Introduction
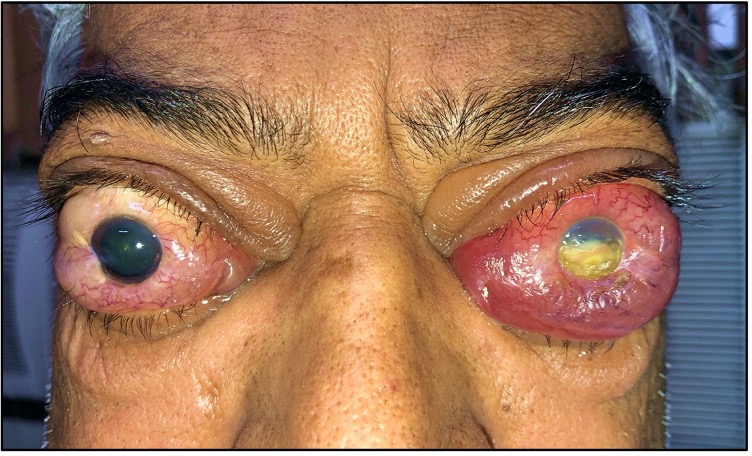
Dysthyroid optic neuropathy (DON) represents the most dreaded manifestation of thyroid eye disease (TED), one of the most common autoimmune diseases of the orbit.[1] DON, previously termed the crowded orbital apex syndrome, is characterized by thyroid-related impairment of optic nerve function that may lead to profound vision loss. The European Group on Graves Orbitopathy (EUGOGO) classifies the severity of TED as mild, moderate-severe, or sight-threatening. Sight-threatening TED, characterized by DON or keratopathy, warrants urgent intervention (see Image. Left Corneal Exposure Keratopathy With Bilateral Optic Nerve Involvement).[2] DON is a multifactorial entity whose diagnosis is extremely challenging owing to its early subclinical stage, the presence of confounding factors, and alternative causes of sight loss in TED. A thorough evaluation of DON and subsequent timely management are crucial to avoid permanent blindness in TED.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Several etiological factors have been implicated in the progression of TED, which may also be linked to DON. Thyroid status impairment is one of the most important associations. The majority of cases of DON are associated with hyperthyroidism. This can be attributed to the fact that hyperthyroidism is the most common form of thyroid dysfunction in TED.[3] It is worth noting that hypothyroidism, autoimmune thyroiditis, or even a euthyroid status may be found with DON. Radio-active Iodine therapy, a well-known risk factor for TED progression, may increase the incidence of DON, although any meaningful evidence is lacking.[4] Studies have demonstrated smoking as an independent modifiable risk factor for the development of the DON.[5][6] A recent study has discovered a correlation between early-onset DON and thyroid-stimulating immunoglobulin positivity.[7] It has also been shown that in patients with diabetic vasculopathy, especially those on injectable insulin, hypoxia of the optic nerve occurs, leading to subsequent nerve damage.[8] Such patients of TED with superimposed comorbidities must closely be monitored for the development of optic neuropathy. Previous studies have linked numerous genes to TED's pathogenetic mechanism; however, none are specific to DON.[9]
Epidemiology
Approximately every 5 in 100 patients with TED progress to sight-threatening optic neuropathy.[10] Younger patients are less likely to develop DON than older age groups. It is found that for every 10-year increment in the onset of ophthalmopathy, the risk of developing optic neuropathy increases by approximately 60%.[11] Increased age at presentation of DON is associated with poorer long-term outcomes.[12] Although the White-race population demonstrates female preponderance in TED, DON is more common in males. Hence, an old male is far more likely to develop DON than a young female.[13][14][15]
Pathophysiology
There are several hypothesized pathophysiological mechanisms. The most widely accepted theory is the direct mechanical compression of the optic nerve by the edematous extra-ocular muscles and intra-orbital fat, leading to the deceleration of the axonal flow and the resultant ischemia.[16][17] The subsequent crowding at the orbital apex also often increases orbital pressure. Substantial evidence to support the above theory can be derived from the fact that the visual acuity and retrobulbar pressure significantly improve after orbital decompression. This surgery relieves the crowding and the congestion in orbit.[18][19] The traditional hypothesis of optic nerve inflammation as the implicated mechanism is obsolete due to a lack of strong evidence of inflammatory cells in the histopathological specimen of the optic nerve in patients with suspected or clinically diagnosed DON.[20] Stretching of the optic nerve is yet another postulated mechanism of optic neuropathy in TED. Unlike the peripheral nerves, the optic nerve has a regular and uniform organization of nerve fibers. This anatomical difference presumably makes it more vulnerable to damage through circumferential straining.[21][22] Impaired venous drainage and hypoperfusion of the apical portion of the optic nerve may also contribute to further optic nerve injury.
