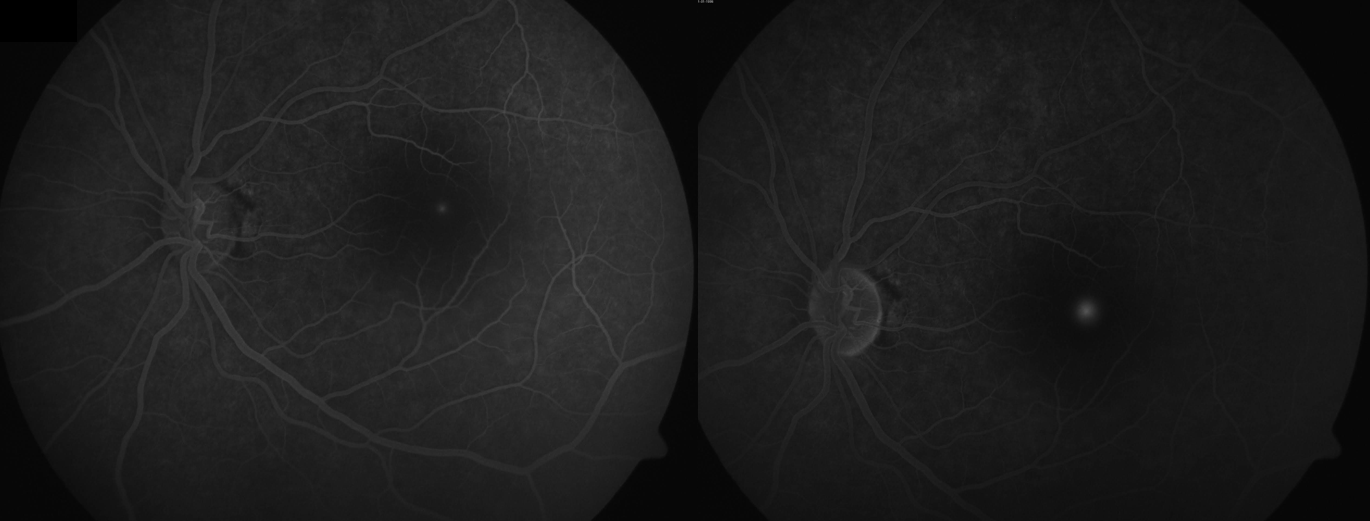
- Transmitted fluorescence: Healthy RPE blocks the choroidal fluorescence. In cases with atrophy of the RPE, the visibility of the underlying choroidal fluorescence is enhanced. This is known as transmitted fluorescence or window defect. This fluorescence follows the same pattern as the flow of dye through normal ocular circulation. It appears early and peaks early during the choroidal filling phase. It is of the same shape as that of pigment epithelial atrophy. Therefore, it doesn't increase in size or shape with time. The fluorescence fades as and when the dye washes out of the choroidal circulation.
- Leak: Fluorescence that shows an increase in size and intensity with time is defined as a leak, which happens due to dye leakage in a potential space.
- Pooling: In this case, the fluorescence shows an increase in intensity with time. However, the size of fluorescence remains the same throughout the angiogram. This is because of the dye collection in a defined anatomic space, either subretinal or sub-RPE space.
- Stain: Certain structures retain the fluorescein dye and show hyperfluorescence in the later stages of the angiography. This is known as staining.
Hypofluorescence
The area of reduced or absent fluorescence is seen as a dark area on the angiogram.
- Blocked fluorescence: This shows reduced visibility of the fluorescence of underlying retinal or choroidal circulation due to a barrier located anterior to the respective circulation. This needs to be correlated clinically or with the color photograph. The shape of the hypofluorescent area will correlate with that of the barrier, for example, bleeding in the subhyaloid space or subretinal space. Opacities involving the cornea, anterior chamber, lens, or vitreous affect the quality of the photograph as a whole and are not seen as hypofluorescent areas. However, asteroid hyalosis does not affect the angiography imaging because the light scattered by the suspended particles is filtered by the barrier filter. This makes FFA a better tool for investigation than the clinical examination with an ophthalmoscope in cases with asteroid hyalosis.[42]
- Vascular filling defect: Absence of visibility of fluorescence due to lack of retinal or choroidal circulation perfusion. A total lack of perfusion results in hypofluorescence that persists through all the phases of the angiogram. A partial lack of perfusion results in delayed filling of the circulation or delayed fluorescence.
A few clinical scenarios have been discussed herewith.
Epiretinal Membrane (ERM)
Abnormal retinal vessel tortuosity and dilation may be seen at the macula, and this is due to the contraction of the internal limiting membrane by the epiretinal membrane. In the late phases of the angiogram, small leakage from these vessels may be seen. Hyperfluorescence at the fovea due to cystoid macular edema may also be present. Other features of retinal vein occlusion, diabetic retinopathy, or coexisting pathology may be seen in cases of secondary epiretinal membranes.
RPE Rip
The RPE tears at one edge of a pigment epithelium detachment, and the RPE layer scrolls at the other end. This is seen in cases of PED with underlying CNVM or PED in cases of CSCR. The characteristic feature of angiography is transmitted hyperfluorescence in the area of the tear. This area of the exposed choroid may show staining in the later phases. The area where the RPE scrolls underneath shows blocked fluorescence.[19] This blocked fluorescence persists throughout the angiogram. In cases with associated CNVM, the CNVM usually lies at the end of the scrolled RPE.
Drusen
Areas of hard drusens show transmitted fluorescence due to atrophy of the overlying RPE. Areas of soft drusen show late mild hyperfluorescence or staining. This is due to the hydrophobic content of the soft drusen. Drusenoid pigment epithelium detachment also shows hyperfluorescence that starts in the mid-arteriovenous phase increases in the late venous phase and persists in the recirculation phase. Cuticular or basal laminar drusen may give a starry sky appearance. Subretinal drusenoid deposits or reticular pseudodrusen may not be evident on FFA or show minimal hypofluorescence.[43]
Serous PED
Serous PED shows fluorescence that starts in the early phase and increases uniformly in intensity in the subsequent phases. The borders of the lesion are well-demarcated with no leakage. The fluorescein dye pools in the sub-RPE space and causes this pattern of fluorescence. Cases of serous PED may show a notch in a few instances. These cases have an underlying CNVM.[44]
Choroidal Neovascular Membrane (CNVM)
Classic CNVM is identified by a well-defined lacy network of early hyperfluorescence, which shows an increase in the intensity and size of the fluorescence in the subsequent phases. This is due to the progressive leakage of the dye. The boundaries of the area of hyperfluorescence become indistinct due to leakage.[45]
Occult CNVM shows two patterns:
- Fibrovascular PED: This is characterized by irregularly elevated RPE with a gradually progressive stippled leak on FFA. Alternatively, a notched serous PED may be seen where the notch or hot spot harbors the CNVM. Such serous PEDs are called vascularized serous PEDs.
