Introduction
The gastrointestinal tract is a highly specialized organ system primarily responsible for nutrient absorption, though it has other roles. From the mouth to the anus, its length is approximately 9 meters (30 feet). The gastrointestinal tract's wide range of functions include the following:[1][2][3][4]
- Nutrient absorption - This comes after the breakdown of carbohydrates, proteins, fats, vitamins, and minerals, which are essential for energy production, growth, and cellular maintenance.
- Egestion of waste and toxins - The process eliminates indigestible components and harmful substances from the body.
- Maintenance of hormonal homeostasis - The gastrointestinal tract influences appetite, satiety, and metabolism.
- Providing immunity - Immune cells line the gastrointestinal mucosa to defend against pathogens and maintain a balance between tolerance and reactivity.
- Influencing behavior - The gastrointestinal tract is a key player in the "gut-brain axis," influencing behavior and cognitive processes.
Cellular Level
The Cells of the Gastrointestinal Tract
Enterocytes: These are the cells that make up most of the intestinal lining. Enterocytes are directly involved in the uptake of ions, water, nutrients, vitamins, and unconjugated bile acid salts.[5]
Goblet cells: These modified epithelial cells are found inserted between enterocytes. They are unicellular glands that mainly produce alkaline mucus, which protects the gastrointestinal lining from shearing forces and acidic secretions.[6]
Enteroendocrine cells: These cells are located in the stomach, pancreas, and small intestine. Enteroendocrine cells have several roles. First, they are responsible for biogenic amine and polypeptide secretion, releasing ghrelin, cholecystokinin, glucagon-like peptide 1, peptide YY, insulin-like peptide 5, and oxyntomodulin. Second, they aid in food digestion and nutrient absorption.[7] Third, they aid in pathogen recognition by expressing toll-like receptors and commensal bacterial metabolite-sensitive receptors.[8]
G-cells: These are neuroendocrine cells in the stomach antrum and the duodenum. They are responsible for secreting the hormone gastrin, which stimulates enterochromaffin-like cells and parietal cells. Enterochromaffin-like cells secrete histamine, while parietal cells release hydrochloric acid in the stomach. Gastrin also promotes gastric mucosal growth and supports gastric motility. G-cells are innervated by the vagus nerve and stimulated by gastrin-releasing peptide and bombesin.[9]
Oxyntic (parietal) cells: These are specialized cells found in the stomach. They are responsible for hydrochloric acid secretion that helps break down food, activates enzymes, and maintains the stomach's low pH for antimicrobial protection. Parietal cells also secrete intrinsic factor, which is necessary for vitamin B12 absorption in the terminal ileum.[10]
Zymogenic (chief) cell: These specialized stomach cells are responsible for pepsinogen, chymosin, and gastric lipase secretion. Pepsinogen is converted to active pepsin by hydrochloric acid. Both pepsin and chymosin break down proteins into amino acids.[11]
Paneth cells: These specialized cells are found in the small intestine's crypts of Lieberkuhn and the large intestine. Paneth cells secrete antimicrobial peptides and proteins. In the large intestine, they play a key role in microbiota regulation and inflammation.[12]
Microfold (M) cells: These cells reside in the Peyer's patches of the small intestine and are crucial to the mucosal immune response to foreign pathogens. They take up antigens and deliver them to antigen-presenting cells, which initiate the immune response.[13]
Tuft cells: These specialized small intestine cells play a role in the immune response against parasite and protozoa infection.[14][15]
Development
Gastrointestinal development begins during the 3rd week of life. The primordial digestive tract, derived from the yolk sac's endodermal lining, develops alongside its arterial blood supply. It divides into the foregut, midgut, and hindgut.
The foregut is supplied by the celiac trunk and forms into the pharynx, esophagus, stomach, liver, gallbladder, bile ducts, pancreas, and proximal duodenum.
