Anatomy and Physiology
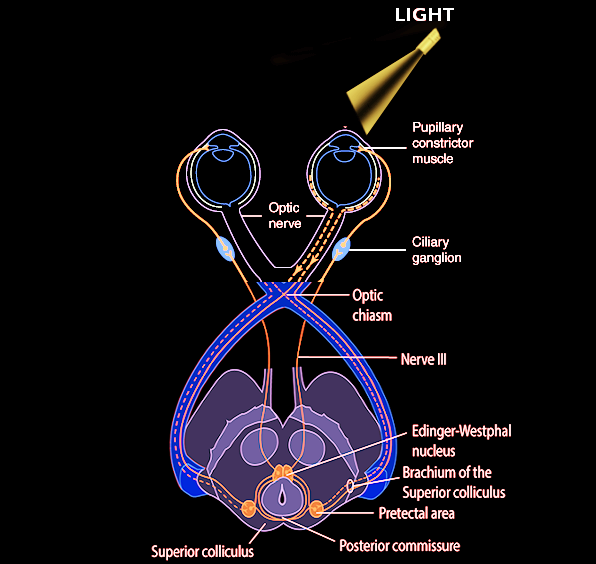
Light travels through the cornea, anterior chamber, pupil, lens, and the posterior chamber, eventually reaching the retina. Photoreceptor cells in the outer layers of the retina, which are called rods and cones, convert light stimuli into neuronal impulses. These signals are then relayed to the bipolar cells, which interact with ganglion cells, which in turn coalesce to form the optic disc and optic nerve (CN II). The optic nerve sends impulses to the brain for further processing and image recognition.[1] These are the first steps of the pupillary light reflex afferent pathway. The optic nerve then forms the optic chiasm, which diverges into a left and right optic tract. At the optic chiasm, nasal retinal fibers will cross to the contralateral side of the optic tract, and the temporal retinal fibers continue on the ipsilateral side. Thus, the right optic tract will contain temporal retinal fibers from the right eye, as well as nasal retinal fibers from the left eye. The optic tracts join the brachium of the superior colliculus, and then signals travel to the pretectal area of the midbrain. Each pretectal area sends bilateral signals to the preganglionic parasympathetic nuclei in the midbrain called Edinger-Westphal nuclei.[2][3] There are a minority of axons that go to the hypothalamus and the olivary pretectal nucleus (OPN).[4] Efferent parasympathetic preganglionic fibers travel on the oculomotor nerve and synapse with the ciliary ganglion, which sends postganglionic axons to directly innervate the iris sphincter muscles. The contraction of the iris sphincter muscles leads to pupillary constriction (miosis).[3] This extensive pathway is being tested when a light is shined in the eyes. And, because of the crossing fibers, there is not only a direct pupillary reflex but also a consensual pupillary light reflex. Of note, the pupillary dark reflex involves a separate pathway, which ends with sympathetic fibers from long ciliary nerves innervating the dilator pupillae muscle.
Technique or Treatment
Gently point the focal light into one eye, this is known as the direct pupillary light reflex. Then, withdraw the light for few seconds, followed by stimulating the same eye again but this time observe the indirect, or consensual, PLR in the opposite eye. It may be helpful to have the nurse control the light stimulus while you observe the unstimulated eye.
Clinical Significance
Pupillary light reflexes are measured based on a 0 to 4+ gradient that considers the magnitude and speed of the light response. A normal, healthy adult patient is expected to have a 4+ response, which indicates a brisk, large response. A 3+ grading indicates a moderate response, 2+ is a small, slowed response, 1+ represents a tiny/just visible response, and a 0 indicates unresponsive pupils. Commonly, clinicians document PERRL–saying the pupils are equal, round, and reactive to light or PEARL - pupils equal and reacting to light.