- Late leakage from an undetermined source: This is not recognizable until the later phases of the angiogram, where hyperfluorescence is seen. However, the exact source of the leakage cannot be distinguished.[45]
ICGA is a better investigation tool for imaging occult CNVM as the visibility of the CNVM network is better than on FFA.[46][47] ICGA also helps identify cases of idiopathic polypoidal choroidal vasculopathy.[48]
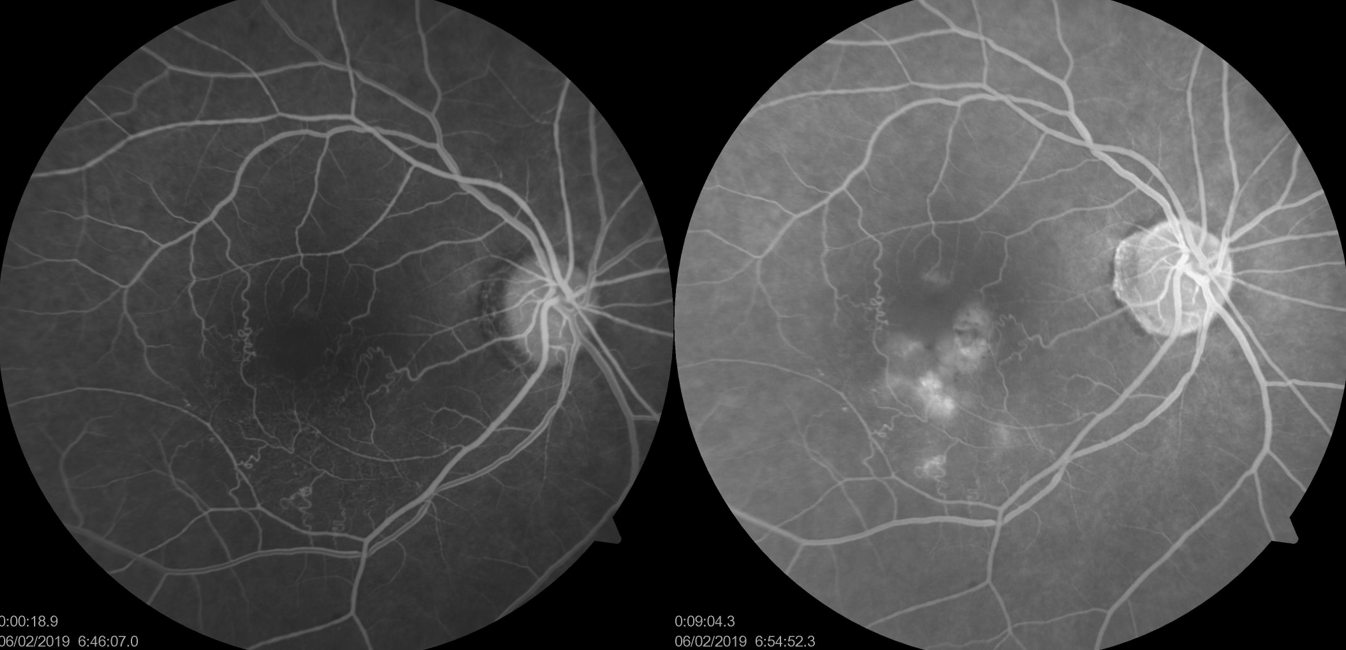
Central Serous Chorioretinopathy (CSCR)
Inkblot leak is seen as dot hyperfluorescence in the early phases, which increases in the following phases. The increase in size is limited as it does not fill the entire area of neurosensory detachment. The number of leaks may vary, and multifocal leakage points may be seen. In a few cases of CSCR, the dot leak is succeeded by a mushroom pattern leak. This is because the dye ascends up and then spreads laterally. The osmotic gradient of the fluid allows rapid diffusion of dye to the superior part of the detachment. Proteins accumulate in the inferior part delaying the entry of dye. This is known as smokestack or umbrella pattern leakage.[49] Chronic cases of CSCR may show transmitted fluorescence at the posterior pole, and a descending RPE track may be noted as a window defect in a teardrop pattern. These are areas of RPE alteration resembling prior fluid collection. The inkblot pattern of leakage is more prevalent than the smokestack pattern. Diffuse type of leakage is the least prevalent.[50][51]
Cystoid Macular Edema (CME)
Leakage from the perifoveal capillaries is seen earlier in the arteriovenous phase, followed by dye accumulation in a petaloid pattern or a more diffuse hyperfluorescence in the later phases.[52]
Diabetic Retinopathy
Microaneurysms appear as dots that hyperfluoresce.[53] These areas of dot hyperfluorescence should correspond with microaneurysms on color photographs of the fundus. This helps differentiate microaneurysms from dot hyperfluorescence due to RPE defects. FFA is a better investigative tool to pick up microaneurysms, and subtle changes in diabetic retinopathy become evident on FFA. An additional number of microaneurysms are visible on FFA compared to clinical examination with the naked eye. Leakage in diabetic macular edema was historically graded as focal or diffuse based on the FFA features. The term focal leak was used for eyes where more than 67 percent of the leakage originated from microaneurysms. The term diffuse leak was used in cases where less than 33 percent of leakage was noted from microaneurysms (most of the leakage was noted from dilated capillaries).[54]
Clinically, focal diabetic macular edema is characterized by a circinate ring of hard exudates around a microaneurysm. Areas of superficial and deep hemorrhages and areas of nonperfusion are visualized as areas of hypofluorescence. The former are areas of blocked fluorescence and need to be correlated clinically. The latter are areas of ischemia. The foveal avascular zone may also show enlargement in size due to ischemia (macular ischemia) and should be examined carefully. In cases with diabetic retinopathy and unexplained vision loss, distortion of the anatomy of perifoveal capillaries indicates macular ischemia.[55][56][57][58]
Abnormal vessels connecting two veins in the areas of capillary nonperfusion may also be seen in cases of diabetic retinopathy. These do not leak, and these are intraretinal microvascular abnormalities (IRMA). IRMA is present within the retina, unlike neovascular vessels, which tend to protrude into the vitreous. Neovascularization networks leak dye from the early phases of the angiogram. Venous beading and loops can also be better appreciated on fluorescein angiography.
Vogt-Koyanagi-Harada Syndrome
Multiple hyperfluorescent point leaks are seen in the arteriovenous phase, increasing intensity in the venous phase. In the later phases, extensive dye pooling is seen in neurosensory detachment, unlike CSCR, where the dye does not fill the entire detachment area, even in the late phases. Another differentiating feature from CSCR is the absence of pigment epithelium detachments in eyes with Vogt-Koyanagi-Harada syndrome. The presence of intraocular inflammation in VKH causes staining of the disc in the late phases.
Best Dystrophy
These are inherited macular dystrophy characterized by yellowish subretinal deposits and sometimes subretinal collections of fluid, depending on the stage of the disease. With time these deposits may be partially absorbed with the gravitation of the remaining deposits. In the early stage, also known as the vitelliform stage, fundus fluorescein angiography shows blocked choroidal fluorescence in the area of the subretinal yellowish deposit.[59]
This area may slightly hyperfluoresce in the later stages or may remain hypofluorescent. In the later stages, when the yellowish deposits have reabsorbed, the FFA shows window defects in the area of absorbed deposits due to RPE changes. The area where the yellowish deposit gravitates shows blocked fluorescence.[60] Fibrous scar, when present in the later stages of the disease, shows staining on FFA. FFA is also useful in identifying CNVM in these cases. In cases of adult-onset vitelliform macular dystrophy, FFA is useful in differentiating these cases from CSCR.
Malignant Melanoma
The tumor shows a characteristic double circulation sign where the intrinsic vascularity of the tumor mass is seen along with normal retinal circulation.