The midgut is supplied by the superior mesenteric artery and forms into the distal duodenum, jejunum, ileum, cecum, ascending colon, and proximal two-thirds of the transverse colon.
The hindgut is supplied by the inferior mesenteric artery and forms into the distal third of the transverse colon, descending colon, sigmoid colon, rectum, and proximal anal canal.
Although organogenesis is achieved by the 8th week, intestinal absorption begins in the 24th week.[16] Defects during embryogenesis can result in congenital anomalies such as atresia and stenosis. While patient outcomes vary depending on the location, extent, and the organ involved, improper development can significantly impair digestion and absorption.[17]
Organ Systems Involved
The gastrointestinal system interacts with every organ system.
Nervous System
Communication between the nervous and gastrointestinal systems is accomplished by hormonal signals and the enteric nerves.[18]
Cardiovascular
Gastrointestinal circulation has the following functions:[19]
- Delivery of nutrients, oxygen, and other substances to the gastrointestinal cells
- Absorption of nutrients
- Clearance of metabolites in the gastrointestinal cells
- Hemodynamic stability
Renal
The interactions between the renal and gastrointestinal systems are crucial to calcium homeostasis. The kidneys activate the pre-hormone 25-hydroxyvitamin D to form 1,25-dihydroxyvitamin D. Activated vitamin D induces the colon to absorb calcium and phosphate. Kidney disease and nutritional deficiencies cause an imbalance in calcium homeostasis and may lead to bone disorders.[20]
Musculoskeletal
The gut absorbs vitamins and minerals that play a crucial role in bone formation. Vitamin D, calcium, magnesium, and phosphate malabsorption can lead to bone loss, inflammation, and pain.[21]
Pulmonary
Interplays between the pulmonary and digestive systems are evident in the following:[22]
- Oxygenation of gastrointestinal cells
- Gut absorption of iron, which is necessary for hemoglobin formation and oxygen delivery
- Liver cirrhosis leading to pleural effusion [23]
Endocrine
Organs of the gastrointestinal system have vital endocrine functions. For example, the pancreas secretes insulin (beta cells) and glucagon (alpha cells), which are crucial in blood glucose regulation and energy expenditure.[24] The intestines absorb cholesterol, which is important in steroid hormone biosynthesis.[25]
Reproductive
The sex hormones are steroid hormones, which derive their central ring structure from cholesterol and its intermediates.[25]
Function
Mouth
The mouth is comprised of the lips, teeth, tongue, salivary glands, hard palate, soft palate, uvula, and oropharynx.
The lips are fleshy, movable structures surrounding the mouth's opening. They play a crucial role in speech, expression, and sealing the mouth during eating and drinking.
The teeth are hard structures responsible for breaking down food into smaller, more digestible particles. They also aid in oral cavity protection and speech.
The tongue is a muscular organ on the oral cavity floor. It is crucial in taste perception, food bolus manipulation, initiating swallowing, and generating the gag reflex.[26]
The salivary glands are located within and around the mouth. Their primary function is to secrete saliva, which contains enzymes that initiate the process of digestion (amylase) and protect against microbes (lysozyme).[27]
The palate is the roof of the mouth and aids in chewing and swallowing. The uvula is a small, fleshy projection originating from the soft palate. It helps prevent food and liquid from entering the nasal cavity during deglutition.[28]
Esophagus
The esophagus is typically can be divided into 3 portions. The upper third predominantly contains somatic-controlled striated muscle. The middle third has a mix of striated and smooth muscles. The lower third mainly has smooth muscle and is autonomic in function. The normal esophageal lining has non-keratinized stratified squamous epithelium.
The esophagus carries food from the mouth to the stomach via muscular contractions known as peristalsis. It has two natural sphincters:
- Upper esophageal sphincter: comprised of the cervical esophagus, cricopharyngeus, and inferior pharyngeal constrictor [29]
- Lower esophageal sphincter: comprised of the diaphragmatic crura, phrenoesophageal ligament, and intrinsic esophageal muscle fibers [30]
Stomach
The stomach is divided into five portions:
- Cardia: the gastric segment that connects with the esophagus. It has a sphincter that prevents gastric contents from refluxing to the esophagus.
