Introduction
Cystitis cystica is a common benign condition of the urinary bladder with a reactive inflammatory change of the bladder mucosa associated with subepithelial vesicles or cysts formation and glandular metaplasia (cystitis glandularis). Invaginations of hyperplastic urothelial extensions into the superficial lamina propria are called von Brunn nests. These inflammatory projections are considered reactive but benign and non-malignant.
Cystitis cystica develops when the urothelial cells in the lumens of von Brunn nests degenerate, causing cystic changes. Additional metaplasia results in cystitis glandularis, characterized by mucin-producing goblet cells that may uncommonly progress to vesical adenocarcinoma.[1][2] Cystitis glandularis is a further proliferative progression of cystitis cystica characterized by glandular metaplasia of the urothelium and goblet cells.[3]
Cystitis cystica and cystitis glandularis occur mainly in response to chronic irritation or inflammation and may occur together. They are usually asymptomatic but may present with non-specific signs and symptoms that require thoughtful attention to exclude other morphologically similar malignant lesions such as bladder adenocarcinoma.[4] A nephrogenic adenoma is a rare, benign bladder tumor that is similar in cystoscopic appearance to cystitis glandularis and represents a type of superficial bladder proliferative disorder. The adenoma is often associated with genitourinary (bladder) stones, trauma, exstrophy, malakoplakia, Mullerian lesions, chronic or recurrent bladder infections, interstitial cystitis, renal transplantation, radiation therapy, pelvic lipohypertrophy, intravesical therapy, foreign bodies, or surgery such as bladder augmentation.[5] The adenoma may grow to cause obstructive urinary symptoms and may be multifocal.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Many studies, including a series of cases, have elucidated no definitive cause of cystitis cystica and cystitis glandularis, although chronic irritation, infection, or inflammation affects many individuals.
Potential causes include the following:
- Bladder exstrophy (frequently associated with diffuse cystitis cystica changes in the bladder mucosa)
- Chronic bladder outlet obstruction (ie, benign prostatic hypertrophy, urothelial carcinoma, and pelvic lipomatosis)
- Chronic urinary tract infections, particularly infections caused by Escherichia coli
- Indwelling catheters
- Mechanical irritation (foreign bodies, calculi)
- Neurogenic bladders [7][8][9]
In addition to the conditions mentioned above, nephrogenic adenoma was reported in many cases with organ transplantation and immunosuppression. A nephrogenic adenoma is characterized by the implantation of desquamated distal renal tubular cells anywhere in the genitourinary tract. However, most cases involve the urinary bladder, usually around the bladder neck and proximal urethra.
In a series of 21 cases of nephrogenic adenoma, the majority had a history of bladder augmentation surgery, which causes recurrent infections and urolithiasis.[10] Nephrogenic adenoma has also been associated with Bacillus Calmette-Guérin and mitomycin-C bladder instillations.[6] Other theories suggest that the adenoma may be a metaplastic lesion, a result of residual embryonic mesonephric tissue, or caused by urothelial trauma in a bladder that had undergone previous surgery or is chronically inflamed.[5] In patients with kidney transplants, nephrogenic adenomas appear to be proliferative growths from implanted renal tubular cells and not derived from the bladder tissue of the recipient.[11]
Epidemiology
Cystitis cystica epidemiology is still poorly documented because of the challenging diagnosis; however, the condition can affect men and women at any age, with a slightly higher prevalence in men. Cystitis cystica is a common incidental finding in biopsies or cystoscopies for any other reason and can be found in 60% of autopsies. Hence, the condition may be a normal variant of the urothelial mucosa.[12]
Cystitis cystica is frequently seen in the bladder, neck, and trigone. The condition is less common in the ureters and renal pelvis. Cystitis glandularis may be diffuse or focal and generally affects about 2% of the population, usually appearing during the fifth and sixth decades of life. Nephrogenic adenomas are more common in men than women, with a 2-to-1 ratio.[5] The incidence is probably more common than is generally reported. While more frequently seen in adults, about 10% of cases have been reported in children.[13]
Histopathology
Cystitis Cystica
In cystitis cystica, the urothelium is invaginated into the lamina propria, forming Von Brunn nests, and subsequently develops into cystically dilated fluid-filled vesicles. In some cases, differentiating cystitis cystica and florid von Brunn nest proliferation from the nested variant of urothelial carcinoma is challenging.[14] Such differentiation can be critical as urothelial carcinoma is an aggressive malignancy. Finding mutations in the TERT promoter is a good method to distinguish a potentially dangerous nested urothelial carcinoma and benign imitators.[15] Grossly, cystitis cystica may demonstrate small (<5 mm) translucent submucosal cysts or appear normal.
Microscopically, cystitis cystica will have abundant von Brunn nests in the urothelium. Other features include:
- Degenerative atypia
- Dilated lumens and cysts with eosinophilic fluid
- Lobular invaginations
- No significant cellular atypia, mitotic activity, muscular invasion, or stromal reaction
- Noninfiltrative growth pattern
In cystitis cystica, the cells are superficial to the lamina propria, while in adenocarcinoma, the deep muscle layer is invaded. Other malignant criteria include atypia, frequent abnormal mitotic figures, increased nuclear-cytoplasmic ratio, and necrosis. Immunohistochemistry can be used in complex cases to differentiate the 2 entities. For example, the urothelial cells in the cystitis cystica nests will positively stain for p63, while nephrogenic adenoma is p63 negative.
Cystitis Glandularis
Grossly, cystitis glandularis may appear as a cobblestone or irregular mucosal pattern with some irregularities or as a polypoidal mass.[16] Blueberry spots may be present. Cystitis glandularis is usually a microscopic diagnosis that rarely presents as a bladder mass. Microscopically, cystitis glandularis will appear the same as cystitis cystica except for the more glandular pattern and columnar or cuboidal cells and mucin-producing goblet cells. The condition may be associated with either glandular or intestinal-type metaplasia.
Cystitis glandularis has been described as a bladder condition where the urothelium lining of the cysts exhibits glandular metaplasia divided into 2 subtypes:
- Typical cystitis glandularis: lined by simple mucinous cells
- Cystitis glandularis intestinal-type: lined by intestinal metaplastic cells in addition to goblet cells and may produce mucin
Cystitis glandularis intestinal-type is of clinical significance because, when extensive, this may mimic well-differentiated adenocarcinoma morphologically and histologically. Furthermore, the condition may exhibit a malignant potential.[17][18][19][20][21][22] The key discriminating element between cystitis glandularis intestinal-type and well-differentiated adenocarcinoma is the invasion of the muscularis propria, which is seen in the latter. Cystitis glandularis with intestinal metaplasia appears similar to a well-differentiated adenocarcinoma, so distinguishing between the 2 entities is clinically significant. The main difference is the extent to which ectopic cells are present and the invasion of the muscularis propria seen in the latter. Also, cystitis glandularis typically exhibits a homogenous membranous expression of β-catenin on immunofluorescence staining.
Traditionally, cystitis glandularis has been considered potentially pre-malignant, although several more recent, long-term studies have demonstrated no such association.[3][23][24] However, the histological finding of dysplasia in cystitis glandularis intestinal-type may still warrant periodic cystoscopic re-evaluations due to the association with malignant transformations.[2][25]
No histological differences are apparent between cases causing ureteral obstruction and hydronephrosis compared to those that do not.[26] Immunohistological staining can differentiate benign conditions like cystitis cystica and cystitis glandularis from the similarly appearing but rare bladder adenocarcinoma (see Image. A Histopathological Examination of Cystitis Cystica and Cystitis Glandularis).