Choroidal Hemangioma
These tumors are vascular hamartomas. They show intense hyperfluorescence in the early arterial phase itself because of their choroidal origin. The fluorescence increases with the leakage of dye in the later phase because of the vascularity of the tumor.[61]
Choroidal Metastasis
The choroidal metastases show hypofluorescence in the arterial phase and then hyperfluoresce in the venous phase of the angiogram. Pinpoint areas of fluorescence appear in the late venous phase at the margins of the lesion. Variable fluorescein patterns may be seen depending on the type of tumor mass.[62] Some lesions block choroidal fluorescence throughout the angiography, some show progressive fluorescence in the late phases, some show fluorescence in the early phase itself, and then show leakage in the subsequent phases.[62] However, FFA may show multiple pinpoint leaks in choroidal metastasis. FFA helps to differentiate it from other causes of choroidal mass, like choroidal hemangioma or melanoma. Other pathologies that may show multiple pinpoint leaks on FFA include[63][64][65][66]
- posterior scleritis,
- toxemia of pregnancy,
- hypertensive choroidopathy,
- IgA nephropathy,
- Vogt-Koyanagi-Harada disease,
- sympathetic ophthalmia,
- punctate inner choroidopathy, and
- leukemia or lymphoma of the uveal tract.
Choroidal Granuloma
They are discrete yellowish-white elevated lesions with indistinct borders. They show hypofluorescence in the early phases and hyperfluorescence in the late phases of angiography.[67] Common causes include sarcoidosis and tuberculosis.
Retinal Capillary Hemangioma
The feeder arteriole fills earlier with the rapid flow of the dye into the venule. The tumor shows intense hyperfluorescence. Angiography is useful in these cases as it identifies smaller angiomas that are not easily visible on naked eye examination.[61][68] These lesions are typically seen in Von Hippel-Lindau's disease.[61]
Branch Retinal Vein Occlusion (BRVO)
Recent vein occlusion cases will show blocked fluorescence corresponding to retinal hemorrhages. Therefore, it is better to wait for the hemorrhages to resolve. In branch retinal vein occlusion (BRVO) cases, a delay in filling the affected vein is noted. Dilated capillaries, microaneurysms, and telangiectatic changes may also be noted. Areas of capillary nonperfusion may be present and may involve the foveal avascular zone. Macular edema is noted as late leakage of dye by the perifoveal capillaries. Late staining of the affected vessels may also be seen. In chronic cases of BRVO, neovascularization of the retina or the optic disc may also be seen. These vessels show fluorescence in the early phases and excessive leakage of the dye subsequently.
Central Retinal Vein Occlusion (CRVO)
Tortuosity and dilatation of the veins are well-demarcated. The capillaries at the disc are also dilated. The filling of dye in the veins is delayed. A delay in the emptying of the veins is also noted. Leakage of dye from the perifoveal capillaries may also be seen. Capillaries may show microaneurysmal changes. Staining of the wall of the vein may be seen. If the size of the capillary nonperfusion area in CRVO is more than ten disc areas, the diagnosis of ischemic CRVO or non-perfused CRVO is confirmed.[69]
Myopic CNVM
FFA is useful in evaluating patients with myopia who have a history of recent vision loss. CNVM in myopia may not always be visible on clinical examination with an ophthalmoscope. Myopic CNVM on FFA shows hyperfluorescence in the early phases, followed by leakage. The hyperfluorescence increases in size and intensity. However, these changes may be very subtle.[70] Associated subretinal hemorrhage present in these cases shows blocked choroidal fluorescence. Lacquer cracks are visible as hyperfluorescent lines due to transmitted fluorescence. Staining of the sclera in the late phases in the areas of RPE atrophy may also be seen at the posterior pole.
Ocular Ischemic Syndrome
A delay in the filling of the choroidal circulation is noted. The retinal arterioles also show delayed entry of dye in the late phases. Due to incomplete filling of the arterioles, the leading edge of the dye may be seen in the arterioles. Microaneurysms may be seen distributed in the mid-periphery of the retina. Other features include capillary nonperfusion, perivascular staining, and staining of the optic disc.[71]
Takayasu Arteritis
It is an autoimmune disease affecting the aorta, aortic arch, and its main branches. Granulomatous inflammation involving all the layers of the arterial wall results in the narrowing of the lumen of the affected blood vessels and ischemia. Involvement of the carotid artery causes ischemic changes in the retinal and choroidal circulation. There is a delay in the circulation time of the dye from the arm to the retina. There is also a delay in the appearance of the dye in the choroidal circulation. After the dye enters the retinal arterioles, a delay in filling the retinal veins may also be noted. Multiple arteriovenous shunts are also seen in these cases.[72] Numerous microaneurysms are observed due to the dilatation of the capillaries. Capillary nonperfusion areas are noted peripheral to the arteriovenous shunts. Profuse leakage from neovascularization at the disc or elsewhere in the retina may also be observed.
Central Retinal Artery Occlusion (CRAO)
A delay in filling the retinal arterioles with no perfusion in the early phases is usually seen. The flow in the retinal arterioles may be very sluggish, and the front edge of the dye may be noted to travel slowly in consecutive FFA images. The choroidal filling is normal—a delay in choroidal filling points towards the obstruction of the ophthalmic artery or ipsilateral carotid artery. A delay in filling the retinal veins is also noted with delayed arteriovenous transit. A patent cilioretinal artery, if present, fills during the choroidal phase of FFA. Dye front reciprocation (systolic advancement and diastolic retraction of the dye front) within the cilioretinal artery may be noted in cases with CRVO and cilioretinal arterial infarction.[73] The FFA may show normal angiography features in cases with transient CRAO or reperfused cases.[74]
Branch Retinal Artery Occlusion (BRAO)
Branch retinal artery occlusions usually occur due to an embolus. A delay in filling the artery distal to the embolus is seen in the early phases of the angiography. Retinal opacification, if present, shows a corresponding area of blocked fluorescence on the FFA. The occluded artery may not show complete fill even in the late phases. Mild staining of the wall of the occluded artery may be seen in the late phases.
Hypertension
Hypertensive retinopathy is characterized by changes at the arteriovenous crossings. Compression of the vein at the area where an arteriole crosses a venule is better appreciated on FFA. Cotton wool spots, when present, show corresponding areas of blocked fluorescence. Leakage from the capillaries at the disc may be seen in cases of malignant hypertension with disc edema. Acute Elschnig spots appear as multiple spots of hyperfluorescence. These are seen in the early phase of the angiogram, with dye leakage seen in the later phases. Siegrist's streaks are areas of fibrinoid necrosis of choroidal arterioles. They are usually tongue-shaped areas that show hypofluorescence due to nonperfusion.[75] If pigmentation is present, then corresponding blocked fluorescence may be seen. Malignant hypertensive choroidopathy may reveal multiple pinpoint leaks and pooling of dye beneath the serous retinal detachment.[65]
Disc Edema
Leakage of dye from the capillaries at the disc is seen in the early phases of the angiogram, followed by staining of the disc in the late phases. Cases with optic neuritis also show dilatation of the capillaries of the disc with leakage and staining of the disc in the subsequent phases. FFA can differentiate cases of papilledema (true disc edema) from pseudopapilledema. The latter does not show early or late dye leakage from the capillaries at the disc, and late hyperfluorescence of the disc is not a characteristic of pseudopapilledema.