- Fundus: lies inferior to the cardia and functions as residual space for gastric contents
- Body: the largest portion of the stomach and the site where food mixes with gastric acid secretions
- Antrum: the inferior portion of the stomach that holds the food-acid mixture before it is moved into the small intestine
- Pyrolus: the portion of the stomach connected to the duodenum. It is comprised of a thick muscular ring that acts as a sphincter controlling gastric emptying. Of note, the stomach is the first site of absorption for lipid-soluble substances such as alcohol and aspirin.[31]
Small Intestine
The small intestine is divided into three portions:
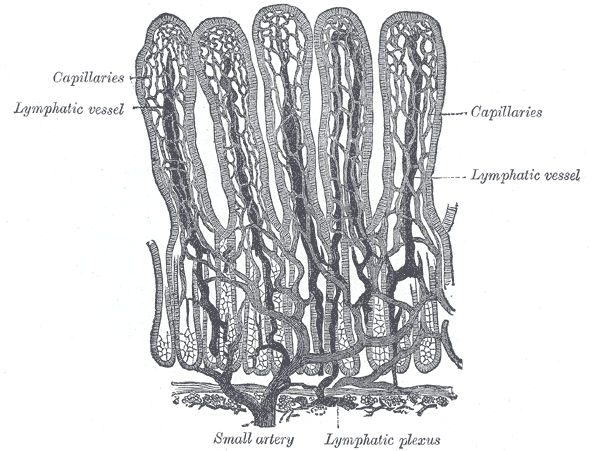
- Duodenum: the segment that attaches to the stomach. It is approximately 30 cm or 1 foot long. The duodenum receives the food-acid mixture from the stomach, which then becomes chyme. Liver, pancreas, and gallbladder secretions come into contact with chyme in this segment, preparing it for further digestion and subsequent absorption. The duodenum absorbs most of the iron, calcium, phosphorus, magnesium, copper, selenium, thiamin, riboflavin, niacin, biotin, folate, and the fat-soluble vitamins A, D, E, and K. Intestinal villi—the small finger-like projections at the epithelial apices—increase the intestinal cells' surface area for absorption.[32]
- Jejunum: measures approximately 244 cm or 8 feet long and is the second portion of the small intestines. The lacteals—the jejunal lymphatic vessels—aid in the absorption of lipids, which have become glycerol and free fatty acids in this segment. Amino acids are also absorbed in the jejunum, entering the bloodstream through the mesenteric capillaries.[33] Other nutrients absorbed by the jejunal cells include thiamine, riboflavin, niacin, pantothenate, biotin, folate, pyridoxine, ascorbic acid, calcium, phosphorus, magnesium iron, zinc, chromium, manganese, molybdenum, lipids, monosaccharides, small peptides, and the fat-soluble vitamins A, D, E, and K.
- Ileum: approximately 150 cm or 5 feet long. It is the most distal segment of the small intestine, terminating at the ileocecal junction. The ileum absorbs bile salts and acids, ascorbic acid, folate, cobalamin, vitamin D, vitamin K, and magnesium.[34]
Large Intestine
The large intestine is the portion of the intestinal tract between the ileocecal valve superiorly and the anus inferiorly. It is comprised of the ascending colon, transverse colon, descending colon, rectum, and anus. Its function is to absorb water, form stools, and eliminate feces.[35]
Mechanism
Digestion is the body's natural process of converting food into products that can be absorbed and used for nourishment. Mechanical digestion involves physically breaking down food into fragments through mastication, deglutition, churning, and segmentation. Chemical digestion breaks down mechanically digested food into small, absorbable particles by pH changes and enzymatic action.[36]
Nutrient absorption occurs through cellular transport, which has two pathways:[37][38]
- Paracellular pathway: molecules move from the intestinal lumen across tight junctions between intestinal epithelial cells to enter the extracellular space (interstitium). This process is unmediated and passively regulated by an electrochemical concentration gradient.
