Continuing Education Activity
Brachial plexitis is characterized by the acute onset of shoulder or upper extremity pain with associated weakness and sensory changes. The condition can result from multiple etiologies, including post-surgery, infection, and inflammatory conditions. A high degree of clinical suspicion, a detailed history, a physical examination, and nerve conduction or electromyography are required for an accurate diagnosis. Imaging modalities such as magnetic resonance imaging may also be helpful. Managing brachial plexitis requires an interprofessional team, including pain specialists, neurologists, physiatrists, and therapists. Treatment focuses on conservative measures, including pain relief and physiotherapy, encompassing techniques such as kinesiotherapy and electrical stimulation to reduce pain and weakness and improve muscle function.
Although recovery can be prolonged with frequent exacerbations, outcomes are generally fair. However, patients with chronic pain may experience a diminished quality of life. This activity reviews the clinical presentation, pathophysiology, diagnostic testing, and management strategies available for brachial plexitis. Participating clinicians are equipped to provide high-quality care to patients with brachial plexitis.
Objectives:
Identify the different etiologies of brachial plexitis guided by presenting symptoms and signs.
Assess the pathophysiology of brachial plexitis utilizing knowledge of the anatomy of the brachial plexus.
Apply the diagnostic modalities and strategies to manage the treatment of brachial plexitis.
Implement care coordination among interprofessional team members to improve outcomes for patients affected by brachial plexitis.
Introduction
Brachial plexitis is characterized by the acute onset of shoulder pain, followed by weakness and a sensory loss of the shoulder and upper extremity. The results of several reports further described the condition. However, the most crucial findings were uncovered in a specific report authored by Parsonage and Turner in 1948 involving 136 patients. The findings strongly characterized the clinical history of the condition. Since then, the condition has been known by many different names, such as Parsonage-Turner syndrome, neuralgic amyotrophy, acute brachial neuropathy, acute brachial plexitis, idiopathic brachial plexopathy, idiopathic brachial neuritis, paralytic brachial neuritis, and brachial radiculitis, among others.[1][2][3][4]
Etiology
Two different forms of brachial plexitis are recognized. One is an idiopathic form, and the other is a hereditary form. The etiology of the idiopathic form is unknown. The idiopathic form is associated with a recent viral infection, upper respiratory tract infection, and recent vaccination. Recent infection has been associated with the development of the disease in 25% to 55% of patients, whereas recent vaccination has been associated with 15% of patients. Infectious causes include smallpox, anaplasmosis, influenza, coxsackievirus, parvovirus B19, cytomegalovirus, HIV, typhoid fever, and Borrelia burgdorferi. Other causes include strenuous exercise, pregnancy, and postsurgical plexopathy. The hereditary form is an autosomal dominant recurrent brachial plexitis. Researchers believe that the condition occurs due to a mutation causing a deficiency in proteins from the septin family.[5][6][7][8][9] Brachial plexitis has also been reported with COVID-19 infection and vaccination.[10][11] The results of reports of brachial plexitis as a presentation of sickle cell disease and as a potential complication of stem cell transplant are also available.[12][13]
Epidemiology
Brachial plexitis is estimated to occur in approximately 1.64 cases per 100,000 person-years. Males are more commonly affected compared to females. Multiple male-to-female ratios have been reported, ranging from 2:1 to 11.5:1. Cases have been documented across a wide age range, from 3 months to 75 years, with the peak onset occurring typically between the third and seventh decades of life.