History and Physical
Besides the clinical features of TED, the most common presenting symptoms specific to DON include blurred vision and impaired perception of color sense.[23][24][25] In subclinical forms, the patients may even be asymptomatic. A comprehensive clinical examination is required that may reveal a decrease in best-corrected visual acuity, relative afferent pupillary defect in unilateral and asymmetric cases, optic disc edema and hyperemia (acute form), optic atrophy (chronic form), which may or may not be accompanied by features of activity, that is, a Clinical Activity Score of more than 2 out of 7 (EUGOGO 2016 guidelines), restricted extra-ocular movements, proptosis, strabismus, and even ptosis.[26][16]
Evaluation
DON is a controversial disease entity whose diagnostic criteria have not been established. Color vision and contrast sensitivity tests may help detect an early onset DON. Characteristic visual defects include a central or paracentral scotoma. Other patterns include an enlarged blind spot, arcuate or altitudinal scotomas, and inferior defects more common than superior.[27] The retinal never fiber layer and macular ganglion cell analysis using optical coherent tomography are gaining popularity due to the test's non-invasive, non-contact, and highly reproducible properties.[28] The clinician must consider electrophysiological assessment to test the integrity of the optic nerve using pattern visual evoked potential signals, which may reveal a decreased amplitude and increased latency of the P-wave.[29] Radiological evaluation using computed tomography and magnetic resonance imaging plays an important role in DON. Imaging may reveal one or more of the various predictors, which include apical crowding, prolapse of the orbital fat through the superior orbital fissure, increased extra-ocular muscle volume (specifically the medial rectus volume that lies close to the optic nerve), and increased superior ophthalmic vein diameter.
Several measurements, such as Barrett’s Muscle Index (MI), Extra-ocular Muscle Volume/ Orbital Volume (MV/OV), and Crowding Index (CI), have been implicated as reliable indicators of the disease in the literature. MV/OV > 20% has potentially come up as a more predictable index of acuity and field loss compared to MI (MI > 60% is indicative of DON).[30][31][32][33][34] Color Doppler ultrasonography has detected orbital blood flow changes such as reduced resistivity index and central retinal artery flow velocities in early-onset DON.[35] However, none of the above parameters is solely diagnostic of DON. In 2007, Dayan and Dayan proposed a useful diagnostic algorithm.[36] The initial step is to look for the clinical features suggestive of TED and the presence of damaged visual function, which is indicated by any one of the following: impairment of color vision, diminished visual acuity, disc edema/atrophy, presence of a relative afferent pupillary defect, specific field defects or electrophysiological abnormalities. If none of these features are present, DON is “unlikely.” If present, rule out an alternative etiology of visual loss in TED. If none could be found in the presence of supporting radiological evidence of compression, DON is “likely” to be present. If no radiological evidence is present or an alternative cause is found, but with radiological compression, DON is still “possible.” In the presence of a different etiology of visual impairment without any compressive features on imaging, DON is again “unlikely.” The key message from the hypothesized flowchart is that DON can never be completely ruled out, and a high index of suspicion must always be maintained, especially in cases with subclinical stage, euthyroid presentation, and bilaterally symmetric DON where most of the ancillary tests may give confusing results.