- Transcellular pathway: molecules first move from the intestinal lumen into the enterocyte by crossing the apical membrane. From inside the cell, the molecules traverse the basolateral membrane and enter the extracellular space. In contrast to the paracellular pathway, transcellular transport is active, requiring energy expenditure in the form of Adenosine Triphosphate (ATP). Apical and basolateral enterocyte transporters help facilitate this process.
While paracellular transport plays a minor role in glucose absorption, the transcellular pathway is responsible for most nutrient absorption.
Once in the interstitium, the absorbed molecules circulate in the venous portal system. Blood from the villous capillaries brings nutrients from the gastrointestinal tract to the liver (see Image. Small Intestinal Villi Schematic Representation). From there, hepatocytes can further process and distribute nutrients to the body through the blood flowing in the inferior vena cava.
Most nutrients get into the bloodstream via the paracellular and transcellular pathways. On the other hand, fats and fat-soluble vitamins enter specialized lymphatic ducts called "lacteals," which distribute them throughout the body.
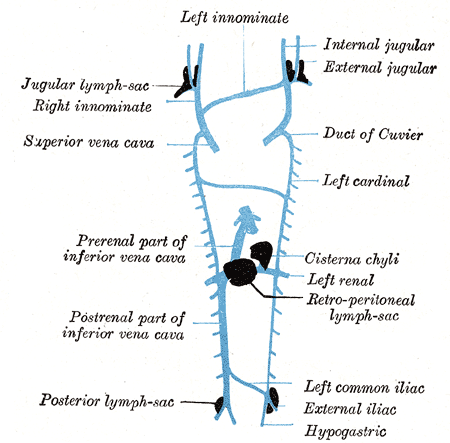
After absorption in the lacteals, lipids enter the intestinal lymphatic system and converge in the cisterna chyli of the retrocrural space. The cisterna chyli is the abdominal origin of the thoracic duct. From there, the thoracic duct will drain 95% of the time into the internal jugular vein, the subclavian vein, or the angle between the two. The rest will drain into the external jugular vein, vertebral vein, brachiocephalic vein, suprascapular vein, or transverse cervical vein (see Image. Thoracic Lymphatic System).[39]
Pathophysiology
Carbohydrate Absorption
Carbohydrate digestion begins in the oral cavity with the mechanical breakdown of food. Chemical digestion occurs once mechanically digested food comes into contact with salivary gland, pancreas, and intestinal brush border enzymes.
Enzymatic glycosidic bond hydrolysis turns complex carbohydrates into their simplest forms: glucose, fructose, and galactose. These monosaccharides exit the intestinal lumen and enter the bloodstream via the transcellular pathway.
Transcellular transport begins with the enterocytic apical symporter, Sodium-Glucose Transporter 1 (SGLT-1). Basolateral sodium-potassium ATPase pumps create a sodium concentration gradient across the enterocytic apical membrane. This gradient drives SGLT-1 symporters, which transport glucose or galactose into the enterocyte paired with 2 sodium ions (secondary active transport).
Glucose Transporter 5 (GLUT-5) is another apical membrane transporter. In contrast to SGLT-1, GLUT-5 has a high affinity for fructose, allowing passive entry into the cell via facilitated diffusion.
A basolateral membrane transporter, Glucose Transporter 2 (GLUT-2), uses facilitated diffusion to transport glucose, galactose, and fructose from the enterocyte body into the interstitial space.[40]
Meanwhile, undigested carbohydrates like cellulose are not absorbed in the gut but remain in the colon and undergo fermentation by colonic bacteria.
Protein Absorption
Chemical protein digestion begins in the stomach and continues into the jejunum.[41] During this process, the stomach, pancreas, and intestinal brush border release enzymes (peptidases), which break down polypeptides into tripeptides, dipeptides, and amino acids.