Arteritic Anterior Ischemic Optic Neuropathy [Giant cell arteritis (GCA)] (A-AION)
A typical feature is a delay in the choroidal filling and prominence of choroidal watershed zones in the early phases. Differential filling of choroidal lobules or persistent non-filling of a few choroidal lobules may be noted.[76] The features suggest that in GCA, the flow impairment involves the short posterior ciliary arteries (SPCA) before these give branches to the optic nerve and choroid.[77]
Non-Arteritic Anterior Ischemic Optic Neuropathy (NA-AION)
Delay in the filling of the choroidal circulation is not usually seen in cases of non-arteritic ischemic optic neuropathy (NAION).[78] The characteristic feature is a difference in the filling of the dye in the capillaries at the disc. Poor filling of a segment of the disc is seen (usually superior or inferior) in the early phases of the angiogram with or without poor filling of the peripapillary choroid. Late phases show leakage at the disc, which may be diffuse (around 70%) and focal (around 30%).[79] Few unusual cases may show macular edema. In NA-AION, the distal branches of SPCA supplying the optic nerve (sparing branches to the choroid) are thought to be involved, which is more distal to the involvement seen in GCA.[77]
White Dot Syndrome
Multiple evanescent white dot syndrome (MEWDS) is identified in FFA by multiple small hyperfluorescent lesions in the early phase. These are arranged in a wreathlike configuration, and this hyperfluorescence persists in the late phase of the FFA.
FFA in cases with acute posterior multifocal placoid pigment epitheliopathy (APMPPE) reveals multiple hypofluorescent lesions in the early phase. These lesions show hyperfluorescence in the late venous phase. The early hypofluorescence results from blocked choroidal fluorescence due to infiltration of inflammatory cells or nonperfusion of the choriocapillaris.[80]
Sympathetic Ophthalmia
Two types of FFA patterns are seen in the acute stages of the disease. Multiple pinpoint hyperfluorescent leaks or multiple hypofluorescent spots in the early phase of the FFA may be seen.[81] The cases with pinpoint leaks[82] in the early phase show dye pooling in the subretinal space in the later phases. The cases with hypofluorescent spots in the early stages show staining of these spots in the late phases, similar to APMPPE.[64] The initial hypofluorescence may be due to blocked fluorescence by Dalen-Fuchs nodules or hypoperfusion of the choriocapillaris.
The chronic stages of sympathetic ophthalmia may show chorioretinal atrophy, nummular scars, subretinal fibrosis, and choroidal neovascularization. The nummular scars show transmitted fluorescence, and the subretinal fibrosis shows staining.
Uveal Effusion Syndrome
This is a less common ocular pathology where retinal detachment and ciliochoroidal detachment are noticed. These eyes are usually hypermetropic or nanophthalmic. Choroidal thickening is noticed on ultrasonography in these cases. FFA, in these cases, reveals a characteristic leopard spot appearance.[83] Multiple hypofluorescent and hyperfluorescence spots are observed in the early phase. These spots do not show any leakage in the late phases. FFA is not diagnostic in these cases. It is useful only to rule out other pathologies.
Intermediate Uveitis
Leakage from perifoveal capillaries leading to macular edema (petaloid leak) is seen in the arteriovenous phase. Staining of the disc is seen in the late phases.[84] The retinal vessels may also show leakage of dye and staining. Media may be hazy due to vitritis, which may limit the quality of captured images.
Retinitis
Retinitis appears as a whitish or yellowish lesion blocking the visibility of the choroid. On FFA, the early phase shows blocked fluorescence, and the late phase may show leakage of dye at the margin of the lesion.[24][67][85] FFA may also show vascular involvement and optic disc stain.[86]
Serpiginous Choroiditis
In its active phase, early hypofluorescence is seen due to blocked fluorescence resulting from the inflammatory process. Later phases of the angiography show staining of the margins of the lesion. Healed areas of the lesion show transmitted fluorescence without staining, and the areas of pigmentation show blocked fluorescence.[80]
Behçet disease
Fern-like fluorescein leakage has been described as a characteristic finding. It is prominent in the arteriovenous phase. It helps in the assessment of the status of inflammation. Leakage from retinal capillaries has been observed in cases of Behçet disease with no ocular symptoms or clinical signs of inflammation.[87] The presence of macular edema, disc hyperfluorescence, capillary nonperfusion areas, occlusion of retinal vessels, and neovascularization are other features observed in angiography, depending on the severity of the disease.
Posterior Scleritis
Ultrasonogram (USG) is the preferred tool for diagnosing posterior scleritis. USG reveals subtenon fluid (hypoechoic area) and a T sign. However, FFA helps rule out differential diagnoses like CSCR or choroidal hemangioma. Early hypofluorescence with pinpoint leaks in later phases may be seen on angiography in cases with posterior scleritis. Disc leakage may be seen in cases where disc edema is present. Choroidal folds may be evident on FFA.[63]
Foveoschisis
In cases with retinal dystrophies with clinically appreciable macular edema, including retinitis pigmentosa, juvenile X-linked retinoschisis, gyrate atrophy of the choroid and retina, no leakage of dye from perifoveal capillaries may be seen even in the late phases suggesting foveoschisis or maculoschisis.[88][89] This helps differentiate these cases from cystoid macular edema caused by inflammatory pathologies, which show a petaloid pattern of the leak around the foveola in the late phase of FFA. Similar non-leaking macular edema may also be seen in various conditions, including:
- Myopic foveoschisis or myopic traction maculopathy
- Vitreomacular traction
- Optic disc pit maculopathy
- Nicotinic acid maculopathy
- Enhanced S-cone syndrome/Goldman-Favre syndrome
- Cohen syndrome
- Hydroxychloroquine toxicity[90][91]
Cases of macular edema showing petaloid leak on FFA are more likely to respond to intraocular, periocular, or systemic steroid therapy. Non-leaking macular edema associated with retinal dystrophies may respond to topical or systemic carbonic anhydrase inhibitors.
Amalric Sign
This is seen in cases of choroidal ischemia. The extent of choroidal ischemia depends on the artery involved. Occlusion of the posterior ciliary artery causes extensive ischemia. These are seen as triangular-shaped lesions with their apex pointing towards the posterior pole and base towards the equator.[92] These are seen in the peripheral fundus. They show hypofluorescence in the early phases due to ischemia and staining in the late phases. Localized segmental involvement is seen in cases of occlusion of the large choroidal artery. Occlusion of smaller choroidal arteries or arterioles causes corresponding smaller ischemic lesions, either geographic in shape or small round ischemic areas depending on the area of supply of the affected vessel.
Anterior Segment Imaging
Fluorescein angiography has also been used to visualize neovascular vessels at the level of the iris, anterior chamber, or cornea.[93] FFA can also show areas of iris ischemia. In cases of neovascular glaucoma, neovascularization of the iris may not be visible clinically in the early pre-rubeotic phase. These cases can be diagnosed timely on fluorescein angiography imaging. These cases show leakage from neovascular vessels at the pupillary margin on imaging of the iris after intravenous fluorescein angiography injection. The aqueous humor may appear greenish on slit-lamp examination in various conditions, including in patients with new vessels in the iris or angle of the anterior chamber and active anterior uveitis.