Treatment / Management
TED complicated with DON requires aggressive management. The treatment options include medical decompression using corticosteroids, radiotherapy, and surgical decompression. There is no single best treatment option; sometimes, even a combination of the above choices is recommended.[37] Corticosteroid therapy is the proposed first-line treatment in DON. EUGOGO recommends intravenous methylprednisolone (IVMP) as the immunosuppressant of choice in DON. The regimen states using 500-1000 mg IVMP daily for 3 consecutive days, followed by a repeat dose after 1 week (if needed). If improvement in visual parameters is observed at 2 weeks, the dosage can be tapered, or alternate forms such as oral steroids may be used. This is referred to as medical decompression. Complete visual recovery has been shown to occur in 43% of cases with this regimen.[38][39][40] It has been shown that almost half of the patients may avoid surgery if treated with IVMP.[41] At no point in time, should the cumulative dose of IVMP exceed 8g, otherwise, fatal systemic adverse effects may occur such as acute heart failure, liver dysfunction, and even sudden death.[42][43] (A1)
The clinician must perform a thorough clinical assessment and conduct all the baseline investigations, such as blood sugar, liver function tests, serum electrolytes, chest x-rays, electrocardiography, and blood pressure, before administering steroids. Inadequate response to IVMP is defined as persistent activity and disc edema even after 2 weeks of treatment.[44] In this scenario, urgent surgical decompression of the orbit is recommended. In general, orbital decompression is advised only after achieving at least 6 months of euthyroid status in inactive TED. DON is probably the only indication of an urgent orbital decompression in the active stage of the disease.[26] Surgical decompression provides excellent outcomes with visual improvement in 70 to 85% of cases.[45][1] It leads to the expansion of the bony volume and a relative decrease in the soft tissue volume so that the apical structures decompress. This can be achieved via infero-medial wall decompression, lateral wall decompression, or even a combination of the two (3-wall), with or without fat decompression. The inferomedial orbit can be approached via the trans-antral, trans-cutaneous, trans-caruncular, or endoscopically through the nasal approach. In the trans-antral approach, the incision is given along the buccal sulcus, and the anterior wall of the maxilla is removed.(B2)
A high number of cases with postoperative diplopia (66%) outranks its rate of success (90%). Alveolar nerve hypoesthesia, cerebrospinal fluid leak, and entropion are rare but possible complications.[46] The trans-cutaneous approach involves giving a subciliary incision along the lower eyelid. A trans-cutaneous Lynch incision, a 3 cm curvilinear incision, is given along the nasal area adjacent to the medial canthus. However, this approach often results in a webbed scar and may potentially damage the underlying lacrimal system. A modification involving a smaller-sized (1.5cm) incision results in minimal webbing and minimizes the risk of iatrogenic damage. Another popular approach is the "swinging lid crease" incision, which gives an excellent view of the inferomedial orbit and provides better visualization of the lateral wall and the orbital fat.[47][48][49] A trans-caruncular approach provides safe, quick, and graded access to the apical area, thus lowering the chances of postoperative diplopia.[50][51] Another safe approach with low rates of postoperative diplopia is lateral wall decompression, which includes removing the deep lateral wall using a drill or aspirator.[52] (B2)
In fat-centric TED complicated with DON, fat decompression alone, via transconjunctival or transcutaneous approach, can alleviate compression.[22] A novel approach describing a combination of endoscopic trans-ethmoidal medial orbital wall decompression and endoscopic trans-ethmoidal fat decompression has been reported in the literature with low rates of postoperative diplopia that resolved within a few months.[53] Fractionated radiotherapy (2000 rad per eye in ten doses over 10 days) is indicated in patients refractory to medical and surgical decompression or patients with several comorbidities, such as hypertension and diabetic retinopathy.[54] However, the scarcity of evidence-based data regarding its definite use in DON makes it debatable. The use of steroid-sparing immunosuppressive agents such as adalimumab, tocilizumab, and teprotumumab, specifically for DON, is yet to be established. (B3)
Differential Diagnosis
The differential diagnoses of DON include other causes of sight-threatening TED, such as ocular surface diseases. Alternative etiologies of optic neuropathy, such as those due to toxic and nutritional causes, must be ruled out, specifically in the presence of central and paracentral scotomas. Unilateral disc edema with the enlarged blind spot and electrophysiological changes may also raise the suspicion of optic neuritis. It is not uncommon to have secondary glaucoma in TED that may cause visual acuity changes, arcuate field defects, retinal never fiber layer defects, and ocular blood flow changes. A meticulous history, clinical evaluation, and dedicated investigative workup help to differentiate each of the above from DON.
Prognosis
It is imperative to understand that the prognosis of DON largely depends upon whether the treatment was received and the stage or time at which the intervention was initiated. A timely intervention in DON saves vision, with full recovery in about 70% of cases. The additional positive prognosticating factors are young age and an elevated Clinical Activity Score.[45][12]
Complications
More than a disease, DON is itself the gravest complication of TED. Misdiagnosed or left untreated, it leads to permanent loss of visual function. It not only leads to visual disability but also results in major psychosocial trauma.