- Positive immunohistological stains for cystitis cystica and cystitis glandularis: GATA3, CK7 (full thickness), CK20 (umbrella cells), p63 (basal cell layer), uroplakin II/III, thrombomodulin, β-catenin (membranous), and E-cadherin
- Negative stains: CDX2, villin, MUC2, MUC5AC, β-catenin (nuclear)
Nephrogenic Adenoma
Nephrogenic adenomas tend to appear after an injury to the urothelium. Cystoscopically, they are generally described as polypoid, papillary, velvety, or fungating bladder lesions grossly resembling bladder malignancies. They tend to form most often in the trigone and bladder neck areas.
Nephrogenic adenoma has multiple histological types and may show mixed patterns. These include:
- A hyaline rim surrounding the tubules
- Basement membrane thickening
- Cells with clear/eosinophilic cytoplasm and small nuclei without prominent nucleoli
- Clear cells rarely present
- A general architecture that is clear, oncocytic, flat, or fibro-myxoidal
- Histological types include cystic, microcystic, papillary, tubular, or signet ring-like
- No mitotic activity, but nuclear atypia is possible
- No necrosis or desmoplasia
- Papillary, cystic, and microcystic patterns are the most common
- Positive PAX2 is a useful, specific, and sensitive immunohistochemical marker for histologically identifying nephrogenic adenoma
- Pseudo-infiltrative growth pattern may occur that closely mimics urothelial carcinoma
- Significant cellular atypia may be seen but without any malignant transformations.
- Solid areas are seen only occasionally
- Lamina propria may be involved, but the muscularis propria is usually spared, although focal involvement may occur
- Tubules lined by eosinophilic cuboidal or hobnail cells [4][27][28][29][30]
The most common histological presentation is small, hollow tubules, similar to mesonephric tubules, usually lined with a single layer of eosinophilic cuboidal or hobnail cells. In difficult cases, the combined expression of napsin A, EMA, PAX-2, and PAX-8 with a negative p63 has been suggested to help distinguish nephrogenic adenoma from a malignancy.[31][32] GATA-3 is usually a reliable marker for urothelial carcinoma but may be positive in 40% of nephrogenic adenoma cases.[6][33]
Adenocarcinoma of the bladder tends to exhibit a positive p63 and PAX-8 but is negative for PAX-2 and GATA-3.[34] Papillary urothelial bladder cancer will have more than a single layer of urothelial cells with atypia (see Image. Urothelial Carcinoma In Situ).[5]
History and Physical
Cystitis cystica and related pathologies have no unique or distinctive symptoms. They may present non-specific symptoms similar to many other urinary diseases and conditions, making the diagnosis more challenging.
The patient may complain of various symptoms, including the following:
- Hematuria
- Lower urinary tract symptoms that may include frequency, urgency, incomplete emptying, and hematuria
- Obstructive urinary symptoms
- Pain (suprapubic or perianal)
- Rarely, urine retention and hydronephrosis
- Symptoms typical of a urinary tract infection [35][36][37]
History taking must exclude other differential diagnoses, including medical, social, sexual, and family history, accompanied by a focused genitourinary examination.
Evaluation
A systematic approach should be adopted to obtain the correct diagnosis of cystitis cystica and related disorders. The definitive diagnosis often requires cystoscopy with a biopsy and histopathological examination.
Laboratory Investigations
Examinations typically include a complete blood count, inflammatory mediators, urinalysis, and a kidney function test. Urinalysis, in particular, is important to detect the presence of hematuria or any urinary tract infection and should be obtained before commencing any antibiotic. If an infection is present, a culture and sensitivity test is required to identify the causative organism and the most effective treatment.
Cystoscopy
Cystitis cystica
Features can vary from a grossly unremarkable appearance to a large bladder cyst or mass. However, the condition typically appears as multiple small mucosal and submucosal translucent cysts, found mainly at the trigone and the bladder neck. The bladder mucosa may demonstrate congestion, irregularity, thickening, multiple nodules, or an exophytic polypoidal mass. A concurrent biopsy is typically performed on both the lesion and the mucosal changes since cystitis cystica typically has inconclusive cystoscopic and radiological features.[38][39]
Cystitis glandularis
Features may appear as an irregular shaggy or fuzzy lesion located in the dome of the bladder. Lesions may also be seen in the bladder neck and trigone and immediately adjacent to the ureteral orifices. The condition tends to appear as an elevated lesion with multiple rounded lumps. Usually, a distinct demarcation is apparent between normal and abnormal urothelium. The lesions may be sessile or pedunculated and may be easily confused cystoscopically with bladder cancer.
Cystitis glandularis may appear either extensive or focal cystoscopically. Urinary symptoms and urological complications are more closely associated with the extensive type of cystitis glandularis, while malignant progression is more likely in the focal version of the disorder.[40] After establishing the diagnosis, patients with cystitis glandularis should be maintained on follow-up visits every 6 months for 2 years and then annually. The follow-up assessments typically involve a cystoscopy and may include a clinical examination, history, urinalysis, cytology, ultrasound, and kidneys, ureter, and bladder (KUB) x-rays.
Nephrogenic adenoma
This adenoma rarely presents with hematuria, but irritative voiding symptoms are common. Cystoscopically, the bladder urothelium may present a velvety appearance, similar to papillary carcinoma of the bladder. The lesions may be papillary (55%), sessile (35%), or polypoidal (10%), and 20% are multiple.[5][41]
Radiographic Imaging Modalities
- Ultrasonography (US) is a noninvasive and reliable option for diagnosing cystitis cystica. The imaging is valuable for recurrent urinary tract infections because measuring the thickness of the bladder wall is possible. Bladder wall thickness values of more than 3 mm suggest cystitis cystica rather than simple recurrent UTIs, which generally have a bladder wall thickness of less than 3 mm (see Image. Ultrasound of Bladder).
- Ultrasonography provides a general idea about the condition of the urinary bladder and the kidneys. The imaging can demonstrate significant papillary projections, nodules, or masses in the bladder and identify urinary retention, incomplete bladder emptying, and hydronephrosis.
- A plain KUB x-ray can be obtained to detect calcific stones.
- Computerized tomography (CT) scanning of the urinary system without and with intravenous contrast media (CT urogram) is used to evaluate the genitourinary system fully. These modalities can visualize bladder lesions of sufficient size, measure the bladder wall thickness, and evaluate for any other lesion that may cause hematuria, such as tumors and calculi.
- In a CT urogram, cystitis cystica may appear as multiple, rounded, small-sized filling defects of 2 to 5 mm diameter in the bladder wall. However, the lesions can be larger or, rarely, appear as a large tumor-like mass.
- Magnetic resonance imaging (MRI) of the urinary system can be used for a more detailed assessment and to exclude other pelvic abnormalities.
- Cystograms can give a more detailed look at the bladder and are beneficial, especially in children, to detect the presence of vesicoureteral reflux, which may coexist with cystitis cystica or mimic the symptoms (see Image. Cystoscopy of Bladder Tumor).[42][43][44][45]
Treatment / Management
Treatment of cystitis cystica can be challenging due to the difficulty of identifying the exact cause. After confirming the diagnosis, the treatment mainly depends on removing the aggravating factor, antibiotics and chemoprophylaxis, symptomatic treatment, and surgical intervention if the previous methods fail.
