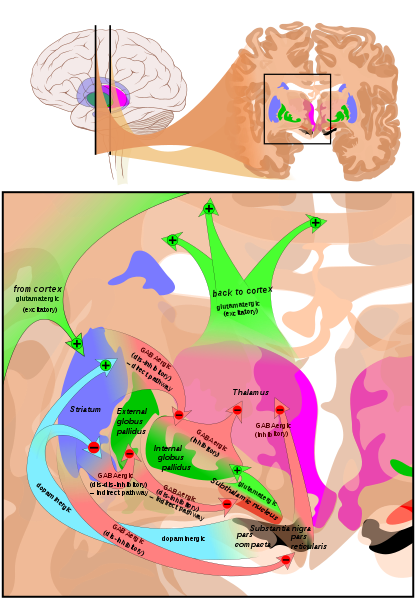
[1]
Mavridis I, Boviatsis E, Anagnostopoulou S. Anatomy of the human subthalamic nucleus: a combined morphometric study. Anatomy research international. 2013:2013():319710. doi: 10.1155/2013/319710. Epub 2013 Dec 15
[PubMed PMID: 24416591]
[2]
Martinez-Ferre A, Martinez S. Molecular regionalization of the diencephalon. Frontiers in neuroscience. 2012:6():73. doi: 10.3389/fnins.2012.00073. Epub 2012 May 25
[PubMed PMID: 22654731]
[3]
Dupont G, Schmidt C, Yilmaz E, Oskouian RJ, Macchi V, de Caro R, Tubbs RS. Our current understanding of the lymphatics of the brain and spinal cord. Clinical anatomy (New York, N.Y.). 2019 Jan:32(1):117-121. doi: 10.1002/ca.23308. Epub
[PubMed PMID: 30362622]
Level 3 (low-level) evidence
[5]
Mahale RR, Mehta A, John AA, Buddaraju K, Shankar AK, Srinivasa R. Acute hemichorea-hemiballism as a sole manifestation of acute thalamic infarct: An unusual occurrence. Journal of neurosciences in rural practice. 2016 Jul-Sep:7(3):478-80. doi: 10.4103/0976-3147.181454. Epub
[PubMed PMID: 27365980]
[6]
Katsumata T, Kitamura S, Inamura K, Terashi A. [Clinical and CT-findings in hemiballismus]. Nihon Ronen Igakkai zasshi. Japanese journal of geriatrics. 1992 Feb:29(2):123-8
[PubMed PMID: 1583799]
[7]
Etemadifar M, Abtahi SH, Abtahi SM, Mirdamadi M, Sajjadi S, Golabbakhsh A, Savoj MR, Fereidan-Esfahani M, Nasr Z, Tabrizi N. Hemiballismus, Hyperphagia, and Behavioral Changes following Subthalamic Infarct. Case reports in medicine. 2012:2012():768580. doi: 10.1155/2012/768580. Epub 2012 Oct 18
[PubMed PMID: 23125861]
Level 3 (low-level) evidence
[8]
Lang AE. Persistent hemiballismus with lesions outside the subthalamic nucleus. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1985 May:12(2):125-8
[PubMed PMID: 3926289]
[9]
Slavin KV, Baumann TK, Burchiel KJ. Treatment of hemiballismus with stereotactic pallidotomy. Case report and review of the literature. Neurosurgical focus. 2004 Jul 15:17(1):E7
[PubMed PMID: 15264776]
Level 3 (low-level) evidence
[10]
Alonso P, Cuadras D, Gabriëls L, Denys D, Goodman W, Greenberg BD, Jimenez-Ponce F, Kuhn J, Lenartz D, Mallet L, Nuttin B, Real E, Segalas C, Schuurman R, du Montcel ST, Menchon JM. Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Meta-Analysis of Treatment Outcome and Predictors of Response. PloS one. 2015:10(7):e0133591. doi: 10.1371/journal.pone.0133591. Epub 2015 Jul 24
[PubMed PMID: 26208305]
Level 1 (high-level) evidence