Introduction
The tendon is a "mechanical bridge," transmitting muscle forces to the bones and joints. This tough, fibrous structure also helps muscles complete joint movements along a plane. The tendon type reflects its associated muscle's morphology and function. Tendon tissue is present throughout an entire muscle's length, not only the tips. The muscle's connective tissue layers (epimysium, perimysium, and endomysium) merge to attach to one or more fixed osseous points. Tendon tissue close to the muscle has contractile fibers. The muscle influences tendon activity, and in turn, the tendon impacts how the muscle functions.
Healthcare professionals must consider these important relationships, especially when performing manual therapy, rehabilitation, and surgery. Tendon tissue architecture can adapt to physiological (eg, training) or pathological (eg, traumatic) stimuli influenced by systemic, hormonal, environmental, and age-related factors.[1][2]
Structure and Function
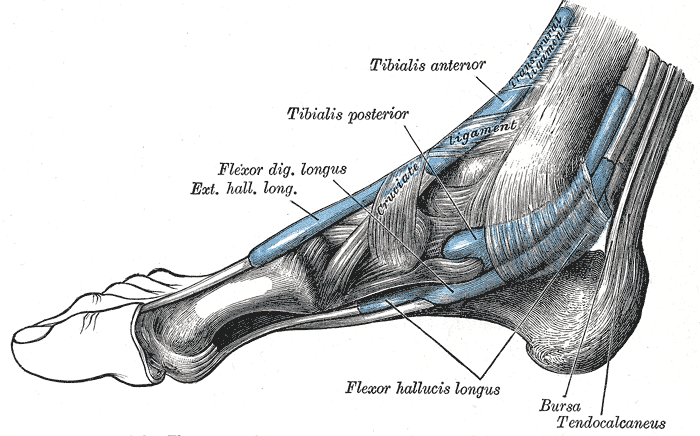
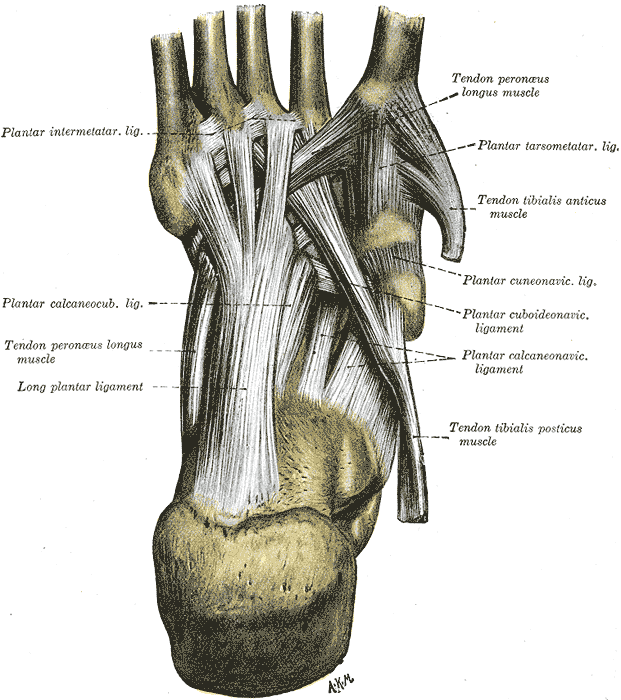
Tendons play an extraordinary role in mechanics and movement. These structures transmit muscle forces to the skeletal levers to facilitate movement and body posture maintenance. Tendons enable muscles to maintain optimal distance from the joint they operate on without needing excessive muscle length between their origin and insertion points. Tendons are stiffer than muscles, have greater tensile strength, and can withstand large loads with minimal deformations. These properties enable tendons to transmit muscle forces to bones efficiently, minimizing energy loss from tendon stretch (see Images. Extrinsic Foot Muscles and Foot Sole Tendons and Ligaments). For example, the foot's flexor tendons can bear more than 8 times the body weight and store about 40% of this weight for elastic hysteresis during walking.[3][4][5][6]
Tendon Sheaths
Tendon sheaths are auxiliary structures that primarily aid in the smooth movement of tendon tissue between neighboring structures and prevent deviation during muscle contraction.