Medical Treatment
The first-line treatment option is treating any active urinary tract infection (UTI) with antibiotics followed by long-term low-dose prophylaxis. Many antimicrobial drugs can be used according to the severity of the case. Oral sulfamethoxazole-trimethoprim, fosfomycin, and nitrofurantoin are considered first-line agents for the initial treatment of UTIs and prophylaxis.[46] Local antibiotic administration in regular, intermittent intravesical instillations can also improve outcomes. The intravesical use of gentamicin is 80 mg in 50 to 60 cc of normal saline as a commonly used dosage.[47] Cranberry products have shown some reduction in recurrent UTIs but have not been shown to benefit cases of cystitis cystica or cystitis glandularis.[48] After treating the current UTI episode, subsequent long-standing prophylaxis is crucial for reducing the incidence of recurrent UTIs and improving the mucosal inflammatory changes in the bladder. Regression of cystitis cystica changes is achievable by compliance with a suitable pharmacotherapy regimen and proper follow-up.[49](A1)
D-mannose is an inert monosaccharide dietary supplement used as a nonantibiotic prophylactic agent for recurrent UTIs. The sugar is excreted in urine and reduces UTIs by inhibiting bacterial adhesion to the urothelial lining. The supplement has demonstrated some UTI reduction activity, but the overall quality of the available studies is low.[50] Patients with cystitis cystica and recurrent urinary infections did appear to benefit from daily D-mannose therapy, significantly reducing UTIs. However, further studies are needed to recommend this treatment routinely.[51][52] The optimal dosage has not been determined, but 1 g twice daily is suggested.(B3)
Long-term low-dose antibiotic UTI prophylaxis typically utilizes nitrofurantoin initially at 50 to 100 mg nightly as effective, has broad coverage, is excreted only in the urine, tends not to cause significant bacterial resistance to other antibiotics, and has minimal side effects when taken long-term. Other antibiotics used for prophylaxis include:
- Fosfomycin 3 g every 10 days
- Norfloxacin 200 mg daily
- Sulfamethoxazole-trimethoprim 200 mg/40 mg nightly or 3 times weekly
- Trimethoprim 100 mg daily [53][54][55][56][57][58] (A1)
Corticosteroids such as prednisolone can be used in otherwise intractable or severe cases. They appear to have a success rate in confirmed, symptomatic cases not responding to alternative treatments but typically require 6 months of therapy.[59] Oral cyclooxygenase-2 (COX-2) inhibitor therapy eliminated gross papillary-type cystitis glandularis previously treated with transurethral resection, which recurred.[60] However, the therapy required 6 months of treatment.(B3)
Intravesical sodium hyaluronate improves the mucosal layer integrity and inhibits the inflammation and proliferation of cystitis cystica changes, leading to symptomatic improvement.[61] Sodium hyaluronate is smaller than hyaluronic acid, penetrating tissues deeply. Sodium hyaluronate is a glycosaminoglycan that forms a viscoelastic solution in water. This acts as protection of the underlying urothelium from irritating urinary chemicals.[62] The substance also helps transport peptide-based growth factors and structural proteins to the injury site. Sodium hyaluronate further reduces bladder inflammation by inhibiting mast cell activation and reducing the secretion of inflammatory chemicals like interleukin (IL-6) and histamine.[63] When enzymatically degraded, sodium hyaluronate releases proteins that promote tissue repair. The usual dose is 40 mg in 50 mL, followed by weekly intravesical treatments until symptomatic control is achieved. Monthly maintenance treatments may be undertaken as needed and are considered optional.[64] Sodium hyaluronate is FDA-approved in the United States for knee installations to treat arthritis, so use for intravesical therapy is off-label. Symptomatic treatment includes physical therapy, pelvic floor exercises with bladder training techniques, non-steroidal anti-inflammatory drugs NSAIDs, and anticholinergic medications, which help reduce the irritative symptoms and improve the patient’s urinary control.(B3)
Surgical Treatment
Surgical intervention is beneficial for cases with unsuccessful medical treatment if the patient presents with a mass or obstructing symptoms and for nephrogenic adenoma.
- The most commonly performed procedure is transurethral resection (TUR) of the lesion. An excisional biopsy should be examined histologically to confirm the diagnosis and exclude other potentially serious disorders.
- Neodymium-YAG laser can be used in more severe cases that do not respond to conservative therapy and transurethral resection.
- Diathermy of the mucosal cystic changes can be performed in patients with recurrent UTIs, although this treatment requires more data and study.
- Extracorporeal shockwave therapy has been used for painful bladder syndrome, but the specific use in cystitis cystica and similar conditions is not well established.
- Partial or total cystectomy can be performed for intractable cases with severe symptoms.[65][66][67] (A1)
Treating nephrogenic adenoma involves either TUR of the lesion or transurethral fulguration, but recurrence is common, especially in children.
- TUR followed by long-term sulfamethoxazole-trimethoprim prophylaxis and NSAIDs have been used successfully.
- Intravesical hyaluronate therapy has also been used successfully for nephrogenic adenoma treatment, but only anecdotally.
- No accepted guidelines exist on follow-up for nephrogenic adenoma of the bladder, but a cystoscopy at 6 to 12 months and then again if symptoms return has been suggested due to a relatively high recurrence rate.[5][68][69][70][71] (B3)
Summary of Treatments
- In most cases, a bladder biopsy is needed to confirm the diagnosis.
- Any urinary calculi that could be a focus of infection should be surgically removed.
- Initial treatment for symptomatic cases includes pelvic floor exercises, bladder training, NSAIDs, and/or overactive bladder medications.
- Intravesical gentamicin or oral D-mannose therapy can be considered in selected cases of recurrent UTIs.
- Intravesical sodium hyaluronate appears to be an effective therapy in otherwise intractable cases.
- Prophylactic therapy can be continued as monthly maintenance after the initial weekly induction period.
- Long-term antibiotic UTI prophylaxis and NSAIDs should be considered in recurrent, symptomatic, or severe cases and after surgical therapy.
- Patients who remain symptomatic may benefit from 6 months of oral corticosteroids or a COX-2 inhibitor.
- Patients with recurrent UTIs should be prescribed long-term low-dose prophylaxis with nitrofurantoin or sulfamethoxazole-trimethoprim.
- Surgical therapy typically involves TUR, but partial and total cystectomies may be required in some cases with severe and intractable symptoms.
- Transurethral resection is the preferred treatment for nephrogenic adenoma of the bladder.
- Other surgical treatments include laser ablation, diathermy, and extracorporeal shockwave therapy, but efficacy data on such modalities is limited.