Imaging of the cornea has also been documented. This is useful for documentation of corneal epithelial defects and their response to treatment at follow-up. In cases with corneal epithelial defects, topical fluorescein is used in the concerned eye, and images are taken with a fundus camera in fluorescein angiography mode with the exciting and barrier filters. The area of epithelial defect is captured as a hyperfluorescent area.[94]
Ultra-wide field retinal imaging
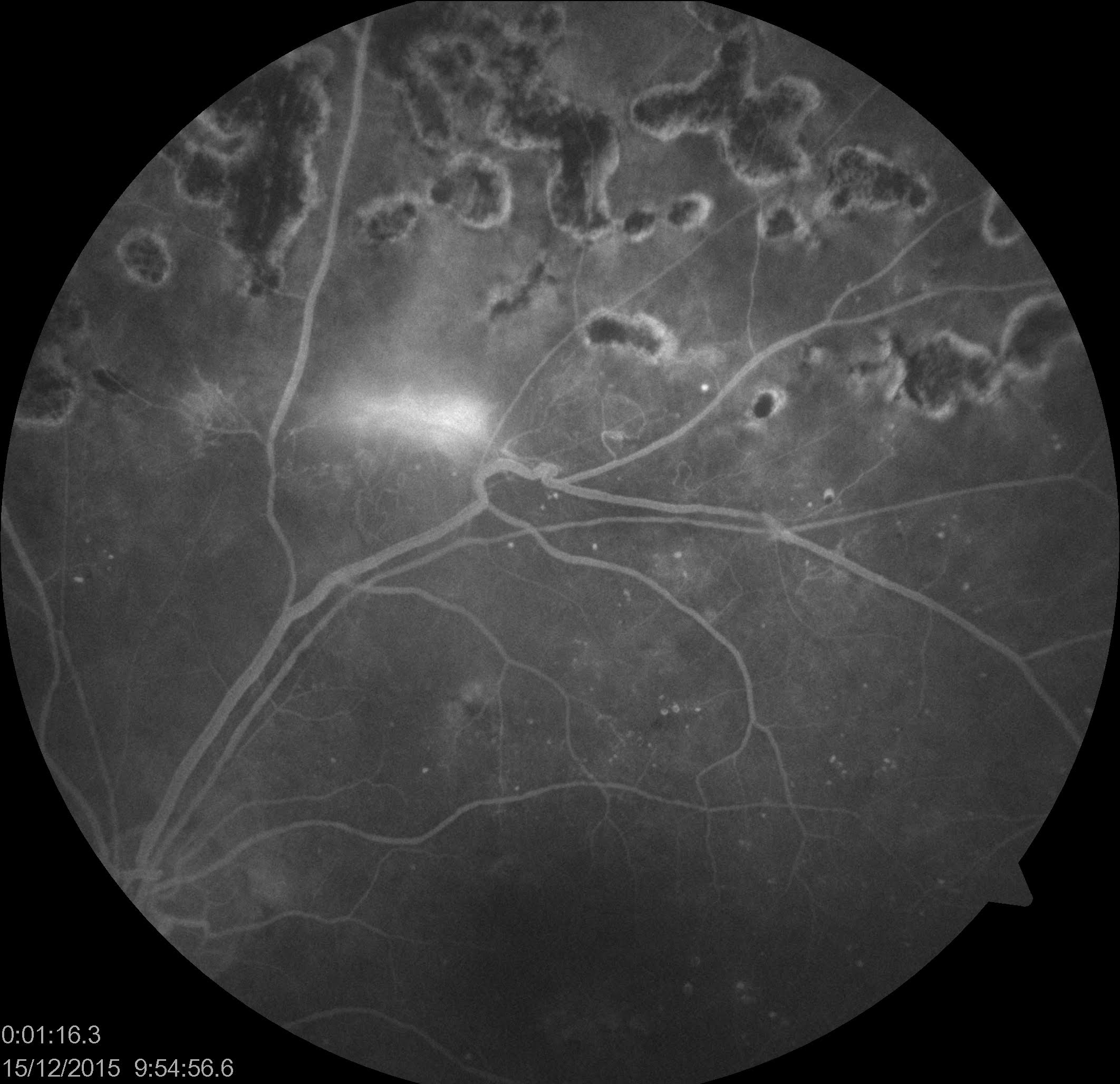
The standard fundus camera captures images of 30-50 degrees of the retina. Several wide-field imaging systems are now available. With the help of wide-field imaging, peripheral vascular leakage, peripheral vascular loops, and capillary nonperfusion areas can be easily assessed.[89][95]
Ultrawide field FFA can vividly show the peripheral avascular area in retinopathy of prematurity (ROP) and familial exudative vitreoretinopathy (FEVR). Both of these diseases can cause temporal dragging of the fovea and straightening of the peripheral retinal vessels. Peripheral avascular areas have also been noted in rhegmatogenous retinal detachments (RRD), possibly proving an explanation of retinal new vessels or iris new vessels seen in chronic RRDs.[96] Ultrawide field FFA using a scanning laser ophthalmoscope and ellipsoid mirror can image the retina through small pupils and hazy media in various conditions, including uveitis and diabetes mellitus.[97]
Ultrawide field FFA showed peripheral retinal vascular involvement and evidence of posterior segment inflammation in a majority of patients with uveitis related to juvenile idiopathic arthritis in a study by Tripathy and colleagues.[98] Targeted retinal photocoagulation to the areas of capillary nonperfusion on ultrawide field FFA has been used successfully in various disorders, including BRVO with macular edema, proliferative diabetic retinopathy, and diabetic macular edema.[99][100][101]
Enhancing Healthcare Team Outcomes
Interprofessional team care can contribute to using fluorescein angiography most efficiently. Nurses are required to assist in preparing the patient for the procedure. Counseling the patient before the procedure, pushing the dye while the ophthalmologist is capturing the images, and observing the patient for any adverse reactions can all be carried out by the nursing staff. Anesthetists or specialists in critical care are required to manage cases that develop an anaphylactic reaction. An interprofessional team approach helps prevent complications. It helps carry out angiography procedures uneventfully and provides optimal diagnostic information. [Level 5].