Fibrous Sheaths
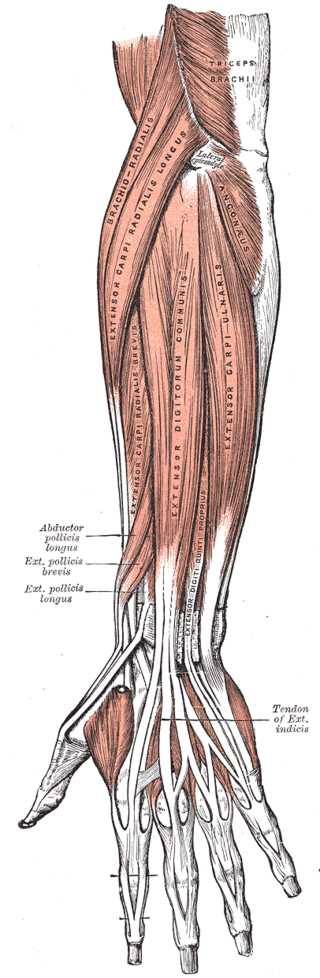
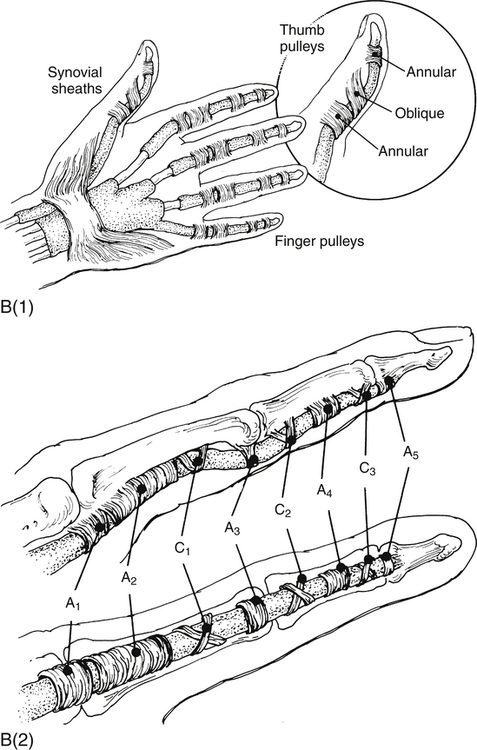
Showers and bone incisions typically have a fibrocartilaginous floor covered by a fibrous fabric roof forming a bridge. This structure, known as the fibrous sheath or retinaculum, is commonly found in the extremities. Examples include the retinacula of the flexor and extensor tendons in the hand and foot at the wrist and instep levels (see Image. Forearm Extensor Muscles and Tendons). Fibrous sheaths, or retinacula, serve as channels for tendon movement, especially for long tendons. These structures prevent significant friction on neighboring tissues, particularly at bone structures, ensuring smooth sliding action. These regions contain tunnels with synovial sheaths surrounding the tendons.[7]
Synovial Sheaths
The synovial sheaths facilitate tendon sliding within the fibrous sheath (see Image. Flexor Tendon Anatomy). These structures comprise 2 thin serous sheets: the parietal sheet covering the fibrous sheath's walls and the visceral sheet covering the tendon's surface. These sheets extend to both ends, enclosing a space with peritendinous fluid that serves mainly as a lubricant. Additionally, loose tissue peduncles detach from osteofibrous canal walls, terminating on the tendon belly and housing vessels and nerves. These structures, collectively called the "mesotenonium," are also covered by synovium and vary in number depending on tendon length. True synovial sheaths are only found in areas necessitating highly efficient lubrication due to sudden direction changes and increased friction.
