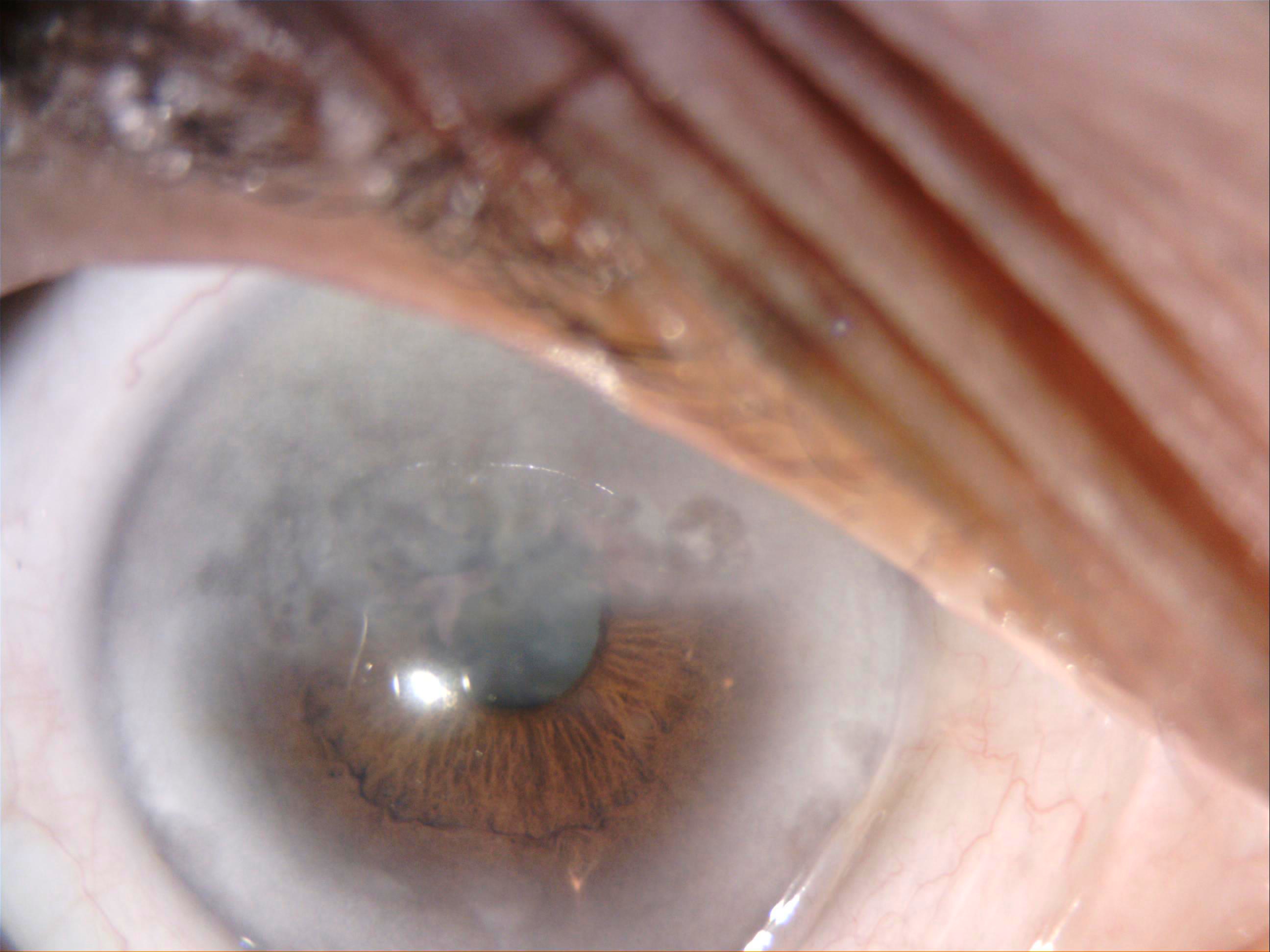
[1]
Kessel A, Vadasz Z, Toubi E. Cogan syndrome--pathogenesis, clinical variants and treatment approaches. Autoimmunity reviews. 2014 Apr-May:13(4-5):351-4. doi: 10.1016/j.autrev.2014.01.002. Epub 2014 Jan 10
[PubMed PMID: 24418297]
[2]
McCabe BF. Autoimmune sensorineural hearing loss. The Annals of otology, rhinology, and laryngology. 1979 Sep-Oct:88(5 Pt 1):585-9
[PubMed PMID: 496191]
[3]
Goodall AF, Siddiq MA. Current understanding of the pathogenesis of autoimmune inner ear disease: a review. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2015 Oct:40(5):412-9. doi: 10.1111/coa.12432. Epub
[PubMed PMID: 25847404]
Level 3 (low-level) evidence
[4]
Iliescu DA, Timaru CM, Batras M, De Simone A, Stefan C. COGAN'S SYNDROME. Romanian journal of ophthalmology. 2015 Jan-Mar:59(1):6-13
[PubMed PMID: 27373108]
[5]
Haynes BF, Kaiser-Kupfer MI, Mason P, Fauci AS. Cogan syndrome: studies in thirteen patients, long-term follow-up, and a review of the literature. Medicine. 1980 Nov:59(6):426-41
[PubMed PMID: 6969345]
[6]
Vollertsen RS. Vasculitis and Cogan's syndrome. Rheumatic diseases clinics of North America. 1990 May:16(2):433-9
[PubMed PMID: 2189159]
[7]
García Berrocal JR, Vargas JA, Vaquero M, Ramón y Cajal S, Ramírez-Camacho RA. Cogan's syndrome: an oculo-audiovestibular disease. Postgraduate medical journal. 1999 May:75(883):262-4
[PubMed PMID: 10533627]
[8]
Tayer-Shifman OE, Ilan O, Tovi H, Tal Y. Cogan's syndrome--clinical guidelines and novel therapeutic approaches. Clinical reviews in allergy & immunology. 2014 Aug:47(1):65-72. doi: 10.1007/s12016-013-8406-7. Epub
[PubMed PMID: 24385257]
[9]
Vollertsen RS, McDonald TJ, Younge BR, Banks PM, Stanson AW, Ilstrup DM. Cogan's syndrome: 18 cases and a review of the literature. Mayo Clinic proceedings. 1986 May:61(5):344-61
[PubMed PMID: 3486332]
Level 3 (low-level) evidence
[10]
Azami A, Maleki N, Kalantar Hormozi M, Tavosi Z. Interstitial Keratitis, Vertigo, and Vasculitis: Typical Cogan's Syndrome. Case reports in medicine. 2014:2014():830831. doi: 10.1155/2014/830831. Epub 2014 Mar 4
[PubMed PMID: 24715922]
Level 3 (low-level) evidence
[11]
Gaubitz M, Lübben B, Seidel M, Schotte H, Gramley F, Domschke W. Cogan's syndrome: organ-specific autoimmune disease or systemic vasculitis? A report of two cases and review of the literature. Clinical and experimental rheumatology. 2001 Jul-Aug:19(4):463-9
