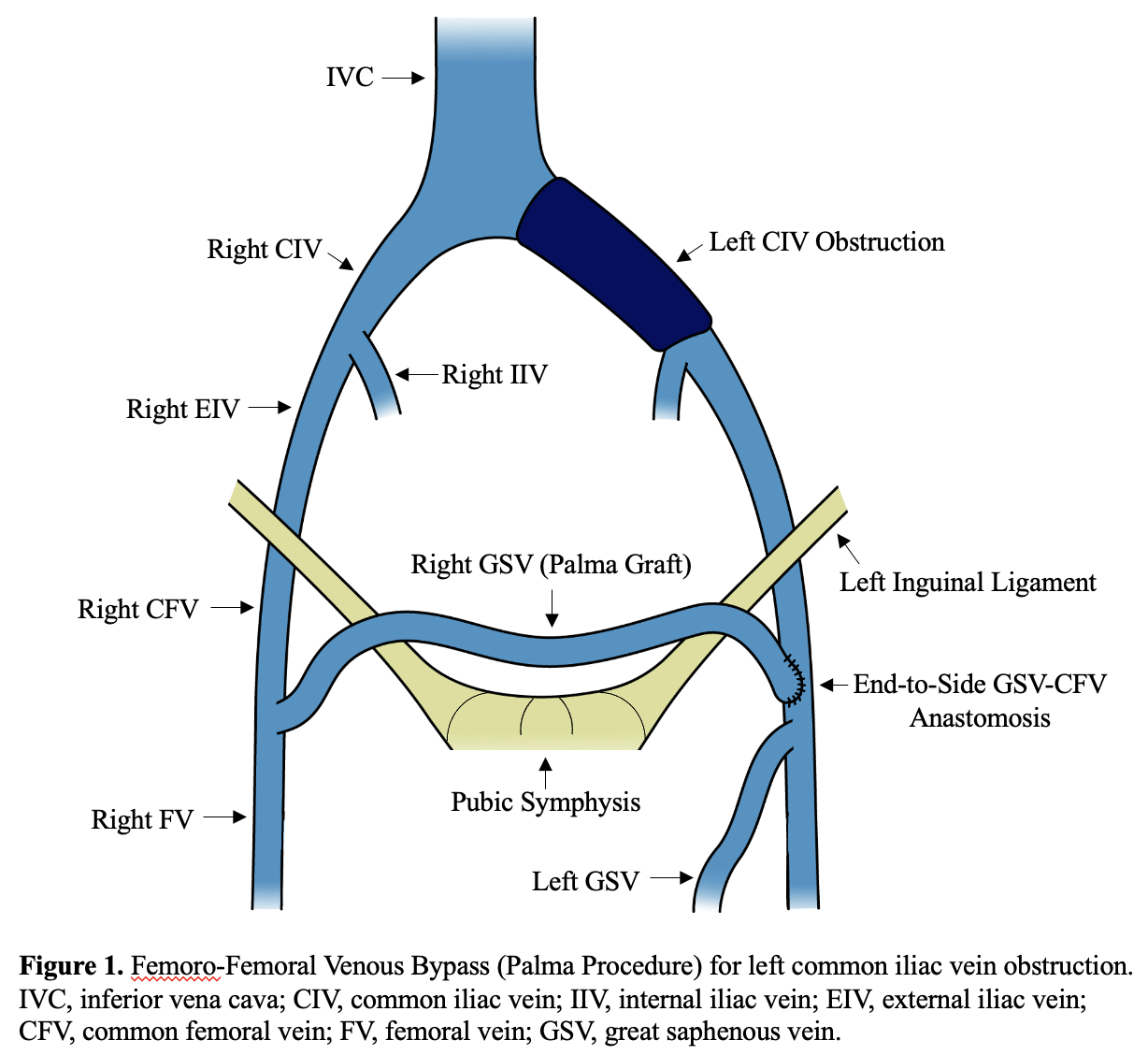
[1]
Harris M, Lim CS. Chronic venous outflow obstruction: An important cause of chronic venous disease. Cleveland Clinic journal of medicine. 2021 Dec 2:88(12):680-688. doi: 10.3949/ccjm.88a.21068. Epub 2021 Dec 2
[PubMed PMID: 34857606]
[2]
Lichtenberg M, Jalaie H. [Endovascular Therapy of Chronic Venous Outflow Obstruction]. Zentralblatt fur Chirurgie. 2017 Oct:142(5):481-486. doi: 10.1055/s-0043-119999. Epub 2017 Oct 27
[PubMed PMID: 29078243]
[3]
Taha MAH, Busuttil A, Bootun R, Thabet BAH, Badawy AEH, Hassan HA, Shalhoub J, Davies AH. A clinical guide to deep venous stenting for chronic iliofemoral venous obstruction. Journal of vascular surgery. Venous and lymphatic disorders. 2022 Jan:10(1):258-266.e1. doi: 10.1016/j.jvsv.2020.12.087. Epub 2021 May 18
[PubMed PMID: 34020107]
[4]
Wittens C, Davies AH, Bækgaard N, Broholm R, Cavezzi A, Chastanet S, de Wolf M, Eggen C, Giannoukas A, Gohel M, Kakkos S, Lawson J, Noppeney T, Onida S, Pittaluga P, Thomis S, Toonder I, Vuylsteke M, Esvs Guidelines Committee, Kolh P, de Borst GJ, Chakfé N, Debus S, Hinchliffe R, Koncar I, Lindholt J, de Ceniga MV, Vermassen F, Verzini F, Document Reviewers, De Maeseneer MG, Blomgren L, Hartung O, Kalodiki E, Korten E, Lugli M, Naylor R, Nicolini P, Rosales A. Editor's Choice - Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2015 Jun:49(6):678-737. doi: 10.1016/j.ejvs.2015.02.007. Epub 2015 Apr 25
[PubMed PMID: 25920631]
[5]
Azar J, Rao A, Oropallo A. Chronic venous insufficiency: a comprehensive review of management. Journal of wound care. 2022 Jun 2:31(6):510-519. doi: 10.12968/jowc.2022.31.6.510. Epub
[PubMed PMID: 35678787]
[6]
Raffetto JD, Mannello F. Pathophysiology of chronic venous disease. International angiology : a journal of the International Union of Angiology. 2014 Jun:33(3):212-21
[PubMed PMID: 24755829]
[7]
Sermsathanasawadi N, Pruekprasert K, Pitaksantayothin W, Chinsakchai K, Wongwanit C, Ruangsetakit C, Mutirangura P. Prevalence, risk factors, and evaluation of iliocaval obstruction in advanced chronic venous insufficiency. Journal of vascular surgery. Venous and lymphatic disorders. 2019 May:7(3):441-447. doi: 10.1016/j.jvsv.2018.10.021. Epub 2019 Feb 11
[PubMed PMID: 30765330]
[8]
Salem AM, AbdelAzeem AboElNeel H, Fakhr ME. Long-term outcome of dedicated venous stents in management of chronic iliofemoral obstruction. Journal of vascular surgery. Venous and lymphatic disorders. 2022 Jan:10(1):52-59. doi: 10.1016/j.jvsv.2021.04.018. Epub 2021 May 18
[PubMed PMID: 34020109]
[9]
Pichon M, Hij A, Wifaq B, Abderrahmane M, El Jarrari M, Menn AM. [Deep venous thrombosis caused by congenital inferior vena cava agenesis]. Journal de medecine vasculaire. 2019 Feb:44(1):79-85. doi: 10.1016/j.jdmv.2018.11.005. Epub 2018 Dec 19
[PubMed PMID: 30770086]
[10]