Peritendon Sheaths (Paratenon)
The peritendinous sheets have overlapping functions with the synovial sheaths despite their histological differences. The paratenonium (paratenon) is composed of type I and type III collagen fibrils and thin elastic fibers. This structure reduces friction and works like an elastic sleeve, allowing free tendon movement within its environment.[8]
Reflection Pulleys
Reflection pulleys are thickened regions of dense fibrillar tissue found within fibrous sheaths, encasing tendons within the sliding bed, particularly around tendon curves. These structures facilitate smooth tendon movement.[9]
Tendon Bursae
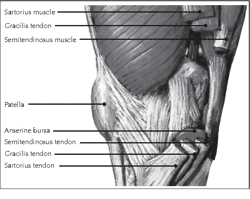
Tendon bursae help minimize friction between the tendon and adjacent bony structures (see Image. Medial View of the Knee). Bursae are small serous vesicles situated where bony protrusions might compress and cause friction on tendons, potentially leading to wear and tear. Examples are the subacromial, infrapatellar, and retrocalcaneal bursae.[10]
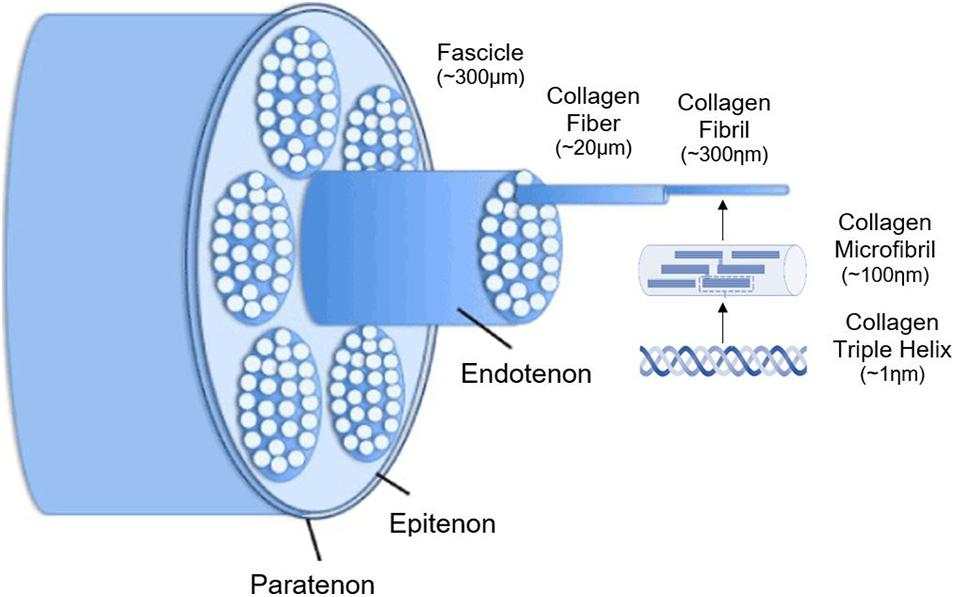
Deep to the paratenon, the entire tendon is surrounded by a thin, dense connective tissue sheath called "epitenonium" (epitenon). The paratenon and epitenon are collectively called "peritenonium." Collagen fibrils within the epitenon are oriented transversely, longitudinally, and obliquely. The epitenon fibrils occasionally appear fused with the superficial tendon fibrils.
The epitenon is connected to the peritenonium externally and endotenonium (endotenon) internally. The endotenon, a thin membrane consisting of loose connective tissue, covers and organizes individual tendon fibers into larger units bundled in various orders. This membrane separates different collagen bundles, allowing neurovascular penetration within the tendon (see Image. Tendon Hierarchical Structure Schematic Diagram).
The musculotendinous junction is the point where the muscle pierces the tendon. The osteotendinous junction is the point where the tendon inserts on the bone.[11][12]
Cell Population and the Extracellular Matrix
Tenocytes and tenoblasts are specialized fibroblasts that coexist in tendinous tissue. Tenocytes are elongated, while tenoblasts are ovoid. Tenoblasts represent approximately 90% to 95% of tendon fibroblasts. These specialized fibroblasts produce extracellular matrix components, including collagen and proteoglycans.[13] Collagen makes up approximately 65% to 80% of the extracellular matrix. The predominant collagen type in tendons is type I. Type III collagen is present in the epitenon and endotenon. Type II collagen occurs in the osteotendinous junction's fibrocartilaginous areas. Elastin, proteoglycans, and glycoproteins constitute 4%, 4%, and 2% of the extracellular matrix, respectively.