Pathophysiology
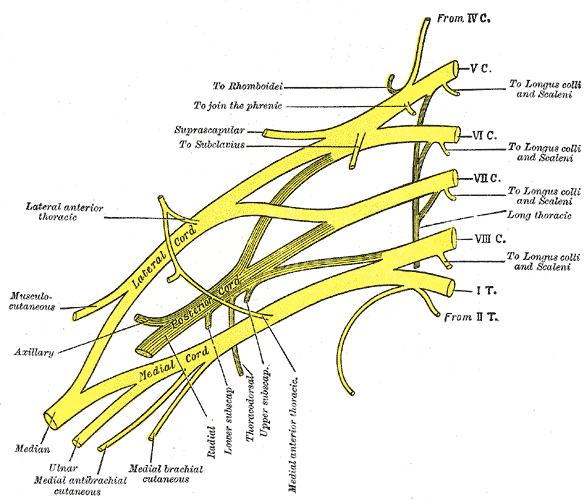
The exact pathophysiological mechanism is unknown, but some explanations exist for the different forms that affect the brachial plexus (see Image. Plan of the Brachial Plexus). A study suggested that patients with the disease have increased immunologic activity of lymphocytes when exposed to brachial plexus nerve extracts compared to sacral plexus nerve extracts. Another study suggested that in patients with early disease, an increase in antibodies occurs to peripheral nerve myelin. These findings, along with the association of disease occurrence with recent infection or vaccination, establish the immunologic mechanism of the disease. The hereditary form is an autosomal dominant brachial plexitis due to various mutations in the septin 9 gene, chromosome 17q25. Septin 9 is a guanosine-5'-triphosphate (GTP)-binding protein highly expressed in glial cells. The gene is involved in cytoskeleton regulation and function. The hereditary form of brachial plexitis is associated with recurrent disease.
History and Physical
Patients may have a history of viral infection or recent vaccination. The most common initial symptom is the acute onset of intense pain, occurring in 95% of patients. The pain associated with this condition can manifest in different ways as follows:
- Shoulder pain, with or without radiation to the upper arm (39.7%)
- Neck pain, with radiation down the arms (35.4%)
- Pain in the scapular or posterior chest wall region radiating to the arm or anterior chest wall or both (18.8%)
- Distribution of the lower brachial plexus (6.1%)
Constant pain with variable quality is expected, exacerbated by shoulder or arm movement. The pain can be somewhat relieved by elbow flexion and shoulder adduction (minimizing shoulder and arm movement). On average, the pain may last from 2 to 3 hours to more than 8 weeks.
Weakness ensues after the acute onset of pain. The onset of the weakness is sudden in 80% of patients. The weakness can coincide with the pain or in a delayed manner. Around 70% of patients report the weakness within 2 weeks of the onset of pain. Approximately 50% of patients have isolated shoulder girdle weakness, and only 10% have weakness localized to a single peripheral nerve. The most commonly affected muscles are the spinati, serratus anterior, deltoid, biceps, and triceps. Despite this, cases of unilateral or bilateral phrenic nerve neuropathy have been reported, resulting in diaphragmatic paralysis. Around 66% of cases are unilateral, and 34% are bilateral. Of the unilateral cases, 54% involve the right side. No statistically significant relationship has been described between the unilaterality of the disease and the patient's dominant side. A winged scapula may be observed in 20% of cases.
Sensory deficits are reported in 78% of cases, while paresthesias are reported in 35% of cases. Isolated paresthesias or a combination of paresthesia and hypoesthesia are the most common sensory complaints, typically affecting the upper arm's deltoid and lateral aspect and the forearm's radial aspect. Patients also reported other, less common symptoms such as autonomic dysfunction, craniofacial dysmorphisms, and unusual skin folds.
Evaluation
No laboratory test may help suggest the diagnosis of brachial plexitis. However, magnetic resonance imaging (MRI) of the shoulder, electromyography, and nerve conduction studies may provide important clues toward the diagnosis. During the acute phase of the disease, an MRI of the shoulder can reveal diffuse T2 signal hyperintensities due to the edema secondary to nerve demyelination. In the subacute or chronic phases, the T2 signal changes persist, and new T1 linear hyperintensities may develop, corresponding with fatty infiltration of the affected muscles. MRI of the brachial plexus is not sufficiently sensitive for appreciating changes related to the disease, but magnetic resonance neurography of the brachial plexus may show thickening and hyperintensity in the acute phase. This hyperintensity may persist into the chronic phase.
Electromyography is pivotal for evaluating demyelination of the brachial plexus. The imaging has to be performed 3 weeks after the onset of symptoms to show any significant findings. Electromyography may show positive sharp waves and fibrillation potentials consistent with acute denervation. Results may show chronic denervation and early reinnervation if performed late into the disease (3 to 4 months). Nerve conduction studies are usually normal but may show proximal conduction blocks. Brachial plexitis is a diagnosis of exclusion, which means clinicians have to rule out other possible pathologies before diagnosing. Most of these tests are good at ruling in or out other diagnoses but not at confirming a diagnosis of brachial plexitis.