Deterrence and Patient Education
Elimination of modifiable risk factors and prompt restoration of euthyroidism is indispensable in every patient of TED. Once the disease has progressed to DON, the patients must be counseled to seek treatment immediately after explaining the risks and benefits. Strict metabolic control and frequent ophthalmological and systemic check-ups must be encouraged for a better prognosis.
Pearls and Other Issues
The occurrence of DON in TED cannot be prevented, but, to a significant extent, the subsequent blindness is avoidable. A systemic multidisciplinary approach is crucial for detecting the subclinical form of DON in patients with thyroid-related ophthalmopathy. A clinician must maintain a low threshold for an aggressive patient work-up.
Enhancing Healthcare Team Outcomes
Every healthcare institution must be motivated to equip themselves with a joint, multidisciplinary “Thyroid Clinic” comprising at least an ophthalmologist and an endocrinologist. This interprofessional approach is highly encouraged as this not only enhances the management outcomes but also reduces the burden of healthcare costs on these patients, especially in lower-to-middle-income countries. Owing to the autoimmune nature of the disease, the patients must be counseled regarding the need for a lifelong follow-up. Randomized multi-center trials are needed to understand the exact etiopathogenesis of DON, the role of steroid-sparing immunosuppressive drugs, especially in co-morbid conditions, and alternate surgical approaches to maximize healthcare outcomes and enhance the patient's quality of life.
Media
(Click Image to Enlarge)
References
Dolman PJ. Dysthyroid optic neuropathy: evaluation and management. Journal of endocrinological investigation. 2021 Mar:44(3):421-429. doi: 10.1007/s40618-020-01361-y. Epub 2020 Jul 29 [PubMed PMID: 32729049]
Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas GE, Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM, European Group on Graves' Orbitopathy (EUGOGO). Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. European journal of endocrinology. 2008 Mar:158(3):273-85. doi: 10.1530/EJE-07-0666. Epub [PubMed PMID: 18299459]
Level 3 (low-level) evidenceBartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, Gorman CA. Clinical features of Graves' ophthalmopathy in an incidence cohort. American journal of ophthalmology. 1996 Mar:121(3):284-90 [PubMed PMID: 8597271]
Level 2 (mid-level) evidenceBahn RS, Graves' ophthalmopathy. The New England journal of medicine. 2010 Feb 25; [PubMed PMID: 20181974]
Wiersinga WM. Management of Graves' ophthalmopathy. Nature clinical practice. Endocrinology & metabolism. 2007 May:3(5):396-404 [PubMed PMID: 17452966]
Lee JH, Lee SY, Yoon JS. Risk factors associated with the severity of thyroid-associated orbitopathy in Korean patients. Korean journal of ophthalmology : KJO. 2010 Oct:24(5):267-73. doi: 10.3341/kjo.2010.24.5.267. Epub 2010 Oct 5 [PubMed PMID: 21052505]
Level 2 (mid-level) evidencePonto KA, Diana T, Binder H, Matheis N, Pitz S, Pfeiffer N, Kahaly GJ. Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. Journal of endocrinological investigation. 2015 Jul:38(7):769-77. doi: 10.1007/s40618-015-0254-2. Epub 2015 Mar 4 [PubMed PMID: 25736545]
Blandford AD,Zhang D,Chundury RV,Perry JD, Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert review of ophthalmology. 2017; [PubMed PMID: 28775762]
Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid : official journal of the American Thyroid Association. 2010 Jul:20(7):777-83. doi: 10.1089/thy.2010.1634. Epub [PubMed PMID: 20578901]
