Continuing Education Activity
Ocular surface squamous neoplasia is a broad entity that comprises the spectrum of squamous neoplasms of conjunctiva and cornea. The varied malignancies include conjunctival intraepithelial neoplasia (CIN), corneal epithelial dysplasia, squamous cell carcinoma (SCC), and mucoepidermoid carcinoma. The clinical manifestation can range from an ocular mass, excessive irritation, congestion, prominent feeder vessels, and reduced visual acuity. The diagnosis rests on histopathological evaluation of the excised mass and imaging to rule out infiltrative neoplasms. A high index of clinical suspicion, prompt diagnosis, meticulous management, and regular follow-up post-surgical excision results in an excellent outcome. This review describes etiology, risk factors, clinical features, investigations, imaging modalities, treatment options, differential diagnosis, and complications of ocular surface squamous neoplasia.
Objectives:
- Describe the etiology of ocular surface squamous neoplasia.
- Summarize the evaluation of ocular surface squamous neoplasia.
- Outline the management of ocular surface squamous neoplasia.
- Review the differential diagnosis and complications of ocular surface squamous neoplasia.
Introduction
Ocular surface squamous neoplasia (OSSN) is an important pathological entity for clinicians since it closely mimics common conjunctival and corneal surface pathologies like pinguecula, pterygium, conjunctival granulomas, and cysts.[1] OSSN should not be neglected because of its high potential to cause ocular and systemic morbidity. The history of OSSN dates back to 1860, when it was first described by Von Graefe.[2]
Since then, OSSN has been extensively studied with the evolution of new treatment modalities. OSSN can have purely conjunctival involvement, purely corneal involvement, or conjunctival tumors extending over the cornea. The malignancies can range from conjunctival intraepithelial neoplasia (CIN) to squamous cell carcinoma.[3]
The squamous neoplasms have further been classified as non-invasive and invasive types.[4] Pizzarello and Jakobiec first described the term CIN that is similar to gynecologic tumor terminology of intraepithelial neoplasia.[1] They subclassified CIN as mild, moderate, and severe dysplasia based on the extent of involvement. The lesion with less than one-third conjunctival involvement is classified as mild, with the inner two-thirds is labeled as moderate, and full-thickness means severe dysplasia. Lee and Hirst were the first to propose a classification system for OSSN encompassing all carcinomatous and dysplastic lesions of the ocular surface.[5] The classification is as follows-
- Benign OSSN- pseudotheliomatous hyperplasia, benign hereditary intraepithelial dyskeratosis and, papilloma
- Preinvasive OSSN - conjunctival/corneal intraepithelial neoplasms grades I–III
- Invasive OSSN- squamous carcinoma, mucoepidermoid carcinoma
Etiology
The etiology of OSSN is multifactorial. Various studies have implicated human papillomavirus (HPV), ultraviolet B (UVB) light rays, human immunodeficiency (HIV) 1 and 2 viruses, and Hepatitis B and C as the most common associations. The various predisposing factors are chronic cigarette smoking, use of petroleum products, hypopigmented hair and eyes, xerophthalmia (vitamin A deficiency), chemicals like arsenic and beryllium, ocular surface trauma, and people from British, Austria, and Switzerland are at high risk.[6][7][8][9]
Another etiological factor is the failure of the DNA repair mechanism as seen in xeroderma pigmentosa, which is linked with OSSN, UVB rays induce p53 gene mutations, and various studies have reported raised nuclear p53 in individuals with OSSN. Lee et al., in their analysis, reported pale skin, hypopigmented iris, and high incidence of sunburns as the probable risk factors for OSSN.[10] HPV 6 and 11 have been reported to cause conjunctival papilloma and other dysplastic and malignant lesions of the ocular surface, and HPV 16 and 18 are closely linked with CIN.[6]