Differential Diagnosis
The differential diagnosis includes:
- Adenocarcinoma of the bladder
- BCG reactive cystitis
- Benign prostatic hyperplasia
- Bladder cancer
- Carcinoma-in-situ of the bladder
- Catheter cystitis (foreign body cystitis)
- Chronic cystitis and other UTIs
- Endocervicosis of the urinary bladder (a rare benign condition that affects women of reproductive age, where ectopic endocervical tissue is found in the bladder wall or the paracervical region)
- Ejaculatory duct cyst
- Fibroepithelial polyp
- Florid von Brunn nest proliferation
- Inverted papilloma
- Keratinizing desquamative squamous metaplasia
- Malakoplakia
- Nephrogenic adenoma
- Painful bladder syndrome
- Papillary urothelial carcinoma of the bladder
- Polypoidal cystitis
- Radiation cystitis
- Schistosomiasis
- Squamous metaplasia
- Transitional cell or squamous cell carcinoma of the bladder
- Trigonitis
- Vaginal metaplasia
- Vaginitis
- Xanthogranulomatous inflammation of the bladder [4]
Prognosis
The clinical course of cystitis cystica is undetermined, though removing the source of the irritation can cause the cystic inflammation to regress. Successful treatment and prevention of UTIs can lead to the disappearance of mucosal changes.[23][72][73] The malignant potential of cystitis cystica remains controversial; however, some anecdotal cases have been reported of cystitis glandularis and nephrogenic adenoma turning into adenocarcinoma.[17][18][19][20][21][22] Cystitis glandularis intestinal type with dysplastic histology most likely still requires periodic cystoscopic surveillance, while other cystitis cystica and cystitis glandularis probably do not. Nephrogenic adenoma has a high recurrence rate, so periodic follow-up cystoscopies are suggested.[5]
Complications
Several possible complications of cystitis cystica, cystitis glandularis, and nephrogenic adenoma are apparent; these include:
- Bladder outlet obstruction
- Bladder pain syndrome
- Hydronephrosis
- Malignant transformation in case of cystitis glandularis intestinal-type with dysplasia
- Psychological impact on the patient due to the chronicity and disturbance to quality of life
- Recurrent UTIs (the most common complication)
- Renal failure from bilateral ureteral obstruction
- Ureterovesical junction obstruction
- Urinary retention
- Urinary urgency, frequency
- Vesicoureteral reflux [74][75][76]
Deterrence and Patient Education
The main action plan to prevent the occurrence and progression of cystitis cystica is to target, avoid, and eradicate the predisposing factors whenever possible. The most effective way of prevention is regular, long-term prophylaxis, especially in chronic or recurrent UTIs. Renal calculi should be removed to eliminate a source of inflammation and recurrent infections. Bladder stones should be treated promptly to avoid long-standing mechanical irritation and inflammation.
Patients should be counseled thoroughly to maintain proper compliance and good personal hygiene. Ensure the patient follows the follow-up visits and evaluations to monitor the condition and detect complications. Encourage patients to follow the general recommendations for preventing cystitis, such as maintaining good genital hygiene and increasing their daily water intake.
Pearls and Other Issues
The following are key points:
- Consider non-invasive therapy with corticosteroids or COX-2 inhibitors in cases with no obvious, otherwise treatable cause.
- D-mannose may help in recurrent UTIs but is not preferred over low-dose prophylactic antibiotic therapy.
- Intravesical treatment with sodium hyaluronate should be considered, especially in complex or intractable cases, due to high efficacy reports, multiple modes of beneficial activity, and safety profile.
- Most cases probably do not need routine surveillance cystoscopy.
- Most recent studies suggest no connection between cystitis cystica and bladder adenocarcinoma.
- Vitamin C may be benefivcial in treating cystitis glandularis, but only limited data is available on this therapy.[47][77]
Enhancing Healthcare Team Outcomes
Since cystitis cystica, cystitis glandularis, and nephrogenic adenomas may have the clinical or radiological appearance of bladder malignancies or ureteral pseudotumors, they should be considered in the differential diagnosis of urinary malignancies.[45] This requires an interprofessional healthcare team that includes primary care clinicians, urologists, gynecologists, radiologists, pathologists, nursing staff, and pharmacists.
The histological examination confirms the final diagnosis and facilitates correct categorization and differentiation from bladder malignancies. Regarding treatment, eliminate any predisposing factors, such as foreign bodies, stones, catheters, and other chronic irritation, infection, or obstruction sources. If these factors are not addressed, the therapeutic and prophylactic measures listed will not be effective. In selected patients, follow-up should be arranged (typically every 6 months) due to the possibility of adenocarcinoma transformation in the bladder and to detect any complications as early as possible. Each team member must contribute from their expertise, monitor the patient's progress, record their observations in the patient's permanent medical record, and reach out to other team members as necessary so therapeutic modifications can be instituted. This interprofessional paradigm will lead to better patient outcomes.
Media
(Click Image to Enlarge)
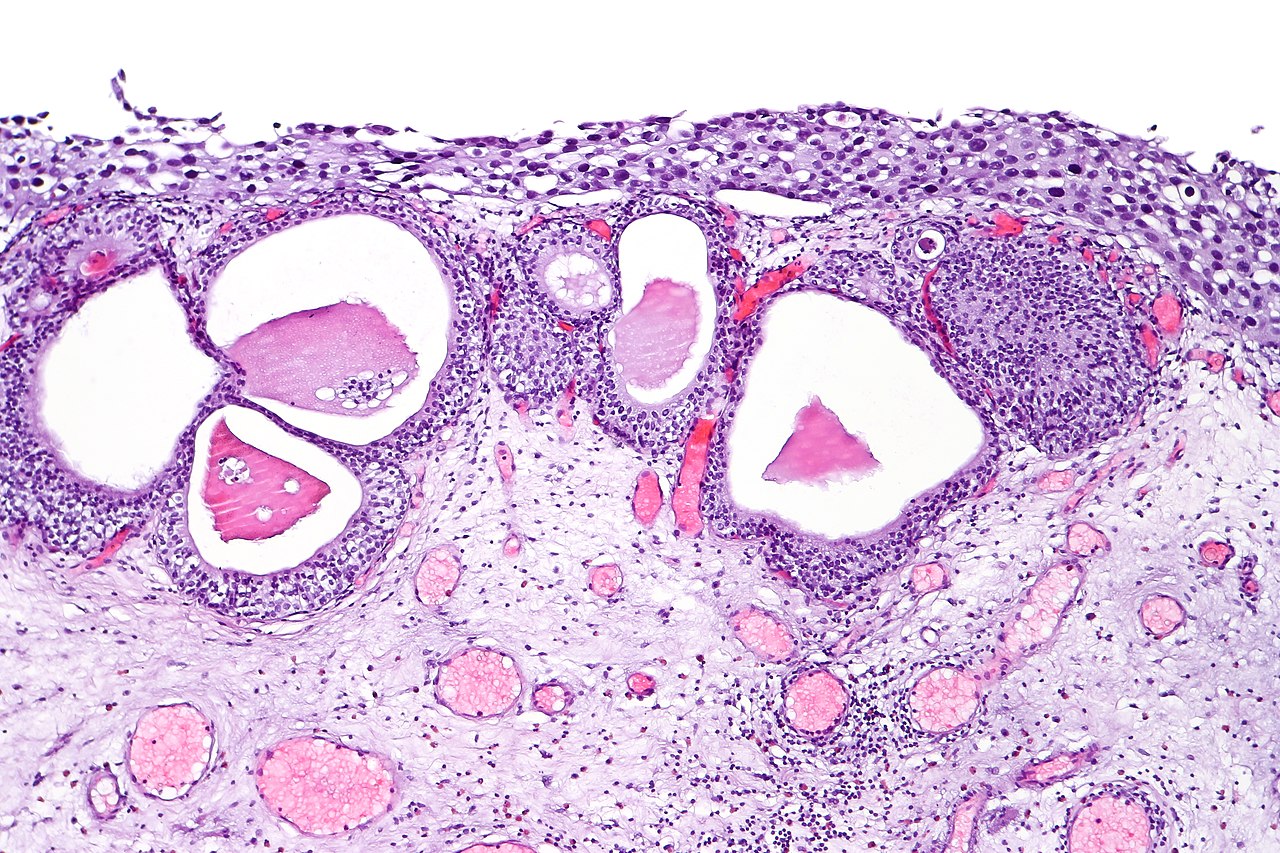
Urothelial Carcinoma In Situ. Urothelial carcinoma in situ in the setting of cystitis cystica glandularis.