Lazarus JH. Epidemiology of Graves' orbitopathy (GO) and relationship with thyroid disease. Best practice & research. Clinical endocrinology & metabolism. 2012 Jun:26(3):273-9. doi: 10.1016/j.beem.2011.10.005. Epub [PubMed PMID: 22632364]
Khong JJ, Finch S, De Silva C, Rylander S, Craig JE, Selva D, Ebeling PR. Risk Factors for Graves' Orbitopathy; the Australian Thyroid-Associated Orbitopathy Research (ATOR) Study. The Journal of clinical endocrinology and metabolism. 2016 Jul:101(7):2711-20. doi: 10.1210/jc.2015-4294. Epub 2016 Apr 7 [PubMed PMID: 27055083]
Miśkiewicz P,Rutkowska B,Jabłońska A,Krzeski A,Trautsolt-Jeziorska K,Kęcik D,Milczarek-Banach J,Pirko-Kotela K,Samsel A,Bednarczuk T, Complete recovery of visual acuity as the main goal of treatment in patients with dysthyroid optic neuropathy. Endokrynologia Polska. 2016; [PubMed PMID: 26884288]
Neigel JM, Rootman J, Belkin RI, Nugent RA, Drance SM, Beattie CW, Spinelli JA. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988 Nov:95(11):1515-21 [PubMed PMID: 3211460]
Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Archives of ophthalmology (Chicago, Ill. : 1960). 1978 Jul:96(7):1199-1209 [PubMed PMID: 666628]
Level 3 (low-level) evidencePerros P, Crombie AL, Matthews JN, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clinical endocrinology. 1993 Apr:38(4):367-72 [PubMed PMID: 8319368]
McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, Hullo AI, Kahaly G, Krassas G, Marcocci C, Marinò M, Mourits MP, Nardi M, Neoh C, Orgiazzi J, Perros P, Pinchera A, Pitz S, Prummel MF, Sartini MS, Wiersinga WM, European Group on Graves' Orbitopathy (EUGOGO). Clinical features of dysthyroid optic neuropathy: a European Group on Graves' Orbitopathy (EUGOGO) survey. The British journal of ophthalmology. 2007 Apr:91(4):455-8 [PubMed PMID: 17035276]
Level 3 (low-level) evidenceGiaconi JA, Kazim M, Rho T, Pfaff C. CT scan evidence of dysthyroid optic neuropathy. Ophthalmic plastic and reconstructive surgery. 2002 May:18(3):177-82 [PubMed PMID: 12021647]
Level 2 (mid-level) evidenceOtto AJ, Koornneef L, Mourits MP, Deen-van Leeuwen L. Retrobulbar pressures measured during surgical decompression of the orbit. The British journal of ophthalmology. 1996 Dec:80(12):1042-5 [PubMed PMID: 9059266]
Riemann CD, Foster JA, Kosmorsky GS. Direct orbital manometry in patients with thyroid-associated orbitopathy. Ophthalmology. 1999 Jul:106(7):1296-302 [PubMed PMID: 10406609]
Day RM, Carroll FD. Corticosteroids in the treatment of optic nerve involvement associated with thyroid dysfunction. Transactions of the American Ophthalmological Society. 1967:65():41-51 [PubMed PMID: 5630622]
Soni CR, Johnson LN. Visual neuropraxia and progressive vision loss from thyroid-associated stretch optic neuropathy. European journal of ophthalmology. 2010 Mar-Apr:20(2):429-36 [PubMed PMID: 20037894]
Level 3 (low-level) evidenceKazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. The British journal of ophthalmology. 2000 Jun:84(6):600-5 [PubMed PMID: 10837384]
Level 3 (low-level) evidenceSaeed P, Tavakoli Rad S, Bisschop PHLT. Dysthyroid Optic Neuropathy. Ophthalmic plastic and reconstructive surgery. 2018 Jul/Aug:34(4S Suppl 1):S60-S67. doi: 10.1097/IOP.0000000000001146. Epub [PubMed PMID: 29927882]
Dolman PJ. Evaluating Graves' orbitopathy. Best practice & research. Clinical endocrinology & metabolism. 2012 Jun:26(3):229-48. doi: 10.1016/j.beem.2011.11.007. Epub [PubMed PMID: 22632361]
Wong Y, Dickinson J, Perros P, Dayan C, Veeramani P, Morris D, Foot B, Clarke L. A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom. Eye (London, England). 2018 Oct:32(10):1555-1562. doi: 10.1038/s41433-018-0144-x. Epub 2018 Jun 18 [PubMed PMID: 29915191]