Epidemiology
OSSN has variable regional incidence. Previous epidemiological studies have reported 0.13/ lakh incidence of OSSN in Uganda and less than 0.2 cases/ million/ year in the United Kingdom. The incidence in Brisbane, Australia, has been reported to be 1.9/100000 population.[5] A high incidence has been reported in Whites, varying from 90 to 100%. Gichuhi et al. reported the highest incidence of OSSN in Africa across the world with equal male and female preponderance.[11][12]
Sun et al., in their analysis, reported a 5 times higher incidence of OSSN in Qhirwa and showed that darker skin individuals residing in tropical climates close to the equator have more incidence of OSSN.[13] Newton et al. reported how OSSN incidence varies with solar UV radiation distribution.[14] They showed that the incidence of squamous cell carcinoma was reduced by 49% with each 10-degree difference in latitude.[14] The OSSN has primarily been reported in older males (approximately 80%).[2]
Lee and Hirst, in their analysis, reported the average age of occurrence of OSSN to be 56 years (range 4 to 96 years).[15] The average age of occurrence of carcinoma is 5 to 9 years younger than intraepithelial neoplasia. In the west, OSSN has been reported frequently in White race men who reside close to the equator in their sixth and seventh decade. In the Asian subcontinent, it is reported more common in younger lineage associated with HIV and xeroderma pigmentosa.[16] Out of the total oculo-orbital tumors, OSSN account for nearly 4 to 29%.[5] OSSN has been listed third most common tumor after melanoma and lymphoma.[15]
Pathophysiology
Limbal Transition Zone/Stem Cell Theory
The nasal limbus in the eye receives the highest intensity of UV B rays. The nasal limbus has epithelial crypts and contains limbal stem cells in the basal epithelial layer. These are labeled as precursor cells for OSSN. OSSN initially arises from basal epithelial cells and then spreads towards the ocular surface and later invades the basement membrane.[17]
UV radiations cause DNA damage. This results in the formation of pyrimidine dimers in the DNA base chain. There is a transformation of the p53 tumor suppressor gene in OSSN. The UV B radiations have been implicated in the reactivation of the HPV virus and also cause systemic and local immunosuppression. It has been reported that E7 proteins of the HPV genome cause proliferation of infected epithelial cells with the assistance of the retinoblastoma gene, and at the same time, E6 proteins restrict the p53 gene in causing cell cycle arrest of infected and damaged cells.[18][19]
Additionally, the immunosuppression induced by HIV, xerophthalmia, and UV B radiations impairs immune surveillance of tumors, thus promoting the survival of aberrant cells. The increase in tumors size and metastasis is promoted by telomerase reactivation. The activation of vascular endothelial growth factors and matrix metalloproteinases also destroy the cellular matrix. The mechanism by which HPV reaches conjunctiva still needs to be elucidated.[20][17]
Histopathology
The specimen for histopathological evaluation (HPE) can be obtained either by in toto excision (small lesions) or incisional biopsy (large infiltrative lesions). The papillomas on HPE show papillary fibrovascular fronds with acanthotic epithelium covering them. Pediatric papillomas are a mixture of goblet cells and neutrophils contained in the epithelium. Adult papillomas are characterized by epithelial dysplasia, nuclear enlargement, raised nuclear-cytoplasmic ratio, and mitotic figures. The preinvasive OSSN is classified on HPE as mild, moderate, and severe based on the degree of dysplastic epithelium involvement.
- CIN grade I: Mild - dysplasia involving the lower one-third of the epithelium.
- CIN grade II: Moderate - dysplasia involving the middle third.
- CIN grade III: Severe - full thickness dysplasia, also labeled as carcinoma-in-situ.