Following digestion, protein absorption occurs in the jejunum and proximal ileum. Dipeptides and tripeptides enter the enterocyte cytoplasm through the Peptide Transporter 1 (PepT1). Single amino acids move from the lumen into the enterocyte by facilitated diffusion in sodium-linked transporters.
PepT1 is a high-capacity, low-affinity proton-dependent transporter. It transports an oligopeptide along with one hydrogen ion. Inside the enterocyte, lysosomes further digest oligopeptides into free amino acids. Oligopeptides cross the basolateral membrane and enter the interstitium as single amino acids.[42]
In contrast, free amino acids in the lumen enter the enterocyte cytoplasm via sodium-linked transporters in a manner similar to glucose. There are different amino acid transporters in the brush border, though they have overlapping affinities for the different amino acids. One example is system B, which co-transports neutral amino acids with sodium ions.
From the cytoplasm, single amino acids cross the basolateral membrane to enter the extracellular space, where they will circulate through the venous portal system, as previously described.[43][44]
Fat Absorption
Lipid breakdown begins early in the gastrointestinal tract as lipase is secreted in the mouth. Lipase cleaves triglycerides into monoglycerides, then glycerol and free fatty acids. The stomach and pancreas also secrete lipase, so lipid digestion continues through to the small intestine.
Bile from the gallbladder enhances lipase efficiency by emulsifying fats in the terminal duodenum and jejunum. The final products of digestion aggregate in the lumen to form lipid-dense particles called "micelles." Fat absorption begins when smaller lipid molecules separate from the micelle, crossing the apical membrane through simple diffusion.[45]
From the enterocyte cytoplasm, fatty acids traverse the basolateral membrane and enter the venous portal system. Meanwhile, monoglycerides assemble in the endoplasmic reticulum to create triglycerides, which are fundamental chylomicron components. Lipoproteins and long-chain fatty acids fuse with the chylomicrons, which then travel to the basolateral surface, bud off, and enter the lacteals.
Thoracic muscle contraction pushes the lipid-filled lymphatic fluid superiorly until it enters the systemic circulation via the right subclavian vein.[46] Upon reaching the tissues, these lipids can be converted and used for insulation, storage, or hormone synthesis.[47]
Secretin and cholecystokinin (CCK) are duodenal hormones that reduce intestinal motility and stimulate the pancreas and gallbladder to enhance fat digestion.
Vitamins and Minerals
Vitamins A, D, E, and K are fat-soluble. [48] As such, they readily dissolve in organic fats and other non-polar solvents. In the small intestine, fat-soluble vitamins fuse with micelles and cross the apical membrane via simple diffusion. Once inside the enterocyte, they integrate with chylomicrons and enter the systemic circulation from there.
Fat-soluble vitamins are absorbed by adipose tissue, where they can stay for long periods and accumulate. Over time, chronically high intake of these vitamins may cause toxicity.
Water-soluble vitamins include thiamine (B1), riboflavin (B2), niacin (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folic acid (B9), cobalamin (B12), and ascorbic acid (C).[49] Water-soluble vitamins do not simply traverse cellular membranes but are absorbed in the digestive tract through specific carrier-mediated pathways. After distribution, they are consumed by the tissues for their metabolic needs.
When water-soluble vitamins reach supra-therapeutic levels, the kidney excretes the excess in urine. These vitamins do not accumulate and are thus less likely to elicit toxicity than lipid-soluble ones. However, that also means they need frequent dietary replacement.[50]
Clinical Significance
Malabsorption occurs when the body cannot effectively absorb nutrients. This condition is often the result of gastrointestinal disease. The most prevalent causes of malabsorption in the United States include pancreatic insufficiency, Celiac disease, and Crohn disease.[51]
Malabsorptive conditions impair either luminal, mucosal, or post-absorptive gastrointestinal processes. Impairment of the luminal processes limits mechanical digestion and chemical hydrolysis, which are needed to break food down into absorbable forms.[52] Mucosal defects reduce nutrient transport across enterocyte membranes. Post-absorptive impairment prevents effective nutrient distribution through the lymphatic and portal systems. Symptoms vary depending on which nutrient is deficient and the extent of intestinal damage.