In standard clinical testing conditions, the diameter of the pupils will usually range from two to five millimeters. Per decade of aging that occurs, there is a 0.3 mm decrease in the standard pupil diameter that has been associated with iris stiffening. The pupillary light response exhibits varying sensitivity to the chromic spectrum, indicating that the process of light recognition is significantly complex; it is not as simple as a binary response with the detection of “light” versus “no light.” While there is a baseline fluctuation in the steady-state conditions for pupillary dilation, a concern for neurological abnormalities is considered in cases of marked pupillary changes, whether with constriction or dilation. One such condition is anisocoria, and it is estimated at 4% of the general population has anisocoria of greater than 1 millimeter, in which case neurological compromise must be ruled out. Pupillary latency occurs when the reaction time of the pupil is inversely related to the increase in light intensity from the stimulus; this can serve as a cue to a potential neurologic cause. Latency increases by approximately 1 millisecond per year with aging. Overall, normal pupillary response times are about one second for initial constriction and 5 seconds for dilation.[4]
Direct and consensual pupillary light reflexes test for appropriate neurological pathway connections and functioning of both cranial nerve II and III. Light entering the eye is processed through the pupillary light reflex, and signals directed to the iris sphincter muscle to adjust the amount of light that reaches the retina. While there are other reasons for variation in pupillary dilation and constriction, such as arousal leading to changes in the balance of the sympathetic and parasympathetic nervous systems, here we will focus on its relation to light exposure. Pupils can become mydriatic, or dilate, in response to potential disease, drug toxicity, trauma, increased intracranial pressure, brainstem damage, or nerve damage to cranial nerve II and/or III.[5]
Abnormalities also depend on where in the track the damage has been done. In the event of optic nerve damage, visual field defects or complete vision loss can occur. If this damage is before the optic chiasm, in the optic nerve, then there are deficits noted to bilateral ipsilateral monocular vision loss. This damage leads to a relative afferent pupillary defect (RAPD), known as a Marcus Gunn pupil, which is examined using the swinging flashlight test. Causes of a Marcus Gunn pupil include ischemic optic neuropathy, optic neuritis, nerve compression, trauma or through asymmetric glaucoma.
Unilateral optic neuropathies, most notably optic neuritis, can cause RAPDs. Optic neuritis is an anterior or posterior inflammatory demyelination of the optic nerve, leading to atrophy of optic nerve fibers and an RAPD. An RAPD can be detected in 96% of acute unilateral cases of optic neuritis.[4] Ischemic optic neuropathies, such as NAION and AION, can cause RAPDs via optic nerve ischemia and infarction secondary to optic nerve edema.[5][6] Asymmetric glaucoma can result in an RAPD, secondary to retinal nerve fiber layer loss.
RAPDs can occur due to ischemic retinal diseases such as BRVO, CRVO, BRAO, and CRAO secondary to the death of photoreceptors and viable retina, ultimately leading to an uneven pupillary response.[2] Via the same mechanism of significant retinal cell death, retinal detachments can cause RAPDs. In 1987, a prediction model quantified the correlation between the sizes of RAPD to the amount of retina detached. A detachment of each peripheral quadrant correlated to 0.36 log units of the pupillary defect. The detachment of the macula caused 0.68 log units of the pupillary defect.
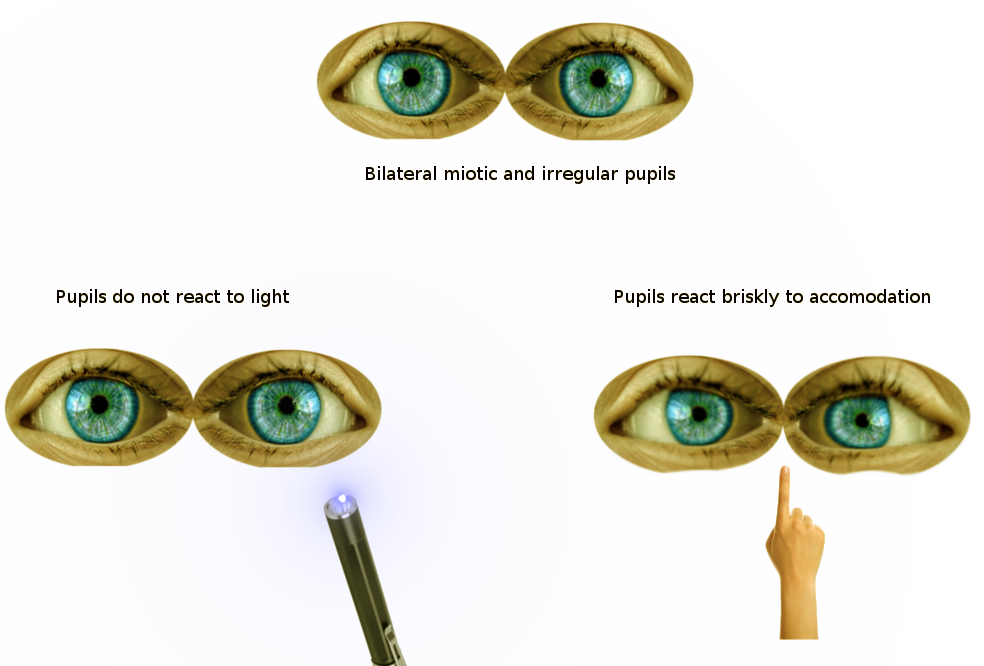
Argyll Robertson pupil, noted in tabes dorsalis from neurosyphilis, is the notable weak-to-absent pupillary light reflex bilaterally, though the pupils will still constrict for the near response. With the near response (accommodation) intact it can be assumed that the afferent and efferent pathways are grossly intact and that the deficit is related to degeneration in bilateral olivary pretectal nuclei or their projections.[6]