Invasive OSSN is characterized by islands of infiltrating cells that usually penetrate the epithelial basement membrane and invade into the conjunctival stroma. These cells can be well- differentiated well and are labeled as squamous or poorly differentiated, which are difficult to distinguish. In squamous cells, two types of cells are seen: mucoepidermoid cells and spindle cells.[21]
Electron Microscopy
OSSN on detailed electron microscopy depicts numerous mitochondria, endoplasmic reticulum, tonofilaments, reduced number of desmosomes, altered basement membrane, and fibrillogranular material between the bowman and basement membrane.[2]
History and Physical
The patient with OSSN typically presents to the outpatient department with complaints of the presence of the fleshy mass or a round lump in the eye, excessive irritation, redness, foreign body sensation, itching, reduced visual acuity due to astigmatism or visual axis involvement. The conjunctival OSSN is present in the bulbar conjunctiva, which may slowly encroach over the limbus and cornea. It can rarely also present as bilateral or multifocal mass.[8]
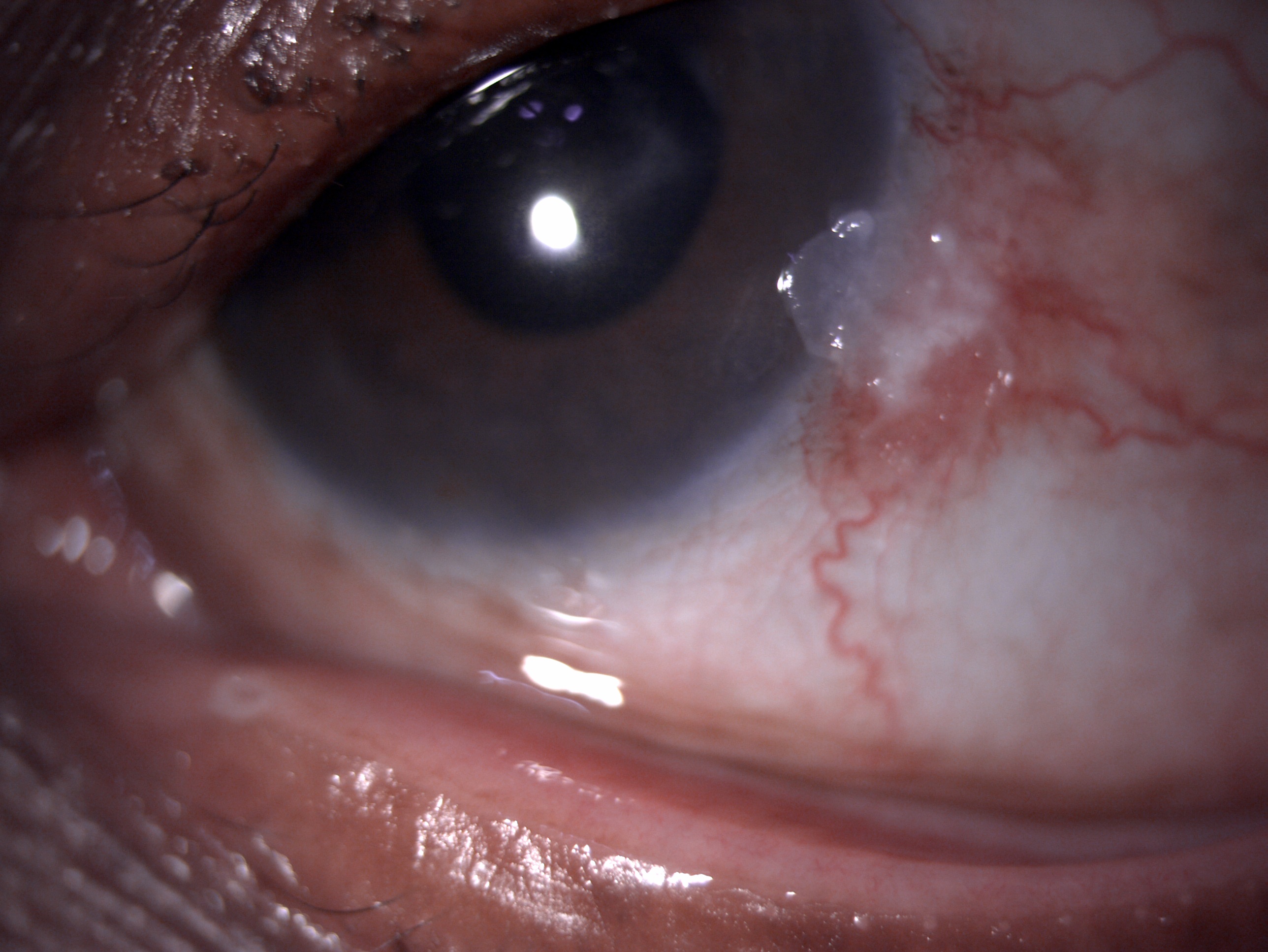
OSSN is clinically defined as an elevated grey white lesion with irregular borders and having a tuft of blood vessels. It usually has a major blood vessel associated with it called the sentinel or feeder vessel.
OSSN Morphological Classification
- Papillomatous - Benign OSSN, pink to cherry red, exophytic, strawberry-like. They are stippled red in appearance, and the red areas are due to the fibrovascular core of the lesion. They have bimodal age distribution and growth characteristics. In the pediatric population, they pedunculated multiple lesions involving fornix and caruncle or eyelid margin. In adults, they are sessile, single present in conjunctiva and limbus.