Chopard R, Albertsen IE, Piazza G. Diagnosis and Treatment of Lower Extremity Venous Thromboembolism: A Review. JAMA. 2020 Nov 3:324(17):1765-1776. doi: 10.1001/jama.2020.17272. Epub
[PubMed PMID: 33141212]
[11]
Comerota A, Lurie F. Pathogenesis of venous ulcer. Seminars in vascular surgery. 2015 Mar:28(1):6-14. doi: 10.1053/j.semvascsurg.2015.07.003. Epub 2015 Jul 17
[PubMed PMID: 26358304]
[12]
Lichtenberg M, de Graaf R, Erbel C. Standards for recanalisation of chronic venous outflow obstructions. VASA. Zeitschrift fur Gefasskrankheiten. 2018 Jun:47(4):259-266. doi: 10.1024/0301-1526/a000696. Epub 2018 Mar 8
[PubMed PMID: 29514591]
[13]
Bałabuszek K, Toborek M, Pietura R. Comprehensive overview of the venous disorder known as pelvic congestion syndrome. Annals of medicine. 2022 Dec:54(1):22-36. doi: 10.1080/07853890.2021.2014556. Epub
[PubMed PMID: 34935563]
Level 3 (low-level) evidence
[14]
Youn YJ, Lee J. Chronic venous insufficiency and varicose veins of the lower extremities. The Korean journal of internal medicine. 2019 Mar:34(2):269-283. doi: 10.3904/kjim.2018.230. Epub 2018 Oct 26
[PubMed PMID: 30360023]
[15]
Needleman L, Cronan JJ, Lilly MP, Merli GJ, Adhikari S, Hertzberg BS, DeJong MR, Streiff MB, Meissner MH. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. Circulation. 2018 Apr 3:137(14):1505-1515. doi: 10.1161/CIRCULATIONAHA.117.030687. Epub
[PubMed PMID: 29610129]
Level 3 (low-level) evidence
[16]
Suehiro K, Morikage N, Ueda K, Samura M, Takeuchi Y, Nagase T, Mizoguchi T, Nakamura K, Hamano K. Venous hemodynamics assessed with air plethysmography in legs with lymphedema. Vascular medicine (London, England). 2018 Apr:23(2):139-142. doi: 10.1177/1358863X17745372. Epub 2018 Jan 11
[PubMed PMID: 29325501]
[17]
Coelho A, O'Sullivan G. Usefulness of Direct Computed Tomography Venography in Predicting Inflow for Venous Reconstruction in Chronic Post-thrombotic Syndrome. Cardiovascular and interventional radiology. 2019 May:42(5):677-684. doi: 10.1007/s00270-019-02161-5. Epub 2019 Jan 9
[PubMed PMID: 30627773]
[18]
Rossi FH, Kambara AM, Rodrigues TO, Rossi CBO, Izukawa NM, Pinto IMF, Thorpe PE. Comparison of computed tomography venography and intravascular ultrasound in screening and classification of iliac vein obstruction in patients with chronic venous disease. Journal of vascular surgery. Venous and lymphatic disorders. 2020 May:8(3):413-422. doi: 10.1016/j.jvsv.2019.09.015. Epub 2020 Mar 17
[PubMed PMID: 32197952]
[19]
Aurshina A, Huber S, Deng Y, Attaran R, Nassiri N, Dardik A, Ochoa Chaar CI. Correlation of venous symptoms with iliac vein stenosis on magnetic resonance imaging. Journal of vascular surgery. Venous and lymphatic disorders. 2021 Sep:9(5):1291-1296.e1. doi: 10.1016/j.jvsv.2020.12.077. Epub 2020 Dec 30
[PubMed PMID: 33387666]
[20]