[PubMed PMID: 11491507]
Level 3 (low-level) evidence
[12]
Lunardi C,Bason C,Leandri M,Navone R,Lestani M,Millo E,Benatti U,Cilli M,Beri R,Corrocher R,Puccetti A, Autoantibodies to inner ear and endothelial antigens in Cogan's syndrome. Lancet (London, England). 2002 Sep 21;
[PubMed PMID: 12354474]
[13]
Yamanishi Y, Ishioka S, Takeda M, Maeda H, Yamakido M. Letter to the Editor. Atypical Cogan's syndrome associated with antineutrophil cytoplasmic autoantibodies. British journal of rheumatology. 1996 Jun:35(6):601-2
[PubMed PMID: 8670587]
Level 3 (low-level) evidence
[14]
FISHER ER, HELLSTROM HR. Cogan's syndrome and systemic vascular disease. Analysis of pathologic features with reference to its relationship to thromboangiitis obliterans (Buerger). Archives of pathology. 1961 Nov:72():572-92
[PubMed PMID: 13893233]
[15]
Rarey KE, Bicknell JM, Davis LE. Intralabyrinthine osteogenesis in Cogan's syndrome. American journal of otolaryngology. 1986 Nov-Dec:7(6):387-90
[PubMed PMID: 3099589]
[16]
Schuknecht HF,Nadol JB Jr, Temporal bone pathology in a case of Cogan's syndrome. The Laryngoscope. 1994 Sep;
[PubMed PMID: 8072362]
Level 3 (low-level) evidence
[17]
Cochrane AD, Tatoulis J. Cogan's syndrome with aortitis, aortic regurgitation, and aortic arch vessel stenoses. The Annals of thoracic surgery. 1991 Nov:52(5):1166-7
[PubMed PMID: 1953144]
[18]
Livingston JZ, Casale AS, Hutchins GM, Shapiro EP. Coronary involvement in Cogan's syndrome. American heart journal. 1992 Feb:123(2):528-30
[PubMed PMID: 1736593]
[19]
Gelfand ML, Kantor T, Gorstein F. Cogan's syndrome with cardiovascular involvement: aortic insufficiency. Bulletin of the New York Academy of Medicine. 1972 May:48(4):647-60
[PubMed PMID: 4503925]
[20]
EISENSTEIN B, TAUBENHAUS M. Nonsyphilitic interstitial keratitis and bilateral deafness (Cogan's syndrome) associated with cardiovascular disease. The New England journal of medicine. 1958 May 29:258(22):1074-9
[PubMed PMID: 13552923]
[21]
Pinals RS. Cogan's syndrome with arthritis and aortic insufficiency. The Journal of rheumatology. 1978 Fall:5(3):294-8
[PubMed PMID: 748553]
[22]
Bonaguri C, Orsoni J, Russo A, Rubino P, Bacciu S, Lippi G, Melegari A, Zavota L, Ghirardini S, Mora P. Cogan's syndrome: anti-Hsp70 antibodies are a serological marker in the typical form. The Israel Medical Association journal : IMAJ. 2014 May:16(5):285-8
[PubMed PMID: 24979832]
[23]