CoRus13, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
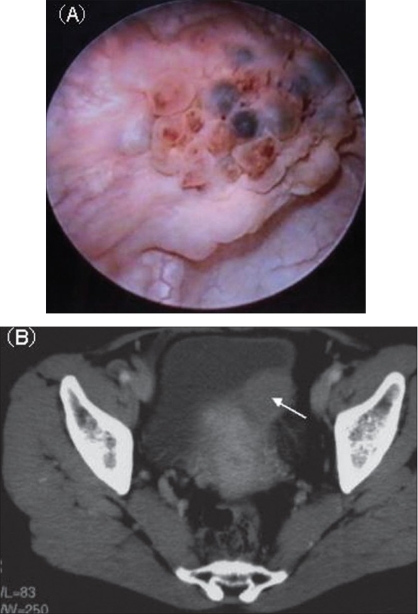
Cystoscopy of Bladder Tumor. (A) Cystoscopy reveals a non-papillary bladder tumor with blueberry spots. (B) CT shows an invasive bladder mass.
Shigehara K, Miyagi T, Nakashima T, Shimamura M. Cystitis glandularis forming a tumorous lesion in the urinary bladder: a rare appearance of disease. Indian J Urol. 2008;24(4):558-560. doi:10.4103/0970-1591.44268.
(Click Image to Enlarge)
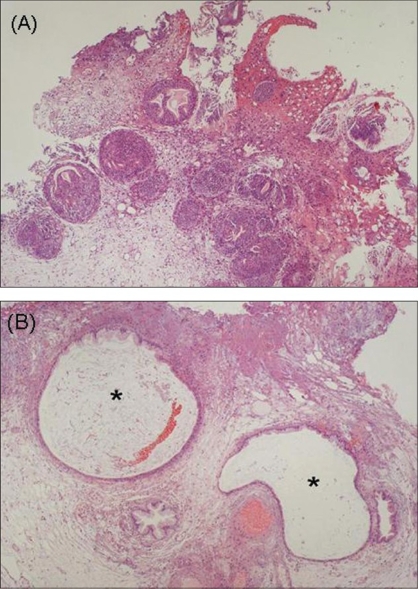
A Histopathological Examination of Cystitis Cystica and Cystitis Glandularis. (A) A histopathological examination shows Brunn's nests, glandular metaplasia, and cystitis glandularis (H & E, ×4). (B) Cystitis cystica is also present in some specimens (H & E, x10).
Shigehara K, Miyagi T, Nakashima T, Shimamura M. Cystitis glandularis forming a tumorous lesion in the urinary bladder: a rare appearance of disease. Indian J Urol. 2008;24(4):558-560. doi:10.4103/0970-1591.44268.
(Click Image to Enlarge)
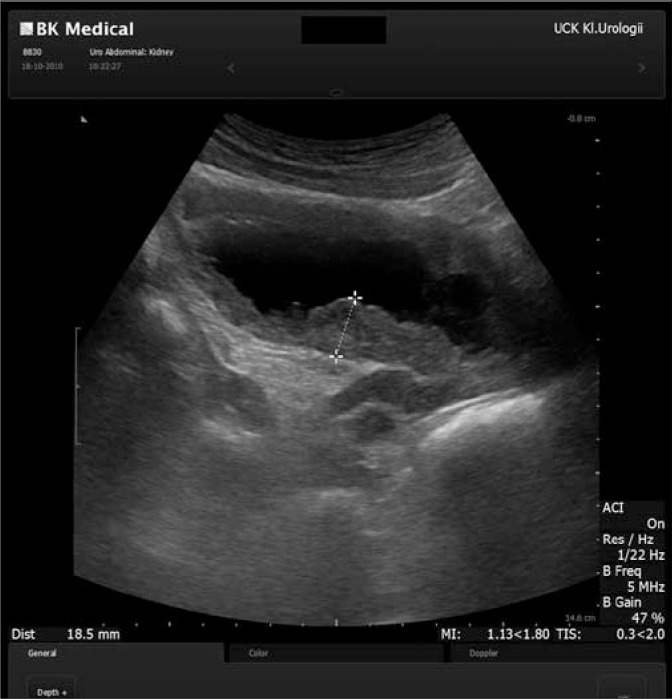
Ultrasound of Bladder. Ultrasound reveals extensive irregular thickening of the urinary bladder wall and two cystic lesions.
Michajłowski J, Matuszewski M, Kłącz J, Gibas A, Biernat W, Krajka K. Acute urinary retention in a patient with extended cystitis glandularis. Cent European J Urol. 2011;64(2):94-96. doi: 10.5173/ceju.2011.02.art11.
References
Eastman R Jr, Leaf EM, Zhang D, True LD, Sweet RM, Seidel K, Siebert JR, Grady R, Mitchell ME, Bassuk JA. Fibroblast growth factor-10 signals development of von Brunn's nests in the exstrophic bladder. American journal of physiology. Renal physiology. 2010 Nov:299(5):F1094-110. doi: 10.1152/ajprenal.00056.2010. Epub 2010 Aug 18 [PubMed PMID: 20719973]
Level 3 (low-level) evidenceXin Z, Zhao C, Huang T, Zhang Z, Chu C, Lu C, Wu M, Zhou W. Intestinal metaplasia of the bladder in 89 patients: a study with emphasis on long-term outcome. BMC urology. 2016 Jun 7:16(1):24. doi: 10.1186/s12894-016-0142-x. Epub 2016 Jun 7 [PubMed PMID: 27267922]
Yi X, Lu H, Wu Y, Shen Y, Meng Q, Cheng J, Tang Y, Wu F, Ou R, Jiang S, Bai X, Xie K. Cystitis glandularis: A controversial premalignant lesion. Oncology letters. 2014 Oct:8(4):1662-1664 [PubMed PMID: 25202387]
Level 3 (low-level) evidenceManini C, Angulo JC, López JI. Mimickers of Urothelial Carcinoma and the Approach to Differential Diagnosis. Clinics and practice. 2021 Feb 25:11(1):110-123. doi: 10.3390/clinpract11010017. Epub 2021 Feb 25 [PubMed PMID: 33668963]
Venyo AK. Nephrogenic Adenoma of the Urinary Bladder: A Review of the Literature. International scholarly research notices. 2015:2015():704982. doi: 10.1155/2015/704982. Epub 2015 Feb 2 [PubMed PMID: 27347540]
Santi R, Angulo JC, Nesi G, de Petris G, Kuroda N, Hes O, López JI. Common and uncommon features of nephrogenic adenoma revisited. Pathology, research and practice. 2019 Oct:215(10):152561. doi: 10.1016/j.prp.2019.152561. Epub 2019 Jul 25 [PubMed PMID: 31358481]
Bannoura S, Putra J. Bladder Exstrophy Polyp: An Uncommon Entity in Surgical Pathology. Fetal and pediatric pathology. 2022 Jun:41(3):523-525. doi: 10.1080/15513815.2020.1854401. Epub 2020 Nov 30 [PubMed PMID: 33252291]
Liu X, Chen Z, Ye Z. Etiological study on cystitis glandularis caused by bacterial infection. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 2007 Dec:27(6):678-80. doi: 10.1007/s11596-007-0615-y. Epub [PubMed PMID: 18231741]
Level 3 (low-level) evidenceChen Z, Ye Z, Zeng W. Clinical investigation on the correlation between lower urinary tract infection and cystitis glandularis. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. 2004:24(3):303-4 [PubMed PMID: 15315357]
Kao CS, Kum JB, Fan R, Grignon DJ, Eble JN, Idrees MT. Nephrogenic adenomas in pediatric patients: a morphologic and immunohistochemical study of 21 cases. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2013 Mar-Apr:16(2):80-5. doi: 10.2350/12-10-1261-OA.1. Epub [PubMed PMID: 23597251]
Level 3 (low-level) evidenceMazal PR, Schaufler R, Altenhuber-Müller R, Haitel A, Watschinger B, Kratzik C, Krupitza G, Regele H, Meisl FT, Zechner O, Kerjaschki D, Susani M. Derivation of nephrogenic adenomas from renal tubular cells in kidney-transplant recipients. The New England journal of medicine. 2002 Aug 29:347(9):653-9 [PubMed PMID: 12200552]
Wiener DP, Koss LG, Sablay B, Freed SZ. The prevalence and significance of Brunn's nests, cystitis cystica and squamous metaplasia in normal bladders. The Journal of urology. 1979 Sep:122(3):317-21 [PubMed PMID: 470001]
Kunju LP. Nephrogenic adenoma: report of a case and review of morphologic mimics. Archives of pathology & laboratory medicine. 2010 Oct:134(10):1455-9 [PubMed PMID: 20923300]
Level 3 (low-level) evidenceManini C, López JI. Unusual Faces of Bladder Cancer. Cancers. 2020 Dec 10:12(12):. doi: 10.3390/cancers12123706. Epub 2020 Dec 10 [PubMed PMID: 33321728]
Zhong M, Tian W, Zhuge J, Zheng X, Huang T, Cai D, Zhang D, Yang XJ, Argani P, Fallon JT, Epstein JI. Distinguishing nested variants of urothelial carcinoma from benign mimickers by TERT promoter mutation. The American journal of surgical pathology. 2015 Jan:39(1):127-31. doi: 10.1097/PAS.0000000000000305. Epub [PubMed PMID: 25118812]
Level 3 (low-level) evidenceWilliamson SR, Lopez-Beltran A, Montironi R, Cheng L. Glandular lesions of the urinary bladder:clinical significance and differential diagnosis. Histopathology. 2011 May:58(6):811-34. doi: 10.1111/j.1365-2559.2010.03651.x. Epub 2010 Sep 21 [PubMed PMID: 20854477]