- Leukoplakic - They are preinvasive. These have surface hyperkeratinization associates with the thickening and whitish appearance of the tumor.
- Gelatinous - They are circumscribed and have the hairpin configuration of conjunctival vessels. They are divided into nodular and diffuse types. The nodular type is localized, has well-defined margins, and spread to nearby lymph nodes. In contrast, the diffuse type is less common and involves conjunctiva masquerading as chronic conjunctivitis. This is slow-growing, with tumefaction occurring late in the disease process.[22]
The various morphological patterns of OSSN show considerable overlap, and it usually becomes difficult to differentiate benign from preinvasive and invasive masses. The large tumor masses invading deeper structures are usually suggestive of malignancy.
Corneal OSSN
They are preinvasive. The surface is mottled, translucent, opalescent, and has a ground glass sheet kind of appearance. The margins are sharply defined and have fimbriated or pseudopodia-like borders. They are avascular. The corneal involvement is due to the spread of abnormal epithelial cells from the nearby limbus. The convex leading edge spread in the form of an advancing arc. In addition, white dots are seen over the greyish epithelium. Corneal OSSN is either stationary or slow-growing, indolent, and known for recurrence.[23]
Squamous cell carcinoma (SCC) has the same morphological presentation as CIN, but the conjunctival mass is more plaque-like elevated, and immobile. Bilateral lesions are rare but may be keratinized and papillary in appearance. They have feeder vessels invading the SCC, which is suggestive of epithelial basement membrane disruption.[24]
Mucoepidermoid carcinoma is a more aggressive variant and mimics SCC clinically. This is usually misdiagnosed and picked up after multiple recurrences of SCC. It can arise anywhere on the conjunctiva and ocular surface. They are more invasive than SCC and can have metastases to regional lymph nodes.[25]
Evaluation
Exfoliative And Impression Cytology
Exfoliative cytology is usually performed using a cytobrush as malignant cells tend to have a low cell to cell adherence and also desquamate when present on the mucosal surface. In contrast, impression cytology is done by using cellulose acetate paper (CAP) and is simple and inexpensive as exfoliative cytology.[26] The main advantage is that the cell-to-cell relationship is maintained. It is the most commonly used non-invasive method for performing a conjunctival biopsy in cases of suspected OSSN. The CAP specimens have shown an 80% correlation between impression cytology, histopathology samples, and suspected diagnosis from the specimens obtained by incisional biopsy. Another method of impression cytology is by using a biopore membrane.[27] The advantage is that the specimen can be stored for several days, and the cell-to-cell relationship is not disturbed. The intraepithelial group shows keratinized dysplastic cells, hyperkeratosis, nonkeratinized dysplastic cells, and syncytial groupings. In the invasive group, significant keratinization and prominent nucleoli have been described. The intraepithelial and invasive groups show numerous keratinized cases. They also help in monitoring the regression of the lesion and response to various chemotherapeutic agents.[28]
Diagnostic Vital Dyes
The various dyes used to assist OSSN diagnosis are rose bengal, toluidine blue, and methylene blue.
Rose bengal helps to satin unhealthy and dead devitalized epithelial cells bright pink. It stains OSSN lesions and helps in distinguishing the abnormal epithelium. The other pathologies with abnormal epithelium also get stained. Hence this has high sensitivity and low specificity.[29]
Due to high affinity, toluidine blue is a metachromatic acidophilic dye stain nuclear material (high density). The OSSN mass having high mitotic rare and less intercellular adhesions also stain well with toluidine blue. Gichuhi et al., in their analysis for OSSN, proved that 0.05% toluidine blue is safe, has no toxicity, and produces only little discomfort with topical application. They found a high sensitivity of 92% and a low specificity of 31% toluidine blue for OSSN diagnosis.[30]
The third dye which aids in the diagnosis of OSSN is methylene blue which is an acidophilic dye. This dye can penetrate cell walls and easily binds to nucleic acid. This has a high affinity for OSSN cells with a rapid metabolic rate. Steffen et al., in their prospective analysis of 75 patients, found 97% sensitivity and 50% specificity of methylene blue for OSSN lesions.[31]
These vital have a good negative predictive value and easily exclude the diagnosis of OSSN. Their main application is during surgery to localize a lesion and margins for excision.