Surgically shortened intestines reduce nutrient absorption time and can also produce malabsorption symptoms.[53]
Carbohydrate Malabsorption
Undigested and unabsorbed carbohydrates move to the large intestine to be fermented by colonic bacteria. Fermentation is accompanied by gas production, which, in excess, can cause abdominal cramping and bloating.
Lactase deficiency impairs the ability to digest lactose-containing food, such as dairy products.[54] The condition affects nearly 65% of the global population. Patients may have insufficient or structurally defective lactase in the gut. The colon is burdened with processing the unabsorbed lactose, so symptoms typically include abdominal pain and diarrhea after lactose ingestion.
Celiac disease is another condition associated with carbohydrate malabsorption. Affected individuals mount an immune reaction to gluten, a substance found in some grain types. The brush border becomes blunted as a result of diffuse mucosal injury, reducing the small intestine's absorptive capacity.
In pediatric patients, carbohydrate malabsorption presents with chronic caloric deficiency, weight loss, and growth delay. Malabsorption of other nutrients often co-exists with this condition, so it may be accompanied by other nutritional deficiencies.[55]
Protein Malabsorption
Impaired protein absorption rarely occurs in isolation and is often a component of global malabsorptive conditions. Protein deficiency affects various body processes, including the absorption and utilization of other nutrients.
For example, protein insufficiency can impair lipoprotein aggregation, which is necessary for lipid and cholesterol metabolism. Hypoalbuminemia reduces the blood's ability to transport fat-soluble vitamins, hormones, and medications. The condition simultaneously lowers plasma oncotic pressure, resulting in third spacing and edematous states.[56][57]
Severe protein malabsorption can lead to Kwarshiorkor syndrome, characterized by edema and skin and hair changes. Hepatomegaly and ascites also manifest due to the liver's inability to produce apoproteins. Patients are prone to infections due to impaired immunoglobulin production.[58] Longstanding protein deficiency from any cause can lead to severe complications, even death.[59]
Fat Malabsorption
Fat malabsorption is most commonly due to the failure of lipolytic enzymes and bile to interact with the fatty contents of partly digested food. Etiologies include pancreatic exocrine insufficiency, biliary obstruction, post-surgical structural changes (eg, after a Whipple procedure), intestinal mucosal injury, and motility disorders.
Pancreatic exocrine insufficiency refers to the pancreas' inability to secrete digestive enzymes, ions, and water. Intestinal mucosal damage can arise from inflammatory disorders, radiation, and infection.[52]
Typical signs of fat malabsorption include loose, bulky, clay-colored stools that tend to float in water. Patients may also experience weight loss, fatigue, and generalized weakness. Fat-soluble vitamin deficiencies may likewise develop and present with bone loss, night blindness, bleeding, and, in rare cases, hemolytic anemia.[60]
Malnutrition in Hospitalized Patients and the Critically Ill
Nutritional support is essential when managing illnesses in the hospital setting. Sepsis, shock, malignancy, and many other critical conditions may lead to intestinal slowing, anorexia, and hypermetabolic states. Surgical patients about to receive sedation are typically advised to fast for several hours or overnight before their procedure.[61] Without proper nutritional support, these patients may become vulnerable to malnutrition-associated conditions like hyponatremia, starvation ketoacidosis, and refeeding syndrome.
To date, evidence showing that modified hospital diets confer any mortality benefit is insufficient. They may even be too restrictive. However, early feeding and nutritional support are not known to have better outcomes, either. Additionally, patients who forego enteric feeding for long periods are more likely to become dependent on non-enteral modalities of feeding.[62]