Lin HY, Wu WJ, Jang MY, Shen JT, Tsai HN, Chou YH, Huang CH. Cystitis glandularis mimics bladder cancer--three case reports and literature review. The Kaohsiung journal of medical sciences. 2001 Feb:17(2):102-6 [PubMed PMID: 11416957]
Level 3 (low-level) evidenceSHAW JL, GISLASON GJ, IMBRIGLIA JE. Transition of cystitis glandularis to primary adenocarcinoma of the bladder. The Journal of urology. 1958 May:79(5):815-22 [PubMed PMID: 13539968]
Lin JI, Yong HS, Tseng CH, Marsidi PS, Choy C, Pilloff B. Diffuse cystitis glandularis. Associated with adenocarcinomatous change. Urology. 1980 Apr:15(4):411-5 [PubMed PMID: 7394970]
García Rojo D, Prera Vilaseca A, Sáez Artacho A, Abad Gairín C, Prats López J, Rosa Bella Cueto M. [Transformation of glandular cystitis into bladder transitional carcinoma with adenocarcinoma areas]. Archivos espanoles de urologia. 1997 Mar:50(2):187-9 [PubMed PMID: 9206946]
Thrasher JB, Rajan RR, Perez LM, Humphrey PA, Anderson EE. Cystitis glandularis. Transition to adenocarcinoma of the urinary bladder. North Carolina medical journal. 1994 Nov:55(11):562-4 [PubMed PMID: 7808523]
Popov H, Stoyanov GS, Ghenev P. Intestinal-Type Adenocarcinoma of the Urinary Bladder With Coexisting Cystitis Cystica et Glandularis and Intestinal Metaplasia: A Histopathological Case Report. Cureus. 2023 Mar:15(3):e36554. doi: 10.7759/cureus.36554. Epub 2023 Mar 22 [PubMed PMID: 37102004]
Level 3 (low-level) evidenceAgrawal A, Kumar D, Jha AA, Aggarwal P. Incidence of adenocarcinoma bladder in patients with cystitis cystica et glandularis: A retrospective study. Indian journal of urology : IJU : journal of the Urological Society of India. 2020 Oct-Dec:36(4):297-302. doi: 10.4103/iju.IJU_261_20. Epub 2020 Oct 1 [PubMed PMID: 33376267]
Level 2 (mid-level) evidenceSmith AK, Hansel DE, Jones JS. Role of cystitis cystica et glandularis and intestinal metaplasia in development of bladder carcinoma. Urology. 2008 May:71(5):915-8. doi: 10.1016/j.urology.2007.11.079. Epub [PubMed PMID: 18455631]
Level 2 (mid-level) evidenceGordetsky J, Epstein JI. Intestinal metaplasia of the bladder with dysplasia: a risk factor for carcinoma? Histopathology. 2015 Sep:67(3):325-30. doi: 10.1111/his.12661. Epub 2015 Mar 8 [PubMed PMID: 25640978]
Level 2 (mid-level) evidenceLi A, Zhou J, Lu H, Zuo X, Liu S, Zhang F, Li W, Fang W, Zhang B. Pathological feature and immunoprofile of cystitis glandularis accompanied with upper urinary tract obstruction. BioMed research international. 2014:2014():872170. doi: 10.1155/2014/872170. Epub 2014 May 29 [PubMed PMID: 25136635]
Diolombi M, Ross HM, Mercalli F, Sharma R, Epstein JI. Nephrogenic adenoma: a report of 3 unusual cases infiltrating into perinephric adipose tissue. The American journal of surgical pathology. 2013 Apr:37(4):532-8. doi: 10.1097/PAS.0b013e31826f0447. Epub [PubMed PMID: 23426119]
Level 3 (low-level) evidenceTong GX, Melamed J, Mansukhani M, Memeo L, Hernandez O, Deng FM, Chiriboga L, Waisman J. PAX2: a reliable marker for nephrogenic adenoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Mar:19(3):356-63 [PubMed PMID: 16400326]
Oliva E, Young RH. Nephrogenic adenoma of the urinary tract: a review of the microscopic appearance of 80 cases with emphasis on unusual features. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1995 Sep:8(7):722-30 [PubMed PMID: 8539229]
Level 3 (low-level) evidenceCheng L, Cheville JC, Sebo TJ, Eble JN, Bostwick DG. Atypical nephrogenic metaplasia of the urinary tract: a precursor lesion? Cancer. 2000 Feb 15:88(4):853-61 [PubMed PMID: 10679655]
López JI, Schiavo-Lena M, Corominas-Cishek A, Yagüe A, Bauleth K, Guarch R, Hes O, Tardanico R. Nephrogenic adenoma of the urinary tract: clinical, histological, and immunohistochemical characteristics. Virchows Archiv : an international journal of pathology. 2013 Dec:463(6):819-25. doi: 10.1007/s00428-013-1497-y. Epub 2013 Oct 19 [PubMed PMID: 24142157]
Level 2 (mid-level) evidenceSharifai N, Abro B, Chen JF, Zhao M, He H, Cao D. Napsin A is a highly sensitive marker for nephrogenic adenoma: an immunohistochemical study with a specificity test in genitourinary tumors. Human pathology. 2020 Aug:102():23-32. doi: 10.1016/j.humpath.2020.05.007. Epub 2020 Jun 17 [PubMed PMID: 32561332]
McDaniel AS, Chinnaiyan AM, Siddiqui J, McKenney JK, Mehra R. Immunohistochemical staining characteristics of nephrogenic adenoma using the PIN-4 cocktail (p63, AMACR, and CK903) and GATA-3. The American journal of surgical pathology. 2014 Dec:38(12):1664-71. doi: 10.1097/PAS.0000000000000267. Epub [PubMed PMID: 24921643]
Mihai I, Taban S, Cumpanas A, Olteanu EG, Iacob M, Dema A. Clear cell urothelial carcinoma of the urinary bladder - a rare pathological entity. A case report and a systematic review of the literature. Bosnian journal of basic medical sciences. 2019 Nov 8:19(4):400-403. doi: 10.17305/bjbms.2019.4182. Epub 2019 Nov 8 [PubMed PMID: 30957722]
Level 1 (high-level) evidenceSon Y, Madison I, Scali J, Chialastri P, Brown G. Cystitis Cystica Et Glandularis Causing Lower Urinary Tract Symptoms in a 29-Year-Old Male. Cureus. 2021 Aug:13(8):e17144. doi: 10.7759/cureus.17144. Epub 2021 Aug 13 [PubMed PMID: 34548967]
Kaya C, Akpinar IN, Aker F, Turkeri LN. Large Cystitis glandularis: a very rare cause of severe obstructive urinary symptoms in an adult. International urology and nephrology. 2007:39(2):441-4 [PubMed PMID: 17171414]
Level 3 (low-level) evidenceel Moussaoui A, Dakir M, Sarf I, Aboutaieb R, Zamiati S, Benjelloun S. [Cystitis cystica glandularis. A study of 2 cases]. Annales d'urologie. 1997:31(4):195-8 [PubMed PMID: 9412342]