Anterior Segment Optical Coherence Tomography (ASOCT)
Although it is very well known that OSSN is sometimes difficult to diagnose clinically on routine slit-lamp examination, excision biopsy and histopathological examination are warranted to subclassify the tumor or when the diagnosis is in doubt. The introduction of ASOCT in OSSN diagnosis came as a boon for all ophthalmologists providing higher axial resolution and rapid scanning speed, thus improving the tumor diagnosis. It is a very vital tool for the cross-sectional evaluation of OSSN. Recently, newly built non-invasive, non-contact, spectral-domain, ultra-high-resolution ASOCT has been built up to map OSSN involving all the corneal layers easily. It reveals any epithelial thickening, epithelial activity, stromal invasion, delineate tumor margin, and normal from abnormal tissue. It also helps to locate the depth of the tumor.[32]
Confocal Microscopy
Cytopathological evaluation of OSSN with the help of confocal microscopy is a relatively safe, simple, and non-invasive technique but has been underused due to limited availability and research in this area. Confocal microscopy can help in early diagnosis, follow-up, and estimating the recurrence of OSSN. It also helps to assess the treatment response of various topical chemotherapeutic agents in corneal and conjunctival OSSN. The newer generations confocal microscopes and high axial resolution of 4, but in contrast to ASOCT, they provide only a transverse view without delineating neighboring corneal layers.[33]
In cases of SCC, high-frequency ultrasound helps assess the extent of invasion into the eye.
Treatment / Management
The main aim of the treatment is to eliminate the tumor, prevent recurrences, safeguard the vision, and prevent any untoward medical or surgical complications. Till a few years back, surgical excision was the only standard treatment for OSSN, but tremendous research in the medical management of OSSN has proved wonders for treating physicians and ophthalmologists.
Medical Treatment
The medical management is subdivided into chemotherapeutic agents and immunotherapy.
Chemotherapy - There are various advantages of topical chemotherapy, which include-
1.The entire ocular surface is treated in a single application; hence there is no need to worry about obtaining clear tissue margins as in the case of excisional biopsy
2. It selectively targets the tumor cells. Hence the risk of limbal stem cell deficiency is eliminated as it is ocular with extensive surgical resection.
3. Chemotherapy is simple, cost-effective, and is useful in recurrent cases as compared to surgery,
The disadvantage can be limited penetration as in cases of invasive squamous cell carcinoma.[34]
Various important chemotherapeutic agents for OSSN include:
1. Mitomycin C (MMC)- It is useful for primary and recurrent cases of OSSN. It is antitumor antibiotics that inhibit DNA synthesis and are effective in the G1 and S phase of the cell cycle. The usual dose of MMC is 0.02% to 0.04% 4 times per day for 1 week, followed by 1 week drug holiday. Three cycles are recommended with a maximum of 8 cycles. Some studies recommend repeat cycles at 4 to 6 weeks intervals. The 1 week on and off regimen prevents damage to slowly proliferating epithelial cells and limbal stem cells, allowing the cells to repair their DNA and prevent corneal epitheliopathy, cataract, glaucoma, and scleral necrosis. MMC induces apoptosis and necrosis and produces cell death. The effect of MMC lasts for at least 8 months, and changes persist on the surface epithelium.[23]
2. 5 Fluorouracil (FU) - It is an antimetabolite and acts on the S phase of the cell cycle. It prevents DNA and RNA synthesis after conversion to 5-F DUMP, which inhibits thymidylate kinase. The dose of 5-FU is four times 1 week on and 1 week off cycle which. This regimen is better tolerated and has good efficacy.[35]
Immunotherapy
Interferon Alpha2b (INF-a2b)
The immunotherapeutic agent employed in the treatment of OSSN is Interferon alpha2b (INF-a2b). It is a natural glycoprotein that attaches to cell surface receptors and affects intracellular events. Interferons have antiviral and antitumor activity. It has been suggested that there is an oncogenetic link between HPV and OSSN; hence it is considered efficacious in the treatment. Off-label topical and subconjunctival interferon-a2b (INF-a2b) has been used for both primary and recurrent OSSN. It has also been used for large, diffuse, recalcitrant, and multifocal lesions. The toxicity profile is more than chemotherapeutic agents; hence it is never used as first-line therapy. Topical IFN-alpha 2b is used as 1 million international unit/ml (IU/ ml) four times a day till the subsidence of the tumor and one month thereafter. The subconjunctival dose is 3 million IU/ml/. The major advantage of interferons is that they help treat the microscopic disease present throughout the ocular surface.[36][37]