Espinoza GM, Wheeler J, Temprano KK, Keller AP. Cogan's Syndrome: Clinical Presentations and Update on Treatment. Current allergy and asthma reports. 2020 Jun 16:20(9):46. doi: 10.1007/s11882-020-00945-1. Epub 2020 Jun 16
[PubMed PMID: 32548646]
[24]
Grasland A, Pouchot J, Hachulla E, Blétry O, Papo T, Vinceneux P, Study Group for Cogan's Syndrome. Typical and atypical Cogan's syndrome: 32 cases and review of the literature. Rheumatology (Oxford, England). 2004 Aug:43(8):1007-15
[PubMed PMID: 15150435]
Level 3 (low-level) evidence
[25]
Kaya M, Erkanlı K, Kılınç F, Sar M, Bakır İ. Surgical Treatment in a Case of Cogan's Syndrome Complicated With Proximal Aortic Vasculitis. The Annals of thoracic surgery. 2015 Oct:100(4):1467-9. doi: 10.1016/j.athoracsur.2015.01.049. Epub
[PubMed PMID: 26434452]
Level 3 (low-level) evidence
[26]
Ndiaye IC, Rassi SJ, Wiener-Vacher SR. Cochleovestibular impairment in pediatric Cogan's syndrome. Pediatrics. 2002 Feb:109(2):E38
[PubMed PMID: 11826248]
[27]
Osborne DC, Jacobson JT, Olsen ML. Cogan's syndrome: auditory and medical management. Journal of the American Academy of Audiology. 1992 May:3(3):225-9
[PubMed PMID: 1581598]
[28]
Shamriz O, Tal Y, Gross M. Autoimmune Inner Ear Disease: Immune Biomarkers, Audiovestibular Aspects, and Therapeutic Modalities of Cogan's Syndrome. Journal of immunology research. 2018:2018():1498640. doi: 10.1155/2018/1498640. Epub 2018 Apr 23
[PubMed PMID: 29850616]
[29]
Matteson EL, Tirzaman O, Facer GW, Fabry DA, Kasperbauer J, Beatty CW, McDonald TJ. Use of methotrexate for autoimmune hearing loss. The Annals of otology, rhinology, and laryngology. 2000 Aug:109(8 Pt 1):710-4
[PubMed PMID: 10961801]
[30]
Allen NB, Cox CC, Cobo M, Kisslo J, Jacobs MR, McCallum RM, Haynes BF. Use of immunosuppressive agents in the treatment of severe ocular and vascular manifestations of Cogan's syndrome. The American journal of medicine. 1990 Mar:88(3):296-301
[PubMed PMID: 2309745]
[31]
Touma Z, Nawwar R, Hadi U, Hourani M, Arayssi T. The use of TNF-alpha blockers in Cogan's syndrome. Rheumatology international. 2007 Aug:27(10):995-6
[PubMed PMID: 17564714]
[32]
Kalogeropoulos C, Karachalios D, Pentheroudakis G, Tsikou-Papafrangou A, Abou-Asabeh L, Argyropoulou M, Drosos A, Pavlidis N. Development of a low grade lymphoma in the mastoid bone in a patient with atypical Cogan's syndrome: A case report. Journal of advanced research. 2015 May:6(3):523-7. doi: 10.1016/j.jare.2014.05.003. Epub 2014 May 16