Level 3 (low-level) evidencePotts S, Calleary J. Cystitis Cystica as a Large Solitary Bladder Cyst. Journal of endourology case reports. 2017:3(1):34-38. doi: 10.1089/cren.2017.0010. Epub 2017 Mar 1 [PubMed PMID: 28466074]
Level 3 (low-level) evidenceLeopold BH, Johnson CM, Makai GEH. Cystitis Cystica on Routine Cystoscopy at Time of Total Laparoscopic Hysterectomy. Journal of minimally invasive gynecology. 2019 Sep-Oct:26(6):999. doi: 10.1016/j.jmig.2019.02.001. Epub 2019 Feb 6 [PubMed PMID: 30735732]
Jeon J, Ha JS, Shin SJ, Ham WS, Choi YD, Cho KS. Differences in clinical features between focal and extensive types of cystitis glandularis in patients without a previous history of urinary tract malignancy. Investigative and clinical urology. 2023 Nov:64(6):597-605. doi: 10.4111/icu.20230210. Epub [PubMed PMID: 37932571]
McIntire TL, Soloway MS, Murphy WM. Nephrogenic adenoma. Urology. 1987 Mar:29(3):237-41 [PubMed PMID: 3548004]
Milosević D, Batinic D, Vrljicak K, Skitarelić N, Potkonjak AM, Turudić D, Bambir I, Roić AC, Spajić M, Spajić B. Ultrasound distinction between simple recurrent urinary tract infections and a specific bladder wall inflammatory entity called cystitis cystica. Collegium antropologicum. 2014 Mar:38(1):151-4 [PubMed PMID: 24851610]
Milošević D, Trkulja V, Turudić D, Batinić D, Spajić B, Tešović G. Ultrasound bladder wall thickness measurement in diagnosis of recurrent urinary tract infections and cystitis cystica in prepubertal girls. Journal of pediatric urology. 2013 Dec:9(6 Pt B):1170-7. doi: 10.1016/j.jpurol.2013.04.019. Epub 2013 May 30 [PubMed PMID: 23725853]
Vrljicak K, Milosević D, Batinić D, Kniewald H, Nizić L. The significance of ultrasonography in diagnosing and follow-up of cystic cystitis in children. Collegium antropologicum. 2006 Jun:30(2):355-9 [PubMed PMID: 16848151]
Sureka B, Jain V, Jain S, Rastogi A. Cystitis Cystica Glandularis: Radiological Imitator of Urothelial Carcinoma. Iranian journal of kidney diseases. 2018 Jan:12(1):10 [PubMed PMID: 29421770]
Li R, Leslie SW. Cystitis. StatPearls. 2024 Jan:(): [PubMed PMID: 29494042]
Reddy M, Zimmern PE. Efficacy of antimicrobial intravesical treatment for uncomplicated recurrent urinary tract infections: a systematic review. International urogynecology journal. 2022 May:33(5):1125-1143. doi: 10.1007/s00192-021-05042-z. Epub 2022 Jan 4 [PubMed PMID: 34982189]
Level 1 (high-level) evidenceJepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. The Cochrane database of systematic reviews. 2012 Oct 17:10(10):CD001321. doi: 10.1002/14651858.CD001321.pub5. Epub 2012 Oct 17 [PubMed PMID: 23076891]
Level 1 (high-level) evidenceMilosević D, Batinić D, Tesović G, Konjevoda P, Kniewald H, Subat-Dezulović M, Grković L, Topalović-Grković M, Turudić D, Spajić B. Cystitis cystica and recurrent urinary tract infections in children. Collegium antropologicum. 2010 Sep:34(3):893-7 [PubMed PMID: 20977079]
Level 2 (mid-level) evidenceDe Nunzio C, Bartoletti R, Tubaro A, Simonato A, Ficarra V. Role of D-Mannose in the Prevention of Recurrent Uncomplicated Cystitis: State of the Art and Future Perspectives. Antibiotics (Basel, Switzerland). 2021 Apr 1:10(4):. doi: 10.3390/antibiotics10040373. Epub 2021 Apr 1 [PubMed PMID: 33915821]
Level 3 (low-level) evidenceSabih A, Leslie SW. Complicated Urinary Tract Infections. StatPearls. 2024 Jan:(): [PubMed PMID: 28613784]
Chiu K, Zhang F, Sutcliffe S, Mysorekar IU, Lowder JL. Recurrent Urinary Tract Infection Incidence Rates Decrease in Women With Cystitis Cystica After Treatment With d-Mannose: A Cohort Study. Female pelvic medicine & reconstructive surgery. 2022 Mar 1:28(3):e62-e65. doi: 10.1097/SPV.0000000000001144. Epub [PubMed PMID: 35272335]
Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2011 Oct:5(5):316-22. doi: 10.5489/cuaj.11214. Epub [PubMed PMID: 22031610]
Stamm WE, Counts GW, Wagner KF, Martin D, Gregory D, McKevitt M, Turck M, Holmes KK. Antimicrobial prophylaxis of recurrent urinary tract infections: a double-blind, placebo-controlled trial. Annals of internal medicine. 1980 Jun:92(6):770-5 [PubMed PMID: 6992677]
Level 1 (high-level) evidencePrice JR, Guran LA, Gregory WT, McDonagh MS. Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta-analysis. American journal of obstetrics and gynecology. 2016 Nov:215(5):548-560. doi: 10.1016/j.ajog.2016.07.040. Epub 2016 Jul 22 [PubMed PMID: 27457111]
Level 1 (high-level) evidenceJent P, Berger J, Kuhn A, Trautner BW, Atkinson A, Marschall J. Antibiotics for Preventing Recurrent Urinary Tract Infection: Systematic Review and Meta-analysis. Open forum infectious diseases. 2022 Jul:9(7):ofac327. doi: 10.1093/ofid/ofac327. Epub 2022 Jul 3 [PubMed PMID: 35899289]
Level 1 (high-level) evidenceAlghoraibi H, Asidan A, Aljawaied R, Almukhayzim R, Alsaydan A, Alamer E, Baharoon W, Masuadi E, Al Shukairi A, Layqah L, Baharoon S. Recurrent Urinary Tract Infection in Adult Patients, Risk Factors, and Efficacy of Low Dose Prophylactic Antibiotics Therapy. Journal of epidemiology and global health. 2023 Jun:13(2):200-211. doi: 10.1007/s44197-023-00105-4. Epub 2023 Jun 5 [PubMed PMID: 37273158]
Aggarwal N, Leslie SW, Lotfollahzadeh S. Recurrent Urinary Tract Infections. StatPearls. 2024 Jan:(): [PubMed PMID: 32491411]
Yuksel OH, Urkmez A, Erdogru T, Verit A. The role of steroid treatment in intractable cystitis glandularis: A case report and literature review. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2015 May-Jun:9(5-6):E306-9. doi: 10.5489/cuaj.2636. Epub [PubMed PMID: 26029302]