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM, European Group on Graves' Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. European thyroid journal. 2016 Mar:5(1):9-26. doi: 10.1159/000443828. Epub 2016 Mar 2 [PubMed PMID: 27099835]
Choi CJ, Oropesa S, Callahan AB, Glass LR, Teo L, Cestari DM, Kazim M, Freitag SK. Patterns of visual field changes in thyroid eye disease. Orbit (Amsterdam, Netherlands). 2017 Aug:36(4):201-207. doi: 10.1080/01676830.2017.1314510. Epub 2017 Apr 28 [PubMed PMID: 28453366]
Micieli JA,Newman NJ,Biousse V, The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Current opinion in neurology. 2019 Feb; [PubMed PMID: 30418197]
Level 3 (low-level) evidenceIao TWU, Rong SS, Ling AN, Brelén ME, Young AL, Chong KKL. Electrophysiological Studies in Thyroid Associated Orbitopathy: A Systematic Review. Scientific reports. 2017 Sep 21:7(1):12108. doi: 10.1038/s41598-017-11998-0. Epub 2017 Sep 21 [PubMed PMID: 28935968]
Level 1 (high-level) evidenceMonteiro ML, Gonçalves AC, Silva CT, Moura JP, Ribeiro CS, Gebrim EM. Diagnostic ability of Barrett's index to detect dysthyroid optic neuropathy using multidetector computed tomography. Clinics (Sao Paulo, Brazil). 2008 Jun:63(3):301-6 [PubMed PMID: 18568237]
Level 2 (mid-level) evidenceBarrett L, Glatt HJ, Burde RM, Gado MH. Optic nerve dysfunction in thyroid eye disease: CT. Radiology. 1988 May:167(2):503-7 [PubMed PMID: 3357962]
Level 2 (mid-level) evidenceNugent RA, Belkin RI, Neigel JM, Rootman J, Robertson WD, Spinelli J, Graeb DA. Graves orbitopathy: correlation of CT and clinical findings. Radiology. 1990 Dec:177(3):675-82 [PubMed PMID: 2243967]
Level 2 (mid-level) evidenceBirchall D, Goodall KL, Noble JL, Jackson A. Graves ophthalmopathy: intracranial fat prolapse on CT images as an indicator of optic nerve compression. Radiology. 1996 Jul:200(1):123-7 [PubMed PMID: 8657899]
Level 2 (mid-level) evidenceRegensburg NI, Wiersinga WM, van Velthoven ME, Berendschot TT, Zonneveld FW, Baldeschi L, Saeed P, Mourits MP. Age and gender-specific reference values of orbital fat and muscle volumes in Caucasians. The British journal of ophthalmology. 2011 Dec:95(12):1660-3. doi: 10.1136/bjo.2009.161372. Epub 2009 Dec 2 [PubMed PMID: 19955201]
Lešin M, Rogošić V, Vanjaka Rogošić L, Barišić I, Pelčić G. Flow Changes in Orbital Vessels Detected with Color Doppler Ultrasound in Patients with Early Dysthyroid Optic Neuropathy. Acta clinica Croatica. 2018 Jun:57(2):301-306. doi: 10.20471/acc.2018.57.02.10. Epub [PubMed PMID: 30431723]
Dayan CM, Dayan MR. Dysthyroid optic neuropathy: a clinical diagnosis or a definable entity? The British journal of ophthalmology. 2007 Apr:91(4):409-10 [PubMed PMID: 17372336]
Xu J, Ye H, Chen G, Chen J, Chen R, Yang H. The Therapeutic Effect of Combination of Orbital Decompression Surgery and Methylprednisolone Pulse Therapy on Patients with Bilateral Dysthyroid Optic Neuropathy. Journal of ophthalmology. 2020:2020():9323450. doi: 10.1155/2020/9323450. Epub 2020 Feb 19 [PubMed PMID: 32148948]
Wakelkamp IM, Baldeschi L, Saeed P, Mourits MP, Prummel MF, Wiersinga WM. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves' ophthalmopathy? A randomized controlled trial. Clinical endocrinology. 2005 Sep:63(3):323-8 [PubMed PMID: 16117821]
Level 1 (high-level) evidenceEckstein A, Schittkowski M, Esser J. Surgical treatment of Graves' ophthalmopathy. Best practice & research. Clinical endocrinology & metabolism. 2012 Jun:26(3):339-58. doi: 10.1016/j.beem.2011.11.002. Epub [PubMed PMID: 22632370]
Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, Bartalena L, European Group of Graves' Orbitopathy. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves' orbitopathy: a questionnaire survey among members of the European Thyroid Association. European journal of endocrinology. 2012 Feb:166(2):247-53. doi: 10.1530/EJE-11-0779. Epub 2011 Nov 4 [PubMed PMID: 22058081]