Tendons consist of collagen fibers arranged parallel to each other and the tendon axis. The bundles of collagen fibers show a wavy pattern with periodic orientation changes known in the literature as crimps. Crimps vary in size and shape within the same tendon, often resembling isosceles or scalene triangles of different dimensions. When observed through a scanning electron microscope, a single crimp is composed of straight fibrillary segments closely packed and connected by nodes or hinges. All fibrils within each fascicle simultaneously change direction at these points. Rather than forming a loop, the fibrils deform similarly to a hollow cylindrical structure. Collagen bends but does not break.[14]
Healing occurs in multiple overlapping phases after a tendon injury. Tenoblasts deposit collagen fibers during the healing phase and transform into tenocytes in the last repair phase.
Mechanical Properties
A tendon's biomechanical behavior is related to its shape and the magnitude of the tension applied to it. Muscles like the finger flexors that perform delicate and precise movements possess long and thin tendons. In contrast, muscles for actions of power and endurance, such as the quadriceps femoris and triceps surae, have shorter and more robust tendons. A short tendon's tensile strength is greater than a long tendon, tolerating more loads with the same diameter. Meanwhile, a long tendon can withstand greater deformation than a short tendon. A tendon's strength and resistance rely on its diameter and length. Tendons' biomechanical properties are linked to collagen fibril diameter and arrangement. Tendons subjected to high stress have fibrils with a larger diameter, which are less flexible than smaller ones.
Tendons' capacity to absorb and transmit muscle forces is linked to their crimps. Research reveals that higher tendon loads produce wider angles at a crimp's base. Tendon stretch gradually flattens the crimps, which act as shock absorbers during the initial pulling stages. Afterward, the crimps allow the tendon to regain its shape when force application ceases. Crimp flattening occurs gradually from the outer edges to the center.
Adaptation to Mechanical Stress
Tendinous adaptations differ between genetic sexes. Genetic females develop less tendon strength but greater length than genetic males when subjected to repeated mechanical stress. The reasons for this tendency are poorly understood.
Tendinous tissue adapts to its mechanical environment. Adaptation is stress-specific. Mechanical tension from muscle contraction and relaxation increases collagen synthesis and tendon diameter. Tendon stiffness and the Young modulus increase with continued tension. The Young modulus is the ratio of tensile stress to tensile strain, expressed in pressure units. This ratio defines how easily the tendon can stretch and deform.[15]
Effect of Aging
The tendons' ability to adapt decreases with age. Aging alters cellular structure and diminishes tendons' capacity for regeneration. Moreover, tendons become less effective in directing muscle forces toward the bone tissue. Collagen fibers become less organized, and calcification can occur. Fibroblasts decrease, and senescent cells appear. Water content and proteoglycans decrease. All these changes reduce tendons' viscoelasticity and strength and increase trauma susceptibility. Decreased estrogen levels in older women loosen tendinous tissue, making it more prone to traumatic injury and inflammation.[16][17]
Embryology
The tendon arises from the ectoderm and mesoderm. Tendons primarily originate from neural crest cells of the ectoderm and the paraxial and lateral plate mesoderm, giving rise to the craniofacial, axial, and limb tendons respectively.[18] Animal models show that cranial and limb tendons form independently from muscle tissue. By comparison, muscle progenitors are required to move the axial tendon precursors from their origin to their destination under the influence of fibroblast growth factor.[19] The progression of cartilage and muscle tissue in the developmental period typically occurs more quickly than the tendon.
Blood Supply and Lymphatics
Tendons have a complex vascular supply. Blood vessels supplying the tendon may originate from the muscle belly, the periosteum around the osteotendinous junction, or the vascular networks within peritendinous leaflets or synovial sheaths.[20][21]
Peritendinous leaflets' circulatory networks vary within and between tendons. These networks may feature primary trunks forming either a mesh structure or irregularly arranged concentric arches, with small to medium-sized arteries and 1 or 2 satellite venous connections.