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Marmor MF,Ravin JG, Fluorescein angiography: insight and serendipity a half century ago. Archives of ophthalmology (Chicago, Ill. : 1960). 2011 Jul; [PubMed PMID: 21746986]
FLOCKS M,MILLER J,CHAO P, Retinal circulation time with the aid of fundus cinephotography. American journal of ophthalmology. 1959 Jul; [PubMed PMID: 13670257]
NOVOTNY HR,ALVIS DL, A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961 Jul; [PubMed PMID: 13729802]
Xu R,Teich W,Frenzel F,Hoffmann K,Radke J,Rösler J,Faust K,Blank A,Brandenburg S,Misch M,Vajkoczy P,Onken JS,Resch-Genger U, Optical Characterization of Sodium Fluorescein {i}In Vitro{/i} and {i}Ex Vivo{/i}. Frontiers in oncology. 2021; [PubMed PMID: 34041024]
O'goshi K,Serup J, Safety of sodium fluorescein for in vivo study of skin. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI). 2006 Aug; [PubMed PMID: 16827689]
Grayson MC,Laties AM, Ocular localization of sodium fluorescein. Effects of administration in rabbit and monkey. Archives of ophthalmology (Chicago, Ill. : 1960). 1971 May; [PubMed PMID: 4996602]
Level 3 (low-level) evidenceCunha-Vaz JG,Shakib M,Ashton N, Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. The British journal of ophthalmology. 1966 Aug; [PubMed PMID: 5915319]
Level 3 (low-level) evidenceHayreh SS, THE OPHTHALMIC ARTERY: III. BRANCHES. The British journal of ophthalmology. 1962 Apr; [PubMed PMID: 18170772]
Anderson B Jr,McIntosh HD, Retinal circulation. Annual review of medicine. 1967; [PubMed PMID: 5337520]
Level 3 (low-level) evidenceSchneider M,Molnar A,Angeli O,Szabo D,Bernath F,Hajdu D,Gombocz E,Mate B,Jiling B,Nagy BV,Nagy ZZ,Peto T,Papp A, Prevalence of Cilioretinal Arteries: A systematic review and a prospective cross-sectional observational study. Acta ophthalmologica. 2021 May; [PubMed PMID: 32833328]
Level 2 (mid-level) evidenceHayreh SS, In vivo choroidal circulation and its watershed zones. Eye (London, England). 1990; [PubMed PMID: 2199236]
Level 3 (low-level) evidenceBringmann A,Syrbe S,Görner K,Kacza J,Francke M,Wiedemann P,Reichenbach A, The primate fovea: Structure, function and development. Progress in retinal and eye research. 2018 Sep; [PubMed PMID: 29609042]
Spaide RF, RETINAL VASCULAR CYSTOID MACULAR EDEMA: Review and New Theory. Retina (Philadelphia, Pa.). 2016 Oct; [PubMed PMID: 27328171]
Provis JM,Hendrickson AE, The foveal avascular region of developing human retina. Archives of ophthalmology (Chicago, Ill. : 1960). 2008 Apr; [PubMed PMID: 18413520]
Shukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32809640]
Tripathy K, Sharma YR, R K, Chawla R, Gogia V, Singh SK, Venkatesh P, Vohra R. Recent advances in management of diabetic macular edema. Current diabetes reviews. 2015:11(2):79-97 [PubMed PMID: 25801496]
Level 3 (low-level) evidenceMishra C, Tripathy K. Asteroid Hyalosis. StatPearls. 2023 Jan:(): [PubMed PMID: 32119262]
Tripathy K. Asteroid Hyalosis. The New England journal of medicine. 2018 Aug 23:379(8):e12. doi: 10.1056/NEJMicm1712355. Epub [PubMed PMID: 30134134]
Tripathy K, Chawla R, Kumar V, Sharma YR, Venkatesh P. A 56-year-old male with unilateral painless diminution of vision. Oman journal of ophthalmology. 2016 May-Aug:9(2):119. doi: 10.4103/0974-620X.184534. Epub [PubMed PMID: 27433043]
Tripathy K, Das A, Chawla R, Temkar S. A young female with subretinal thread-like structures. Oman journal of ophthalmology. 2019 Jan-Apr:12(1):67. doi: 10.4103/ojo.OJO_152_2016. Epub [PubMed PMID: 30787543]
Tripathy K, Quint JM. Angioid Streaks. StatPearls. 2024 Jan:(): [PubMed PMID: 30844178]
Ruiz-Medrano J,Montero JA,Flores-Moreno I,Arias L,García-Layana A,Ruiz-Moreno JM, Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Progress in retinal and eye research. 2019 Mar [PubMed PMID: 30391362]
Quaranta M,Arnold J,Coscas G,Français C,Quentel G,Kuhn D,Soubrane G, Indocyanine green angiographic features of pathologic myopia. American journal of ophthalmology. 1996 Nov; [PubMed PMID: 8909205]
Gupta N, Tripathy K. Retinitis. StatPearls. 2024 Jan:(): [PubMed PMID: 32809355]
Kornblau IS,El-Annan JF, Adverse reactions to fluorescein angiography: A comprehensive review of the literature. Survey of ophthalmology. 2019 Sep - Oct; [PubMed PMID: 30772364]
Level 3 (low-level) evidenceMaquire AM,Bennett J, Fluorescein elimination in human breast milk. Archives of ophthalmology (Chicago, Ill. : 1960). 1988 Jun; [PubMed PMID: 3369981]
Level 3 (low-level) evidenceBalny C,Douzou P, Production of superoxide ions by photosensitization of dyes. Biochemical and biophysical research communications. 1974 Jan 23 [PubMed PMID: 4823873]
Kearns GL,Williams BJ,Timmons OD, Fluorescein phototoxicity in a premature infant. The Journal of pediatrics. 1985 Nov [PubMed PMID: 4056983]
Level 3 (low-level) evidenceAutor AP,Frank L,Roberts RJ, Developmental characteristics of pulmonary superoxide dismutase: relationship to idiopathic respiratory distress syndrome. Pediatric research. 1976 Mar [PubMed PMID: 1250644]
Level 3 (low-level) evidenceAlemzadeh-Ansari MJ,Beladi-Mousavi SS,Feghhei M, Effect of fluorescein on renal function among diabetic patients. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2011 [PubMed PMID: 21959735]
Level 3 (low-level) evidenceKameda Y,Babazono T,Haruyama K,Iwamoto Y,Kitano S, Renal function following fluorescein angiography in diabetic patients with chronic kidney disease. Diabetes care. 2009 Mar [PubMed PMID: 19246582]
Level 3 (low-level) evidenceKwiterovich KA,Maguire MG,Murphy RP,Schachat AP,Bressler NM,Bressler SB,Fine SL, Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology. 1991 Jul [PubMed PMID: 1891225]
Justice J Jr,Paton D,Beyrer CR,Seddon GG, Clinical comparison of 10 percent and 25 percent intravenous sodium fluorescein solutions. Archives of ophthalmology (Chicago, Ill. : 1960). 1977 Nov [PubMed PMID: 336013]
Level 1 (high-level) evidenceAzad R,Chandra P,Khan MA,Darswal A, Role of intravenous fluorescein angiography in early detection and regression of retinopathy of prematurity. Journal of pediatric ophthalmology and strabismus. 2008 Jan-Feb [PubMed PMID: 18286961]
Agarwal K,Vinekar A,Chandra P,Padhi TR,Nayak S,Jayanna S,Panchal B,Jalali S,Das T, Imaging the pediatric retina: An overview. Indian journal of ophthalmology. 2021 Apr [PubMed PMID: 33727440]
Level 3 (low-level) evidenceAzad RV,Baishya B,Pal N,Sharma YR,Kumar A,Vohra R, Comparative evaluation of oral fluorescein angiography using the confocal scanning laser ophthalmoscope and digital fundus camera with intravenous fluorescein angiography using the digital fundus camera. Clinical & experimental ophthalmology. 2006 Jul [PubMed PMID: 16872337]