Level 3 (low-level) evidenceZhao LQ, Yu DY, Cheng JW. Intravenous glucocorticoids therapy in the treatment of Graves' ophthalmopathy: a systematic review and Meta-analysis. International journal of ophthalmology. 2019:12(7):1177-1186. doi: 10.18240/ijo.2019.07.20. Epub 2019 Jul 18 [PubMed PMID: 31341811]
Level 1 (high-level) evidenceEguchi H, Tani J, Hirao S, Tsuruta M, Tokubuchi I, Yamada K, Kasaoka M, Teshima Y, Kakuma T, Hiromatsu Y. Liver Dysfunction Associated with Intravenous Methylprednisolone Pulse Therapy in Patients with Graves' Orbitopathy. International journal of endocrinology. 2015:2015():835979. doi: 10.1155/2015/835979. Epub 2015 Jun 28 [PubMed PMID: 26221141]
Yong KL, Chng CL, Htoon HM, Lim LH, Seah LL. Safety Profile and Effects of Pulsed Methylprednisolone on Vital Signs in Thyroid Eye Disease. International journal of endocrinology. 2015:2015():457123. doi: 10.1155/2015/457123. Epub 2015 Nov 22 [PubMed PMID: 26681940]
Currò N, Covelli D, Vannucchi G, Campi I, Pirola G, Simonetta S, Dazzi D, Guastella C, Pignataro L, Beck-Peccoz P, Ratiglia R, Salvi M. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid : official journal of the American Thyroid Association. 2014 May:24(5):897-905. doi: 10.1089/thy.2013.0445. Epub 2014 Mar 6 [PubMed PMID: 24417307]
Level 2 (mid-level) evidenceJeon C, Shin JH, Woo KI, Kim YD. Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean journal of ophthalmology : KJO. 2012 Apr:26(2):73-9. doi: 10.3341/kjo.2012.26.2.73. Epub 2012 Mar 22 [PubMed PMID: 22511831]
Level 2 (mid-level) evidenceGarrity JA, Fatourechi V, Bergstralh EJ, Bartley GB, Beatty CW, DeSanto LW, Gorman CA. Results of transantral orbital decompression in 428 patients with severe Graves' ophthalmopathy. American journal of ophthalmology. 1993 Nov 15:116(5):533-47 [PubMed PMID: 8238212]
McCord CD Jr. Orbital decompression for Graves' disease. Exposure through lateral canthal and inferior fornix incision. Ophthalmology. 1981 Jun:88(6):533-41 [PubMed PMID: 6894974]
Level 3 (low-level) evidenceLeone CR Jr, Bajandas FJ. Inferior orbital decompression for thyroid ophthalmopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1980 May:98(5):890-2 [PubMed PMID: 6892879]
Level 3 (low-level) evidenceTimoney PJ, Sokol JA, Hauck MJ, Lee HB, Nunery WR. Transcutaneous medial canthal tendon incision to the medial orbit. Ophthalmic plastic and reconstructive surgery. 2012 Mar-Apr:28(2):140-4. doi: 10.1097/IOP.0b013e318248e62c. Epub [PubMed PMID: 22410662]
Level 2 (mid-level) evidencePerry JD, Kadakia A, Foster JA. Transcaruncular orbital decompression for dysthyroid optic neuropathy. Ophthalmic plastic and reconstructive surgery. 2003 Sep:19(5):353-8 [PubMed PMID: 14506419]
Level 2 (mid-level) evidenceShorr N, Baylis HI, Goldberg RA, Perry JD. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology. 2000 Aug:107(8):1459-63 [PubMed PMID: 10919889]
Level 2 (mid-level) evidenceGoldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthalmic plastic and reconstructive surgery. 2000 Jul:16(4):271-7 [PubMed PMID: 10923974]
Level 2 (mid-level) evidenceLv Z, Selva D, Yan W, Daniel P, Tu Y, Wu W. Endoscopical Orbital Fat Decompression with Medial Orbital Wall Decompression for Dysthyroid Optic Neuropathy. Current eye research. 2016:41(2):150-8. doi: 10.3109/02713683.2015.1008640. Epub 2015 Apr 2 [PubMed PMID: 25835075]
Donaldson SS, Bagshaw MA, Kriss JP. Supervoltage orbital radiotherapy for Graves' ophthalmopathy. The Journal of clinical endocrinology and metabolism. 1973 Aug:37(2):276-85 [PubMed PMID: 4198257]