Three types of microvascular capillary units exist within tendons. One involves capillaries traveling a long distance parallel to the tendon's longitudinal axis before looping back to join a venule. Another type originates from a single arteriole, giving rise to several capillary loops running in different planes within the tendon. The 3rd type functions as arteriovenous shunts, featuring short capillaries with a straight course. Variability in these microvascular units' organization facilitates gas and metabolite diffusion within individual tendon bundles.
Tendinous lymphatic drainage exists as a network, with lymph draining toward tendon veins or other neighboring venous structures. Blood flow to the tendon rises under mechanical stress, but lymphatic drainage does not increase proportionally.
Nerves
Tendons are innervated by nerve branches from the muscle belly and skin, though innervation is scarce. Nerves are localized to the paratenon, endotenon, and epitenon. Nerve branches parallel the tendon's central axis, connecting with transverse and oblique branches. Golgi tendon organs, Pacini or Ruffini corpuscles, and free arborizations lie in the nerve endings.[22] Innervation accompanies the vascular supply. Tendons' sympathetic innervation mirrors the vascular supply, serving to induce vasoconstriction. Sensory fibers similar to parasympathetic nerves or separate small-diameter sensory fibers stimulate vasodilation.
Muscles
The tendon adapts to its associated muscle's morphology. Flat muscles have flattened tendons or aponeuroses, while round ones have cordiform tendons. Healthy tendons enable muscles to function effectively and adapt to competing demands. Such tendons also facilitate optimal mechanotransduction mechanisms in contractile fibers, enhancing overall performance.
Physiologic Variants
Tendon anatomy variations are common. For instance, the tendon of the biceps brachii's long head can vary in shape and origin or even be absent, with a prevalence ranging from 1.9% to 7.4% in the population.[23] The popliteal muscle tendon bifurcates, producing 2 muscular bellies, in approximately 0.4% of individuals.[24] Length and shape variability of the flexor hallucis longus tendon is seen in about 9.8% of cases.[25] The patellar tendon, which stabilizes the patella, may appear as 2 distinct tendons or be absent.
Surgical Considerations
Tendon injuries may be managed conservatively or operatively. The decision to perform surgery depends on many factors. A review of specific tendon injury management is beyond the scope of this activity. However, the key principles guiding the comprehensive clinical analysis and management of diverse tendon injuries include the following:
- Injury severity, ie, partial or complete
- Body region affected
- Capacity of the surrounding structures to compensate (to assess the clinical impact of a tendon injury and predict functional outcome)
- Patient age
- Functional goals (sedentary versus active or sport-involved lifestyle)
- Patient medical comorbidities (diabetes, inflammatory arthropathies, systemic conditions)
- Hand dominance
- Resulting cosmetic deformity
- Injury chronicity and resulting tissue quality (warranting consideration of direct repair versus allograft or autograft tendon reconstruction)
- Available surgical alternatives (navigating tendon transfer principles)
- Social factors (patient compliance, smoking status, history of illicit drug use)
- Current medications (chronic steroid use, fluoroquinolones)
Clinical Significance
Tendinopathy
Tendinopathy results in vascular changes and adaptations to the muscle and tendon's macroscopic and microscopic environment. Increased vascularization is due to hypertrophy and hyperplasia induced by an angiogenic cytokine, such as vascular endothelial growth factor (VEGF). Inflammation due to injury increases local VEGF concentrations. Greater perfusion reduces tendon strength by decreasing the sliding capacity of its various connective layers. Nerve fibers also increase superficially in the tendon and deeper structures. Nociceptive nerve branches also proliferate, causing pain.[26][27][28][29] Sympathetic nerve fibers also multiply near the blood vessels. Research suggests that altered tenocytes may produce nociceptive neurotransmitters, which can subsequently trigger pain signals recorded by the sympathetic nervous system.
Ehlers-Danlos Syndrome
Ehlers-Danlos Syndrome (EDS) is a group of genetically borne connective tissue disorders that result in abnormal collagen synthesis, increasing skin elasticity and joint mobility. Collagen gives strength and elasticity to connective tissues, including the components of tendons, joints, skin tissue, and blood vessels. EDS incidence is approximately 1 in 5000 cases.[30][31] The condition develops in childhood but is often undiagnosed until later in life.