[PubMed PMID: 26257951]
Level 3 (low-level) evidence
[33]
Orsoni JG, Laganà B, Rubino P, Zavota L, Bacciu S, Mora P. Rituximab ameliorated severe hearing loss in Cogan's syndrome: a case report. Orphanet journal of rare diseases. 2010 Jun 16:5():18. doi: 10.1186/1750-1172-5-18. Epub 2010 Jun 16
[PubMed PMID: 20550723]
Level 3 (low-level) evidence
[34]
Peters BR, Wyss J, Manrique M. Worldwide trends in bilateral cochlear implantation. The Laryngoscope. 2010 May:120 Suppl 2():S17-44. doi: 10.1002/lary.20859. Epub
[PubMed PMID: 20422715]
[35]
Wang JR, Yuen HW, Shipp DB, Stewart S, Lin VY, Chen JM, Nedzelski JM. Cochlear implantation in patients with autoimmune inner ear disease including cogan syndrome: a comparison with age- and sex-matched controls. The Laryngoscope. 2010 Dec:120(12):2478-83. doi: 10.1002/lary.21060. Epub
[PubMed PMID: 21082747]
[36]
Coelho DH, Lalwani AK. Medical management of Ménière's disease. The Laryngoscope. 2008 Jun:118(6):1099-108. doi: 10.1097/MLG.0b013e31816927f0. Epub
[PubMed PMID: 18418279]
[37]
Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmunity reviews. 2011 May:10(7):410-5. doi: 10.1016/j.autrev.2011.01.005. Epub 2011 Jan 20
[PubMed PMID: 21256250]
[38]
Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in Ophthalmology: A Comprehensive Review. Seminars in ophthalmology. 2017:32(2):237-250. doi: 10.3109/08820538.2015.1045150. Epub 2015 Jul 6
[PubMed PMID: 26148300]
[39]
Brogan K, Eleftheriou D, Rajput K, Edelsten C, Sebire NJ, Brogan PA. Tubulointerstitial nephritis, uveitis, hearing loss and vestibular failure: TINU-atypical Cogan's overlap syndrome. Rheumatology (Oxford, England). 2012 May:51(5):950-2. doi: 10.1093/rheumatology/ker443. Epub 2012 Jan 11
[PubMed PMID: 22240503]
[40]
Scharl M, Frei P, Fried M, Rogler G, Vavricka SR. Association between Cogan's syndrome and inflammatory bowel disease: a case series. Journal of Crohn's & colitis. 2011 Feb:5(1):64-8. doi: 10.1016/j.crohns.2010.09.003. Epub 2010 Oct 29
[PubMed PMID: 21272808]
Level 2 (mid-level) evidence
[41]
Vavricka SR, Greuter T, Scharl M, Mantzaris G, Shitrit AB, Filip R, Karmiris K, Thoeringer CK, Boldys H, Wewer AV, Yanai H, Flores C, Schmidt C, Kariv R, Rogler G, Rahier JF, ECCO CONFER investigators. Cogan's Syndrome in Patients With Inflammatory Bowel Disease--A Case Series. Journal of Crohn's & colitis. 2015 Oct:9(10):886-90. doi: 10.1093/ecco-jcc/jjv128. Epub 2015 Jul 17