Level 2 (mid-level) evidenceYannuzzi LA,Rohrer KT,Tindel LJ,Sobel RS,Costanza MA,Shields W,Zang E, Fluorescein angiography complication survey. Ophthalmology. 1986 May [PubMed PMID: 3523356]
Level 3 (low-level) evidenceLee KM,Woo SJ, Fundus autofluorescence in the buried optic disc drusen: optical coherence tomography findings. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2017 Apr; [PubMed PMID: 28457302]
Tripathy K, Chawla R, Meena S, Agarwal P. Unilateral giant peripapillary drusen and retinal drusenoid deposits in a case of X-linked retinoschisis. BMJ case reports. 2016 Feb 23:2016():. doi: 10.1136/bcr-2016-214558. Epub 2016 Feb 23 [PubMed PMID: 26907824]
Level 3 (low-level) evidenceSato T,Mrejen S,Spaide RF, Multimodal imaging of optic disc drusen. American journal of ophthalmology. 2013 Aug [PubMed PMID: 23677136]
Level 2 (mid-level) evidenceMennel S,Meyer CH,Eggarter F,Peter S, Autofluorescence and angiographic findings of retinal astrocytic hamartomas in tuberous sclerosis. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2005 Nov-Dec; [PubMed PMID: 16286794]
Hampton GR, Nelsen PT, Hay PB. Viewing through the asteroids. Ophthalmology. 1981 Jul:88(7):669-72 [PubMed PMID: 7267035]
Spaide RF,Curcio CA, Drusen characterization with multimodal imaging. Retina (Philadelphia, Pa.). 2010 Oct [PubMed PMID: 20924263]
Gass JD. Serous retinal pigment epithelial detachment with a notch. A sign of occult choroidal neovascularization. Retina (Philadelphia, Pa.). 1984 Fall-Winter:4(4):205-20 [PubMed PMID: 6085179]
Level 3 (low-level) evidenceSubfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Macular Photocoagulation Study Group. Archives of ophthalmology (Chicago, Ill. : 1960). 1991 Sep [PubMed PMID: 1718252]
Level 1 (high-level) evidenceDestro M,Puliafito CA, Indocyanine green videoangiography of choroidal neovascularization. Ophthalmology. 1989 Jun [PubMed PMID: 2472588]
Guyer DR,Yannuzzi LA,Slakter JS,Sorenson JA,Hope-Ross M,Orlock DR, Digital indocyanine-green videoangiography of occult choroidal neovascularization. Ophthalmology. 1994 Oct [PubMed PMID: 7524004]
Ahuja RM,Stanga PE,Vingerling JR,Reck AC,Bird AC, Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelial detachments. The British journal of ophthalmology. 2000 May [PubMed PMID: 10781511]
Spitznas M,Huke J, Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1987 [PubMed PMID: 3678855]
Turchetti R,de Moraes HV Jr,Maia HS, [Number, shape, and topography of leakage points in patients with central serous chorioretinopathy]. Arquivos brasileiros de oftalmologia. 2005 May-Jun [PubMed PMID: 16059561]
Level 2 (mid-level) evidenceHow AC,Koh AH, Angiographic characteristics of acute central serous chorioretinopathy in an Asian population. Annals of the Academy of Medicine, Singapore. 2006 Feb [PubMed PMID: 16565758]
Level 2 (mid-level) evidenceTripathy K. Cystoid Macular Edema in Retinitis Pigmentosa with Intermediate Uveitis Responded Well to Oral and Posterior Subtenon Steroid. Seminars in ophthalmology. 2018:33(4):492-493. doi: 10.1080/08820538.2017.1303521. Epub 2017 Mar 29 [PubMed PMID: 28353369]
Norton EW,Gutman F, Diabetic retinopathy studied by fluorescein angiography. Transactions of the American Ophthalmological Society. 1965; [PubMed PMID: 5859782]
Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 Sep; [PubMed PMID: 7661748]
Arend O,Wolf S,Harris A,Reim M, The relationship of macular microcirculation to visual acuity in diabetic patients. Archives of ophthalmology (Chicago, Ill. : 1960). 1995 May [PubMed PMID: 7748131]
Bresnick GH,Condit R,Syrjala S,Palta M,Groo A,Korth K, Abnormalities of the foveal avascular zone in diabetic retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1984 Sep [PubMed PMID: 6477244]
. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991 May:98(5 Suppl):807-22 [PubMed PMID: 2062514]
Level 1 (high-level) evidenceSim DA,Keane PA,Zarranz-Ventura J,Fung S,Powner MB,Platteau E,Bunce CV,Fruttiger M,Patel PJ,Tufail A,Egan CA, The effects of macular ischemia on visual acuity in diabetic retinopathy. Investigative ophthalmology & visual science. 2013 Mar 28 [PubMed PMID: 23449720]
Level 2 (mid-level) evidenceTripathy K,Chawla R,Mittal K,Temkar S, Egg yolk in the eye: an ultrawide field evaluation. BMJ case reports. 2016 Jan 27 [PubMed PMID: 26818692]
Level 3 (low-level) evidenceTripathy K,Salini B, Best Disease StatPearls. 2021 Jan [PubMed PMID: 30725975]
Shanmugam PM,Ramanjulu R, Vascular tumors of the choroid and retina. Indian journal of ophthalmology. 2015 Feb; [PubMed PMID: 25827544]
Davis DL,Robertson DM, Fluorescein angiography of metastatic choroidal tumors. Archives of ophthalmology (Chicago, Ill. : 1960). 1973 Feb; [PubMed PMID: 4683617]
Benson WE. Posterior scleritis. Survey of ophthalmology. 1988 Mar-Apr:32(5):297-316 [PubMed PMID: 3043740]
Level 3 (low-level) evidenceCasella AM,Farah ME,Martins MC,Hasegawa A,Oguido AP, Sympathetic ophthalmia - histopathological correlation with fluorescein and indocyanine green angiography: case report. Arquivos brasileiros de oftalmologia. 2008 Nov-Dec [PubMed PMID: 19169528]
Level 3 (low-level) evidenceVerstappen M,Draganova D,Judice L,Makhoul D,Papadaki M,Caspers L,Willermain F, Hypertensive Choroidopathy Revealing Malignant Hypertension in a Young Patient. Retina (Philadelphia, Pa.). 2019 May [PubMed PMID: 30893276]
Mabie WC,Ober RR, Fluorescein angiography in toxaemia of pregnancy. The British journal of ophthalmology. 1980 Sep [PubMed PMID: 7426588]
Level 3 (low-level) evidenceGeetha R, Tripathy K. Chorioretinitis. StatPearls. 2024 Jan:(): [PubMed PMID: 31869169]
Tripathy K,Sharma YR, Retinal vascular lesions. Journal of paediatrics and child health. 2017 Jan [PubMed PMID: 28070944]
. Baseline and early natural history report. The Central Vein Occlusion Study. Archives of ophthalmology (Chicago, Ill. : 1960). 1993 Aug:111(8):1087-95 [PubMed PMID: 7688950]
Level 1 (high-level) evidenceCheung CMG, Arnold JJ, Holz FG, Park KH, Lai TYY, Larsen M, Mitchell P, Ohno-Matsui K, Chen SJ, Wolf S, Wong TY. Myopic Choroidal Neovascularization: Review, Guidance, and Consensus Statement on Management. Ophthalmology. 2017 Nov:124(11):1690-1711. doi: 10.1016/j.ophtha.2017.04.028. Epub 2017 Jun 24 [PubMed PMID: 28655539]
Level 3 (low-level) evidenceTripathy K, Mazumdar S. Recurrent retinal and choroidal ischemia in a case of ocular ischemic syndrome. Therapeutic advances in ophthalmology. 2019 Jan-Dec:11():2515841419848926. doi: 10.1177/2515841419848926. Epub 2019 Jul 12 [PubMed PMID: 31321381]