Pegylated Interferon Alpha 2b
Pegylated interferon alpha 2b has also been employed as a pilot trial in treating OSSN. It was concluded that Pegylated Interferon Alpha 2b effectively treats OSSN with no to minimal side effects. A total of 3 injections were required for the complete elimination of the tumor. The practical disadvantage is the high cost of the drug, which is nearly 3 times that of interferons.[38]
Radiotherapy
Earlier radium (gamma radiation) and strontium-90 (beta irradiation) were tried for OSSN treatment, but due to many side effects, longer duration of treatment, and varied complications, their use is restricted now.[39]
Surgical Treatment
Surgical excision is the gold standard treatment for OSSN as it assists in tissue diagnosis and targeted adjunctive medical therapy. Excision or incisional biopsy allows for tumor removal, debulking, and also histopathological analysis and diagnosis. Primary excision entails a recurrence rate of approximately 15 to 52%
No-touch Technique
The surgical technique employed for perfect OSSN removal is a no-touch technique with a margin clearance of 3 to 4 mm of uninvolved conjunctiva. The primary aim is to avoid direct manipulation of the tumor to prevent tumor seeding in the surrounding. A good conjunctival margin is needed as the uninvolved tissue may still contain the residual dysplastic cells. Absolute alcohol is used to loosen the corneal epithelium from underneath the basement membrane. Post alcohol application, the surface is rinsed with saline for 30 to 40 seconds to prevent toxicity due to absolute alcohol. The loosened tumor tissue is scraped from the corneal surface with the help of either a crescent blade or Beaver blade or surgical sponges to prevent damage to underlying Bowman’s membrane and stroma. The residual conjunctival defect can be either closed primarily if the defect is less than 3 clock hours. In case of large defects (>3 clock hours), either conjunctival limbal autograft, conjunctival autograft, or amniotic membrane graft can be used. After excision, frozen sections can be used. This will help in knowing the adequacy of excision and locate the horizontal spread of the tumor. The Mohs technique of tumor excision (Bunn’s modification) can also be used for tumor surveillance.[40]
Cryotherapy
Cryotherapy is used intraoperatively to destroy the residual tumor cells at the conjunctival margin. It acts by reducing the temperature and ischemic necrosis of cells. A cryoprobe tip of nitrous oxide (2.5 to 5 mm) is used. It forms an ice ball over 2 mm conjunctival tissue, 1 mm of episcleral, and 0.5 mm of the cornea. A double-free thaw technique is also recommended (freeze-thaw-freeze). The limbal application is avoided to prevent damage to limbal stem cell deficiency. The recommended duration of the contact is 3 seconds in one single application.[41]
Enucleation and Exenteration
Enucleation and exenteration are rarely required in cases of intraocular or intraorbital spread.[42]
Current Recommended Therapeutic Strategy
Basti et al. made recommendations based on recent advances and current treatment modalities in OSSN.[2]
- In case of suspected OSSN of 1 to 3 clock hours – The recommendation is complete excision biopsy. In case of residual tumor in margins, MMC is administered. The patient should be followed up at 3 months to evaluate tumor resolution. The next follow-up should be every 6 months. In another scenario where tumor margins have no residual cell, a 3 monthly follow-up should be done for a year and every 6 months thereafter.
- In case of suspected OSSN of 3 to 6 clock hours - The recommendation is a biopsy to assess the invasiveness of the tumor. If preinvasive: administer MMC. Follow the patient every month with 3 monthly follow up for tumor resolution. If there is a complete resolution, 6 monthly follow-ups should be done. In case of an invasive tumor, initial chemo reduction is recommended, followed by surgical excision. The defect must be covered with an amniotic membrane graft. Follow up with the patient monthly, followed by 3 monthly evaluations for tumor recurrence.