[PubMed PMID: 26188351]
Level 2 (mid-level) evidence
[43]
Durtette C, Hachulla E, Resche-Rigon M, Papo T, Zénone T, Lioger B, Deligny C, Lambert M, Landron C, Pouchot J, Kahn JE, Lavigne C, De Wazieres B, Dhote R, Gondran G, Pertuiset E, Quemeneur T, Hamidou M, Sève P, Le Gallou T, Grasland A, Hatron PY, Fain O, Mekinian A, SNFMI and CRI. Cogan syndrome: Characteristics, outcome and treatment in a French nationwide retrospective study and literature review. Autoimmunity reviews. 2017 Dec:16(12):1219-1223. doi: 10.1016/j.autrev.2017.10.005. Epub 2017 Oct 14
[PubMed PMID: 29037902]
Level 2 (mid-level) evidence
[44]
Pasanisi E, Vincenti V, Bacciu A, Guida M, Berghenti T, Barbot A, Orsoni JG, Bacciu S. Cochlear implantation and Cogan syndrome. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003 Jul:24(4):601-4
[PubMed PMID: 12851552]
[45]
Kawamura S, Sakamoto T, Kashio A, Kakigi A, Ito K, Suzuki M, Yamasoba T. Cochlear implantation in a patient with atypical Cogan's syndrome complicated with hypertrophic cranial pachymeningitis. Auris, nasus, larynx. 2010 Dec:37(6):737-41. doi: 10.1016/j.anl.2010.04.005. Epub 2010 Jun 25
[PubMed PMID: 20576375]
[46]
Greco A, Gallo A, Fusconi M, Magliulo G, Turchetta R, Marinelli C, Macri GF, De Virgilio A, de Vincentiis M. Cogan's syndrome: an autoimmune inner ear disease. Autoimmunity reviews. 2013 Jan:12(3):396-400. doi: 10.1016/j.autrev.2012.07.012. Epub 2012 Jul 28
[PubMed PMID: 22846458]
[47]
Su JW, Low AH, Tay KH, Sebastian MG, Thumboo J, Sin KY. Recurrent aortic aneurysms following thoracic aortic stent-graft repair in a patient with Cogan syndrome. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2006 Dec:13(6):779-82
[PubMed PMID: 17154705]
[48]
Gasparovic H, Djuric Z, Bosnic D, Petricevic M, Brida M, Dotlic S, Biocina B. Aortic root vasculitis associated with Cogan's syndrome. The Annals of thoracic surgery. 2011 Jul:92(1):340-1. doi: 10.1016/j.athoracsur.2010.12.068. Epub
[PubMed PMID: 21718871]
[49]
Karni A, Sadeh M, Blatt I, Goldhammer Y. Cogan's syndrome complicated by lacunar brain infarcts. Journal of neurology, neurosurgery, and psychiatry. 1991 Feb:54(2):169-71
[PubMed PMID: 2019845]
[50]
D'Aguanno V, Ralli M, de Vincentiis M, Greco A. Optimal management of Cogan's syndrome: a multidisciplinary approach. Journal of multidisciplinary healthcare. 2018:11():1-11. doi: 10.2147/JMDH.S150940. Epub 2017 Dec 22
[PubMed PMID: 29317827]