EDS' clinical manifestations vary widely. Thus, disease severity also differs individually. Diagnosis hinges on medical history and physical examination, noting the patient’s movements.[32] Symptoms may include abnormal scar formation, stretchy skin, and loose joints.
Joint hypermobility or laxity is characteristic of EDS. Small joints are typically more affected than large joints. Joint pain, dislocations, and sprains are common. Other EDS complications include scoliosis, osteoarthritis, chronic pain, and aortic dissection.
Thirteen EDS subtypes have already been identified, with 19 known causal genes involved in extracellular matrix and collagen development. Inheritance is often autosomal dominant. Collagen abnormalities are present in most known EDS variants.
Classical EDS, consisting of former types I and II, is characterized by abnormal type V collagen production. Vascular EDS, formerly EDS type IV, exhibits abnormal type III collagen. This EDS type is the deadliest, as it can manifest with arterial rupture, though only 4% to 5% of patients have this variant. Arthrochalasia EDS, comprised of former types VIIA and VIIB, exhibits type I collagen deficiencies. Cardiac-valvular EDS also involves collagen type I abnormalities, though this condition is autosomal recessive and may present with severe cardiac valve defects. Myopathic EDS is associated with collagen type XII abnormalities and manifests with congenital muscle atrophy, proximal joint contractures, joint hypermobility, and developmental motor delay. The causative gene of hypermobile EDS, formerly type III EDS, is unknown in most cases, though a few individuals have tenascin-X and type III collagen mutations. This EDS variant is the least severe and most common.[33]
Diagnosis of EDS is difficult because joint hypermobility is multifactorial, with gender, age, training, and body weight all contributing to the symptom. Patients with hypermobile EDS exhibit joint hypermobility, dislocation, and cutaneous manifestations. Additional symptoms include chronic pain, fatigue, anxiety, and dysautonomia. The musculoskeletal pain associated with this condition is often diagnosed as fibromyalgia.
Pain is a common EDS symptom, which may present as variable arthralgias during childhood. Concurrent pain patterns, such as paresthesias, burning sensations, allodynia, cramps, and myalgias, may suggest a neurological component alongside joint and skin pathologies. Small fiber neuropathy is common.[34] Pain may be classified as nociceptive, arising from pain receptor stimulation, or neuropathic, caused by nerve dysfunction. Musculoskeletal nociceptive pain occurs in EDS, but neuropathic pain seems more common.[35] Pain may also be initially nociceptive, but neuropathic pain can become a significant factor as pain intensifies. Central sensitization is also significant, indicating a generalized hyperalgesia among patients. Loss of proprioception, muscle weakness, and alterations in the extracellular matrix further contribute to chronic pain and its perpetuation.[36]
Patients with EDS often present with chronic, severe pain. The condition must be differentiated from other genetic connective tissue syndromes such as Marfan syndrome, cutis laxa, and skeletal dysplasias.