Level 3 (low-level) evidenceKoyama J, Namiki S, Kudo T, Aizawa M, Ioritani N, Nakamura Y. [TYPICAL TYPE CYSTITIS GLANDULARIS PRESENTING URINARY RETENTION IN A YOUNG MAN: ADJUVANT THERAPY USING ORAL CYCLOOXYGENASE-2 INHIBITOR: A CASE REPORT]. Nihon Hinyokika Gakkai zasshi. The japanese journal of urology. 2019:110(2):148-151. doi: 10.5980/jpnjurol.110.148. Epub [PubMed PMID: 32307385]
Level 3 (low-level) evidenceNi Y, Zhao S, Yin X, Wang H, Guang Q, Hu G, Yang Y, Jiao S, Shi B. Intravesicular administration of sodium hyaluronate ameliorates the inflammation and cell proliferation of cystitis cystica et glandularis involving interleukin-6/JAK2/Stat3 signaling pathway. Scientific reports. 2017 Nov 21:7(1):15892. doi: 10.1038/s41598-017-16088-9. Epub 2017 Nov 21 [PubMed PMID: 29162939]
Klingler CH. Glycosaminoglycans: how much do we know about their role in the bladder? Urologia. 2016 Jun 25:83 Suppl 1():11-4. doi: 10.5301/uro.5000184. Epub 2016 Jun 23 [PubMed PMID: 27405344]
Boucher WS, Letourneau R, Huang M, Kempuraj D, Green M, Sant GR, Theoharides TC. Intravesical sodium hyaluronate inhibits the rat urinary mast cell mediator increase triggered by acute immobilization stress. The Journal of urology. 2002 Jan:167(1):380-4 [PubMed PMID: 11743360]
Level 3 (low-level) evidenceMadurga Patuel B, González-López R, Resel Folkersma L, Machado Fernández G, Adot Zurbano JM, Bonillo MÁ, Vozmediano Chicharro R, Zubiaur Líbano C. Recommendations on the use of intravesical hyaluronic acid instillations in bladder pain syndrome. Actas urologicas espanolas. 2022 Apr:46(3):131-137. doi: 10.1016/j.acuroe.2022.02.007. Epub 2022 Mar 4 [PubMed PMID: 35256323]
Samdal F, Brevik B, Halgunset J. Cystitis cystica treated with the neodymium-YAG laser. Case report. Scandinavian journal of urology and nephrology. 1991:25(2):163-4 [PubMed PMID: 1871562]
Level 3 (low-level) evidenceShen YC, Tyagi P, Lee WC, Chancellor M, Chuang YC. Improves symptoms and urinary biomarkers in refractory interstitial cystitis/bladder pain syndrome patients randomized to extracorporeal shock wave therapy versus placebo. Scientific reports. 2021 Apr 6:11(1):7558. doi: 10.1038/s41598-021-87040-1. Epub 2021 Apr 6 [PubMed PMID: 33824389]
Level 1 (high-level) evidenceNasrallah OG, Balaghi A, El Sayegh N, Mahdi JH, Sinno S, Nasr RW. Florid Cystitis Glandularis with Intestinal Metaplasia in the Prostatic Urethra: a case report and review of the literature. International journal of surgery case reports. 2024 Mar:116():109416. doi: 10.1016/j.ijscr.2024.109416. Epub 2024 Feb 28 [PubMed PMID: 38422750]
Level 3 (low-level) evidenceHeidenreich A, Zirbes TK, Wolter S, Engelmann UH. Nephrogenic adenoma: A rare bladder tumor in children. European urology. 1999 Oct:36(4):348-53 [PubMed PMID: 10473997]
Level 3 (low-level) evidenceVoss K, Peppas D. Recurrent nephrogenic adenoma: a case report of resolution after treatment with antibiotics and nonsteroidal anti-inflammatory medication. Urology. 2013 Nov:82(5):1156-7. doi: 10.1016/j.urology.2013.04.024. Epub 2013 Jun 20 [PubMed PMID: 23791211]
Level 3 (low-level) evidenceSantoni N, Cottrell L, Talia Jones JE, Bekarma HJ. Multifocal nephrogenic adenoma treated by intravesical sodium hyaluronate. Urology annals. 2020 Apr-Jun:12(2):187-189. doi: 10.4103/UA.UA_74_19. Epub 2020 Apr 14 [PubMed PMID: 32565661]
Yi Y, Wu A, Cameron AP. Nephrogenic adenoma of the bladder: a single institution experience assessing clinical factors. International braz j urol : official journal of the Brazilian Society of Urology. 2018 May-Jun:44(3):506-511. doi: 10.1590/S1677-5538.IBJU.2017.0155. Epub [PubMed PMID: 29493186]
Vuckov S, Subat-Dezulović M, Nikolić H. [Relation between successful treatment of urinary tract inflammation and the disappearance of changes in the bladder mucosa in children and adolescents with cystoscopically proven cystitis cystica]. Lijecnicki vjesnik. 1997 Nov:119(10):266-9 [PubMed PMID: 9531758]
Vrljicak K, Turudić D, Bambir I, Gradiski IP, Spajić B, Batinić D, Topalović-Grković M, Spajić M, Batinić D, Milosević D. Positive feedback loop for cystitis cystica: the effect of recurrent urinary tract infection on the number of bladder wall mucosa nodules. Acta clinica Croatica. 2013 Dec:52(4):444-7 [PubMed PMID: 24696993]
Maeda M, Hirabayashi T, Inuzuka Y, Kondo A, Tanaka K. [Case of cystitis glandularis causing bilateral hydronephrosis]. Nihon Hinyokika Gakkai zasshi. The japanese journal of urology. 2013 Sep:104(5):671-3 [PubMed PMID: 24187856]
Level 3 (low-level) evidenceAbasher A, Abdel Raheem A, Aldarrab R, Aldurayhim M, Attallah A, Banihani O. Bladder outlet obstruction secondary to posterior urethral cystitis cystica & glandularis in a 12-year-old boy. A rare case scenario. Urology case reports. 2020 Nov:33():101425. doi: 10.1016/j.eucr.2020.101425. Epub 2020 Sep 23 [PubMed PMID: 33102121]
Suzuki T, Furuse H, Matsumoto R, Ito T, Sugiyama T, Nagata M, Otsuka A, Takayama T, Mugiya S, Ozono S. [A case of proliferative cystitis forming ureterovesical junction obstruction]. Hinyokika kiyo. Acta urologica Japonica. 2011 Oct:57(10):573-6 [PubMed PMID: 22089157]
Level 3 (low-level) evidenceGe B, Guo C, Liang Y, Liu M, Wu K. Network analysis, and human and animal studies disclose the anticystitis glandularis effects of vitamin C. BioFactors (Oxford, England). 2019 Nov:45(6):912-919. doi: 10.1002/biof.1558. Epub 2019 Aug 30 [PubMed PMID: 31469455]
Level 3 (low-level) evidence