Peng C, Wu H, Kim S, Dai X, Jiang X. Recent Advances in Transducers for Intravascular Ultrasound (IVUS) Imaging. Sensors (Basel, Switzerland). 2021 May 19:21(10):. doi: 10.3390/s21103540. Epub 2021 May 19
[PubMed PMID: 34069613]
Level 3 (low-level) evidence
[21]
Gagne PJ, Tahara RW, Fastabend CP, Dzieciuchowicz L, Marston W, Vedantham S, Ting W, Iafrati MD. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. Journal of vascular surgery. Venous and lymphatic disorders. 2017 Sep:5(5):678-687. doi: 10.1016/j.jvsv.2017.04.007. Epub 2017 Jun 28
[PubMed PMID: 28818221]
[22]
Gloviczki P, Pairolero PC, Toomey BJ, Bower TC, Rooke TW, Stanson AW, Hallett JW Jr, Cherry KJ Jr. Reconstruction of large veins for nonmalignant venous occlusive disease. Journal of vascular surgery. 1992 Nov:16(5):750-61
[PubMed PMID: 1433663]
[23]
Papadakis KG, Christopoulos D, Hobbs JT, Nicolaides AN. Descending phlebography in patients with venous ulceration: hemodynamic implications. International angiology : a journal of the International Union of Angiology. 2015 Jun:34(3):263-8
[PubMed PMID: 25877427]
[24]
Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. Journal of vascular surgery. 2002 Apr:35(4):694-700
[PubMed PMID: 11932665]
[25]
Raju S. New approaches to the diagnosis and treatment of venous obstruction. Journal of vascular surgery. 1986 Jul:4(1):42-54
[PubMed PMID: 3522942]
[26]
Raju S, Knight A, Lamanilao L, Pace N, Jones T. Peripheral venous hypertension in chronic venous disease. Journal of vascular surgery. Venous and lymphatic disorders. 2019 Sep:7(5):706-714. doi: 10.1016/j.jvsv.2019.03.006. Epub 2019 Jun 10
[PubMed PMID: 31196767]
[28]
Shaydakov E, Porembskaya O, Geroulakos G. The May-Husni Procedure: A Reappraisal. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2015 Oct:50(4):513-7. doi: 10.1016/j.ejvs.2015.05.010. Epub 2015 Jul 31
[PubMed PMID: 26238309]
[29]
Husni EA. Reconstruction of veins: the need for objectivity. The Journal of cardiovascular surgery. 1983 Sep-Oct:24(5):525-8
[PubMed PMID: 6654967]
[30]
PALMA EC, ESPERON R. Vein transplants and grafts in the surgical treatment of the postphlebitic syndrome. The Journal of cardiovascular surgery. 1960 Jul:1():94-107
[PubMed PMID: 14429961]
[31]
Ulloa JH, Glickman M. One-Year First-in-Human Success for VenoValve in Treating Patients With Severe Deep Venous Insufficiency. Vascular and endovascular surgery. 2022 Apr:56(3):277-283. doi: 10.1177/15385744211073730. Epub 2022 Feb 7
[PubMed PMID: 35129407]
[32]
Medical Advisory Secretariat. Community-based care for chronic wound management: an evidence-based analysis. Ontario health technology assessment series. 2009:9(18):1-24
[PubMed PMID: 23074522]
[33]
Vu T, Harris A, Duncan G, Sussman G. Cost-effectiveness of multidisciplinary wound care in nursing homes: a pseudo-randomized pragmatic cluster trial. Family practice. 2007 Sep:24(4):372-9
[PubMed PMID: 17602174]
Level 1 (high-level) evidence