Marfan Syndrome
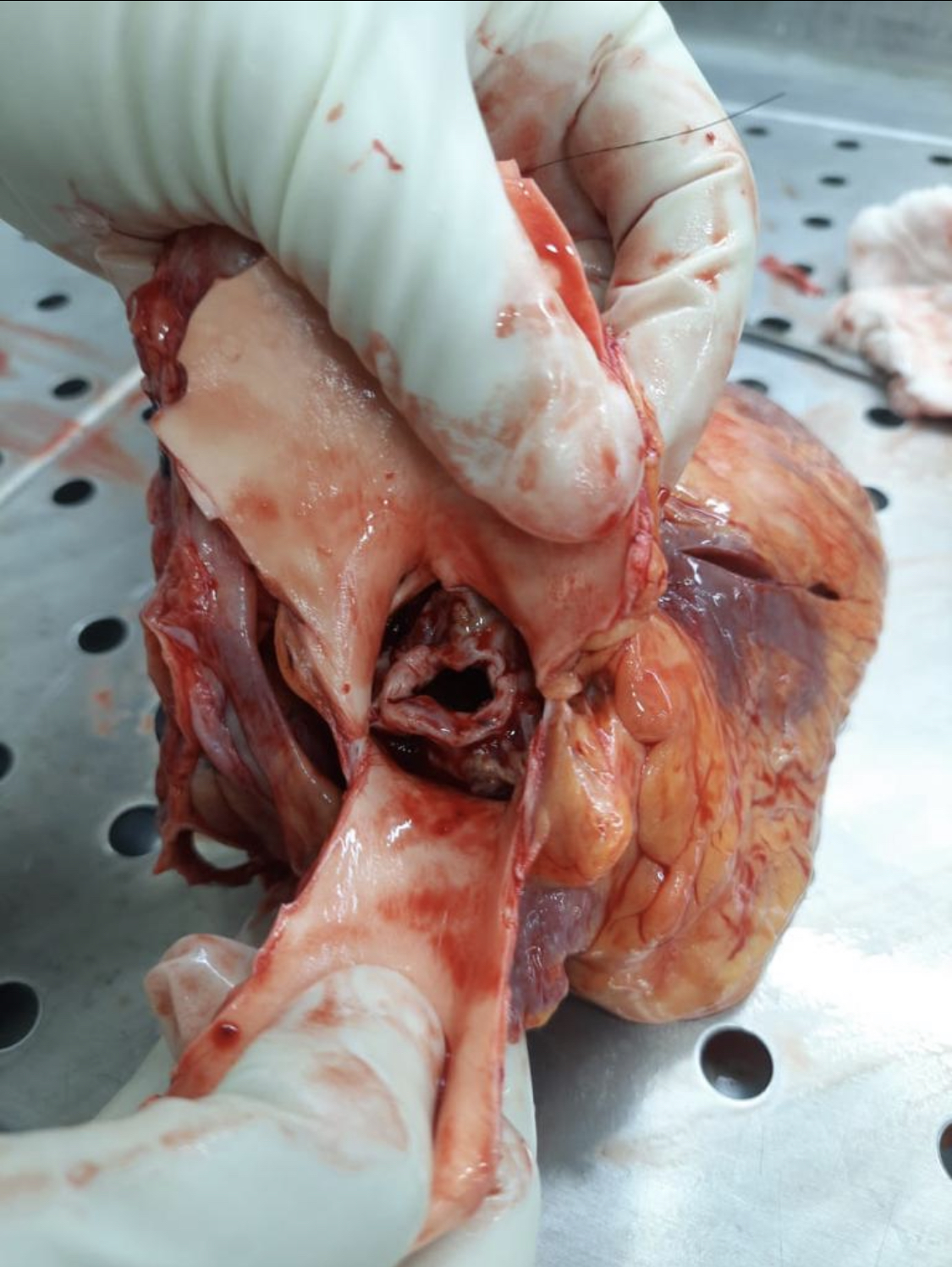
Marfan syndrome is an autosomal dominant connective tissue disease with variable penetrance, resulting in many symptoms. The most dangerous complications of this condition include aortic dissection and regurgitation (see Image. Marfan Syndrome-Associated Aortic Regurgitation). The primary genetic abnormality is a mutation in the fibrillin-1 gene, the product of which coats elastins.[37] Musculoskeletal system abnormalities are often the presenting physical signs of Marfan syndrome. Increased joint laxity, excessive long bone growth, scoliosis, and sternal deformities such as pectus excavatum are common.
The Steinberg or “thumb sign” may indicate Marfan syndrome during the physical examination. The sign is positive if the entire distal phalanx of the thumb protrudes beyond the hand's ulnar border while the thumb is abducted with the fingers curled over it. The Walker-Murdoch or “wrist" sign is evaluated by having the patient encircle the wrist proximal to the styloid process with the thumb and finger of the other hand. This sign is positive if the thumb overlaps and completely covers the nail of the encircling digit.[38]
Other Issues
Tendon tissue plays a crucial role in fascial continuity, actively and passively contributing to muscle performance and joint movements.[39][40] The tendon-bone junction typically lacks nerves, yet a loss of innervation in the rest of the tendon can lead to chronic tendinopathies, especially near such junctions.[41]