Treatment / Management
The treatment of brachial plexitis is conservative, based on analgesia and physiotherapeutic rehabilitation. Analgesia is best achieved with non-steroidal anti-inflammatory drugs. Some studies have suggested that corticosteroid use early in the disease may reduce pain and weakness recovery time, but their clinical significance remains unknown. Physiotherapeutic rehabilitation, which may include kinesiotherapy, transcutaneous electrical nerve stimulation, deep dermal therapy, cryotherapy, and functional electric stimulation, plays a crucial role in reducing pain and weakness and regaining muscle trophism and functional status.[14][15]
Differential Diagnosis
The differential diagnosis for brachial plexitis is broad and encompasses several potential etiologies, including but not limited to:
- Acute poliomyelitis
- Amyotrophic lateral sclerosis
- Brachial plexus tumor
- Cervical disc disease
- Cervical lesions
- Mononeuritis multiplex
- Neoplastic infiltration of the brachial tube
- Non-traumatic compressive nerve injuries
- Traction injury to the brachial plexus
- Traumatic compressive nerve injury
A thorough evaluation involving a detailed history, a comprehensive clinical examination, and appropriate investigations is essential for differentiating brachial plexitis from the aforementioned differential diagnoses.
Prognosis
Although the prognosis for brachial plexitis was typically considered positive in the past, a study by Alfen et al showed that out of the 246 cases that they followed over 3 years or more, approximately two-thirds had persistent pain and paresis. Severe fatigue may be present in one-third of the cases.[16] Most cases of idiopathic brachial plexitis are expected not to recur. A case series by Tsairis et al reported recurrence of pain and weakness in only 4 out of 84 patients (4.8%).[17] However, Alfen et al observed a higher recurrence rate of 26.1% over a 6-year follow-up period.[18] However, this also suggests that more than 70% of the cases do not recur. In the setting of multiple recurrences, clinicians should consider the possibility of hereditary neuralgic amyotrophy.
Pearls and Other Issues
The differential diagnosis of brachial plexitis includes the following:
- Cervical disc disease with or without radiculopathy
- Cervical lesions
- Mononeuritis multiplex
- Transverse myelitis
- Acute poliomyelitis
- Amyotrophic lateral sclerosis
- Traumatic compressive nerve injury
- Traction injury to the brachial plexus
- Non-traumatic compressive nerve injuries
- Spinal cord tumor
- Brachial plexus tumor
- Neoplastic infiltration of the brachial plexus
- Pancoast tumor
- Thoracic outlet syndrome
- Radiation plexopathy, rotator cuff injury
- Adhesive capsulitis
- Acute calcific tendonitis
- Diaphragmatic paralysis
- Myocardial infarction
- Pulmonary embolism
Around 60% of upper plexus lesions may recover in less than 1 year, whereas lower plexus lesions may take 1 to 3 years. The estimated recovery rate is 36%, 75%, and 89% within 1, 2, and 3 years, respectively. Idiopathic reoccurrence is estimated at 5%, although other figures have been proposed.
Enhancing Healthcare Team Outcomes
The diagnosis and management of brachial plexitis are best addressed within an interprofessional team consisting of a pain specialist, neurologist, physiatrist, physical and occupational therapists, and sports clinicians. Each member brings unique skills and expertise, contributing to patient-centered care and optimal outcomes.
For most patients, the treatment of brachial plexitis is conservative, based on analgesia and physiotherapeutic rehabilitation. Physiotherapeutic rehabilitation plays a crucial role in managing brachial plexitis, encompassing various techniques such as kinesiotherapy, transcutaneous electrical nerve stimulation, deep dermal therapy, cryotherapy, and functional electric stimulation. These interventions have demonstrated effectiveness in reducing pain and weakness associated with brachial plexus, as well as promoting the restoration of muscle trophism and functional status. However, the outcomes for most patients with brachial plexitis are generally fair. Recovery is often prolonged, and exacerbations are common. The quality of life in chronic cases is poor.[19][20]