Level 3 (low-level) evidenceKaram EZ,Muci-Mendoza R,Hedges TR 3rd, Retinal findings in Takayasu's arteritis. Acta ophthalmologica Scandinavica. 1999 Apr [PubMed PMID: 10321541]
Level 3 (low-level) evidenceRavani R,Chawla R,Jain S,Kumar A, "Dye front reciprocation" in combined central retinal vein occlusion with cilioretinal artery infarction. Indian journal of ophthalmology. 2017 Nov [PubMed PMID: 29133654]
Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina (Philadelphia, Pa.). 2007 Mar:27(3):276-89 [PubMed PMID: 17460582]
DOLLERY CT,HODGE JV, HYPERTENSIVE RETINOPATHY STUDIED WITH FLUORESCEIN. Transactions of the ophthalmological societies of the United Kingdom. 1963 [PubMed PMID: 14123138]
Littlewood R,Mollan SP,Pepper IM,Hickman SJ, The Utility of Fundus Fluorescein Angiography in Neuro-Ophthalmology. Neuro-ophthalmology (Aeolus Press). 2019 Aug [PubMed PMID: 31528186]
Arnold AC,Costa RM,Dumitrascu OM, The spectrum of optic disc ischemia in patients younger than 50 years (an Amercian Ophthalmological Society thesis). Transactions of the American Ophthalmological Society. 2013 Sep [PubMed PMID: 24167327]
Level 2 (mid-level) evidenceArnold AC,Hepler RS, Fluorescein angiography in acute nonarteritic anterior ischemic optic neuropathy. American journal of ophthalmology. 1994 Feb 15 [PubMed PMID: 8116751]
Kim MK,Kim US, Analysis of Fundus Photography and Fluorescein Angiography in Nonarteritic Anterior Ischemic Optic Neuropathy and Optic Neuritis. Korean journal of ophthalmology : KJO. 2016 Aug [PubMed PMID: 27478356]
Crawford CM, Igboeli O. A review of the inflammatory chorioretinopathies: the white dot syndromes. ISRN inflammation. 2013:2013():783190. doi: 10.1155/2013/783190. Epub 2013 Oct 31 [PubMed PMID: 24294536]
Chawla R, Kapoor M, Mehta A, Tripathy K, Vohra R, Venkatesh P. Sympathetic Ophthalmia: Experience from a Tertiary Care Center in Northern India. Journal of ophthalmic & vision research. 2018 Oct-Dec:13(4):439-446. doi: 10.4103/jovr.jovr_86_17. Epub [PubMed PMID: 30479714]
Tripathy K, Mittal K, Chawla R. Sympathetic ophthalmia following a conjunctival flap procedure for corneal perforation. BMJ case reports. 2016 Mar 14:2016():. doi: 10.1136/bcr-2016-214344. Epub 2016 Mar 14 [PubMed PMID: 26976837]
Level 3 (low-level) evidenceUyama M,Takahashi K,Kozaki J,Tagami N,Takada Y,Ohkuma H,Matsunaga H,Kimoto T,Nishimura T, Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology. 2000 Mar [PubMed PMID: 10711879]
Chauhan K, Tripathy K. Pars Planitis. StatPearls. 2024 Jan:(): [PubMed PMID: 28613790]
Gupta V, Gupta A. Ancillary investigations in uveitis. Indian journal of ophthalmology. 2013 Jun:61(6):263-8. doi: 10.4103/0301-4738.114093. Epub [PubMed PMID: 23803477]
Tripathy K,Chawla R,Mittal K,Farmania R,Venkatesh P,Gulati S, Ophthalmic examination as a means to diagnose Subacute Sclerosing Panencephalitis: an optical coherence tomography and ultrawide field imaging evaluation. Eye and vision (London, England). 2017 [PubMed PMID: 28116334]
Tugal-Tutkun I,Ozdal PC,Oray M,Onal S, Review for Diagnostics of the Year: Multimodal Imaging in Behçet Uveitis. Ocular immunology and inflammation. 2017 Feb [PubMed PMID: 27541278]
Elnahry AG, Tripathy K. Gyrate Atrophy of the Choroid and Retina. StatPearls. 2024 Jan:(): [PubMed PMID: 32491691]
Tripathy K, Chawla R, Sharma YR, Gogia V. Ultrawide field fluorescein angiogram in a family with gyrate atrophy and foveoschisis. Oman journal of ophthalmology. 2016 May-Aug:9(2):104-6. doi: 10.4103/0974-620X.184529. Epub [PubMed PMID: 27433038]
Beck KD,Wong RW,Gibson JB,Harper CA 3rd, Nonleaking cystoid macular edema in Cohen syndrome. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2019 Feb [PubMed PMID: 30144585]
Parikh VS,Modi YS,Au A,Ehlers JP,Srivastava SK,Schachat AP,Singh RP, Nonleaking Cystoid Macular Edema as a Presentation of Hydroxychloroquine Retinal Toxicity. Ophthalmology. 2016 Mar [PubMed PMID: 26459999]
Hayreh SS, Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Investigative ophthalmology [PubMed PMID: 14985286]
Level 3 (low-level) evidenceBrancato R,Bandello F,Lattanzio R, Iris fluorescein angiography in clinical practice. Survey of ophthalmology. 1997 Jul-Aug [PubMed PMID: 9265702]
Level 3 (low-level) evidenceTripathy K. Documentation of corneal epithelial defects with a fluorescein angiographic imaging system. Clinical case reports. 2019 Sep:7(9):1815-1816. doi: 10.1002/ccr3.2350. Epub 2019 Jul 30 [PubMed PMID: 31534763]
Level 3 (low-level) evidenceTripathy K, Chawla R, Venkatesh P, Vohra R, Sharma YR, Gogia V, Jain S, Behera A. Ultra-wide Field Fluorescein Angiography in Retinitis Pigmentosa with Intermediate Uveitis. Journal of ophthalmic & vision research. 2016 Apr-Jun:11(2):237-9. doi: 10.4103/2008-322X.183929. Epub [PubMed PMID: 27413510]
Tripathy K, Chawla R, Wadekar BR, Venkatesh P, Sharma YR. Evaluation of rhegmatogenous retinal detachments using Optos ultrawide field fundus fluorescein angiography and comparison with ETDRS 7 field overlay. Journal of current ophthalmology. 2018 Sep:30(3):263-267. doi: 10.1016/j.joco.2018.06.006. Epub 2018 Jul 3 [PubMed PMID: 30197958]
Tripathy K, Chawla R, Vohra R. Evaluation of the fundus in poorly dilating diabetic pupils using ultrawide field imaging. Clinical & experimental optometry. 2017 Nov:100(6):735-736. doi: 10.1111/cxo.12484. Epub 2016 Oct 5 [PubMed PMID: 27704602]
Tripathy K, Chawla R, Venkatesh P, Sharma YR, Vohra R. Ultrawide Field Imaging in Uveitic Non-dilating Pupils. Journal of ophthalmic & vision research. 2017 Apr-Jun:12(2):232-233. doi: 10.4103/2008-322X.205360. Epub [PubMed PMID: 28540019]
Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, Turner GS, Stanga PE. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta ophthalmologica. 2013 May:91(3):251-8. doi: 10.1111/j.1755-3768.2011.02307.x. Epub 2011 Dec 16 [PubMed PMID: 22176513]
Level 2 (mid-level) evidenceGoel S,Kumar A,Ravani RD,Chandra P,Chandra M,Kumar V, Comparison of ranibizumab alone versus ranibizumab with targeted retinal laser for branch retinal vein occlusion with macular edema. Indian journal of ophthalmology. 2019 Jul [PubMed PMID: 31238421]
Brown DM, Ou WC, Wong TP, Kim RY, Croft DE, Wykoff CC, DAVE Study Group. Targeted Retinal Photocoagulation for Diabetic Macular Edema with Peripheral Retinal Nonperfusion: Three-Year Randomized DAVE Trial. Ophthalmology. 2018 May:125(5):683-690. doi: 10.1016/j.ophtha.2017.11.026. Epub 2018 Jan 11 [PubMed PMID: 29336896]
Level 1 (high-level) evidence