Compression damage to the optic chiasm leads to bitemporal hemianopia and is frequently related to a pituitary adenoma. Downstream to the optic chiasm, damage to the optic tract will produce contralateral homonymous hemianopia; for example, if there is damage to the left optic tract, there are right visual field deficits to both eyes. In the case of comatose patients, it has been noted that a majority of the patients had non-reactive, dilated pupils, and the one patient that had pinpoint pupils became vegetative. [4][7] Uncal herniation, in which the uncus protrudes over the edge of the tentorium, can lead to compression of CN III, suggesting current or impending brainstem compromise. Lesions within the efferent pathway, specifically the preganglionic fibers of the oculomotor nerve, can cause ipsilateral mydriasis and accommodation paralysis. One syndrome noted to have this finding is Weber Syndrome. If there is damage to the postganglionic fibers, tonic dilated pupil or Adie syndrome develops so that the constrictor muscles are hypersensitive to a cholinergic stimulus. If there is a disruption between the balance of parasympathetic and sympathetic innervation, such as in Horner syndrome where there is a loss of sympathetic stimulation, leading to miosis of the ipsilateral pupil.[6]
Transient mydriasis can be associated with tricyclic antidepressants, typical antipsychotics, and selective serotonin reuptake inhibitors, but usually, these are not long-term consequences. Topiramate, used for migraines, has been associated with acquired myopia and angle-closure glaucoma.[8] The fixed dilation of pupils noted in coma patients was related to the increased intracranial pressure (ICP) in which an association was noted in 1866 from Von Leyden’s animal experiments. Through continued research over the next 50 years or so, it was noted that the fixed dilation of pupils was noted to be a sign of acute mass effect in relation to the ICP.[9]
Tumors in the retina, optic nerve, and brain can also cause RAPDs. In children, the most common intraocular tumors are benign, developmental cysts. The most common malignant intraocular tumor is retinoblastoma.[9] Tumors or lesions affecting the optic chiasm or midbrain can cause decreased signals reaching the Edinger-Westphal nuclei, leading to pupillary constriction. In children, the most common intracranial tumor detected is a glioma. They account for 75% of intracranial tumors in children. Also common in children are astrocytomas, medulloblastomas, and ependymomas.
Another cause of RAPD is severe amblyopia, characterized as amblyopia of 20/100 to 20/400. Clinically, RAPDs are found in severe amblyopia with BCVA 20/400 or worse. While the etiology of an RAPD in amblyopia is poorly understood, significant risk factors include anisometropia, early age of onset with a history of strabismus, level of visual acuity at the conclusion of treatment, and extended periods of occlusion therapy.[10]
Pupillary escape is a phenomenon that can occur in the setting of a diseased optic nerve or retina. When light is shone onto the affected pupil, there will be a transient pupillary constriction and then a slow dilation to the original size.[11]
In cases in which one pupil is unable to constrict (such as due to a third nerve palsy), the “reverse RAPD test” can be performed, with direct and consensual responses compared in the reactive pupil. If the reactive pupil constricts more during the direct response, then the RAPD is in the unreactive eye. If the reactive pupil constricts more during the consensual response, then the RAPD is in the unreactive eye.[12] Emergency clinicians often encounter patients with the triad of pinpoint pupils, respiratory depression, and coma related to opioid overuse. Opioids are used for pain relief by interacting with opioid receptors, including mu, delta, and kappa. With significant respiratory depression resulting in hypoxia, pupils can become dilated. Oxygenation causes the pupils to revert to the original pinpoint presentation caused by the opioid. For stabilization, one of the medications given to these patients is naloxone, an opioid antagonist, which has a peak effect at approximately 10 minutes. Repeated dosing is frequently required and can be given up to 5 mg per hour. If there is a dilation of the pupil with the administration of naloxone, this also eliminates organophosphate poisoning, which can present similarly. Pupillary changes are used to recognize when the effects of naloxone are waning because of how the pupils will begin to constrict again, indicating that the opioid has not yet been metabolized out of the body. There is also a concern in the event of minimal reaction that the patient may be affected by other central nervous system depressants or have hypoxic brain damage.[10]