- In case of suspected OSSN of >6 clock hours, the recommendation is a biopsy to decide whether the tumor is invasive or preinvasive. The patient should be followed up monthly with 3 monthly evaluations for tumor resolution. If there is a complete resolution, a 6 monthly follow-up must be done. In case of invasive tumor- high-dose chemotherapy with MMC is recommended. If there is complete resolution, monthly follow-up for a year followed by 3 monthly thereafter. In case of partial resolution, chemo reduction followed by surgical excision with cryotherapy is recommended. The residual defect is covered with an amniotic membrane graft.
- Thereafter monthly follow-up, with 3 monthly evaluations to confirm the absence of recurrence. Later, every 3 months, follow up. In the case of greater than 6 months after chemotherapy, palliative radiotherapy must be done.
Differential Diagnosis
- Actinic keratosis
- Benign intraepithelial dyskeratosis
- Conjunctival/ tarsal cyst
- Conjunctival haemangioma
- Keratoacanthoma
- Malignant melanoma and nevi
- Pannus
- Pinguecula
- Pterygium
- Pseudoepitheliomatous hyperplasia
- Pyogenic granuloma
- Xerophthalmia (vitamin A deficiency)[2]
Prognosis
The overall prognosis of OSSN with perfect margin clearance is usually good and has a very less recurrence rate. Incompletely excised tumor margins, old age, deeper tissue penetration, corneal OSSN, and large tumors (greater than 2 mm) are factors that are usually responsible for recurrence, and the prognosis is guarded in these cases. Recent medical and surgical treatment modalities have proven to be effective. The reported local recurrence rate is 5%, and lymph node metastasis is less than 2%. The invasive and mucoepidermoid carcinoma have a worse prognosis.[43]
Complications
Medical
- Drug toxicity
- Keratitis
- Scleral melt
- Sclerokeratitis[44]
Surgical
- Recurrence
- Scleral necrosis
- Scleral melt
- Dellen
- Corneal thinning
- Corneal perforation[45]
Postoperative and Rehabilitation Care
Post excisional biopsy, the patient must be managed with topical steroids and lubricants in tapering doses. Mitomycin C 0.02 to 0.04% one week on and one week off cycle is usually started once the histopathological diagnosis has confirmed OSSN. The patient must be followed up at regular intervals to look for any signs of recurrences or toxicity due to chemotherapeutic agents. The treating ophthalmologist, optometrists, and nurses have a key role in the overall visual and cosmetic rehabilitation of the patient. The patient must be explained the side effects of topical steroids, chemotherapy, and immunotherapy and report to the ophthalmologist for any complications.[46]
Consultations
Ocular surface squamous neoplasia (OSSN) commonly mimics numerous ocular surface pathologies, as listed above. It requires a high index of clinical suspicion to pick it up early. All cases of OSSN must be referred to a cornea and ocular surface specialist for expert opinion and appropriate medical and surgical management.
Deterrence and Patient Education
All patients must be educated regarding the need for surgical excision and targeted medical therapy. The patients should be explained that excised mass can be a surface tumor and need timely and appropriate treatment. The need for regular follow-up post-surgery must be stressed. Additionally, since OSSN is associated with HIV, HPV, and Hepatitis B, the need for blood investigations must be explained to the patient. The patient should be educated to prevent direct excessive sunlight exposure and the side effects of medical therapy. In the end, the patient should be told that OSSN has an overall good prognosis and outcome is good in the majority of the cases.
Pearls and Other Issues
To conclude, ocular surface squamous neoplasia is a disease affecting the limbal stem cells and can have conjunctival, corneal, or conjunctivocorneal involvement. The patient usually presents with a slow-growing ocular lesion to the outpatient department. It required a high index of clinical suspicion to diagnose it. However, timely and adequate medical and surgical treatment usually salvages the eye in the majority of the cases.
Enhancing Healthcare Team Outcomes
Any case of suspected OSSN presenting to the general ophthalmologist in the outpatient department must be dealt with with a high index of clinical suspicion. The patient must be referred to a cornea and ocular surface expert who plays a critical role in early diagnosis, meticulous management, and regular follow-up with these patients. Microbiologists and pathologists are instrumental in histopathological tumor diagnosis. The nursing team also plays a critical role in explaining the drug schedule to the patient, side effects, and the prognosis of the disease. They also play an important role in uplifting the emotional and psychological well-being of the patient. Therefore, an interprofessional approach is mandated for an optimal outcome.