[1]
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International journal of oral science. 2020 Feb 24:12(1):8. doi: 10.1038/s41368-020-0074-x. Epub 2020 Feb 24
[PubMed PMID: 32094336]
[2]
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16:181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5
[PubMed PMID: 32142651]
[3]
Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. Journal of leukocyte biology. 2020 Jul:108(1):17-41. doi: 10.1002/JLB.3COVR0520-272R. Epub 2020 Jun 13
[PubMed PMID: 32534467]
[4]
Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of biological regulators and homeostatic agents. 2020 March-April,:34(2):327-331. doi: 10.23812/CONTI-E. Epub
[PubMed PMID: 32171193]
[5]
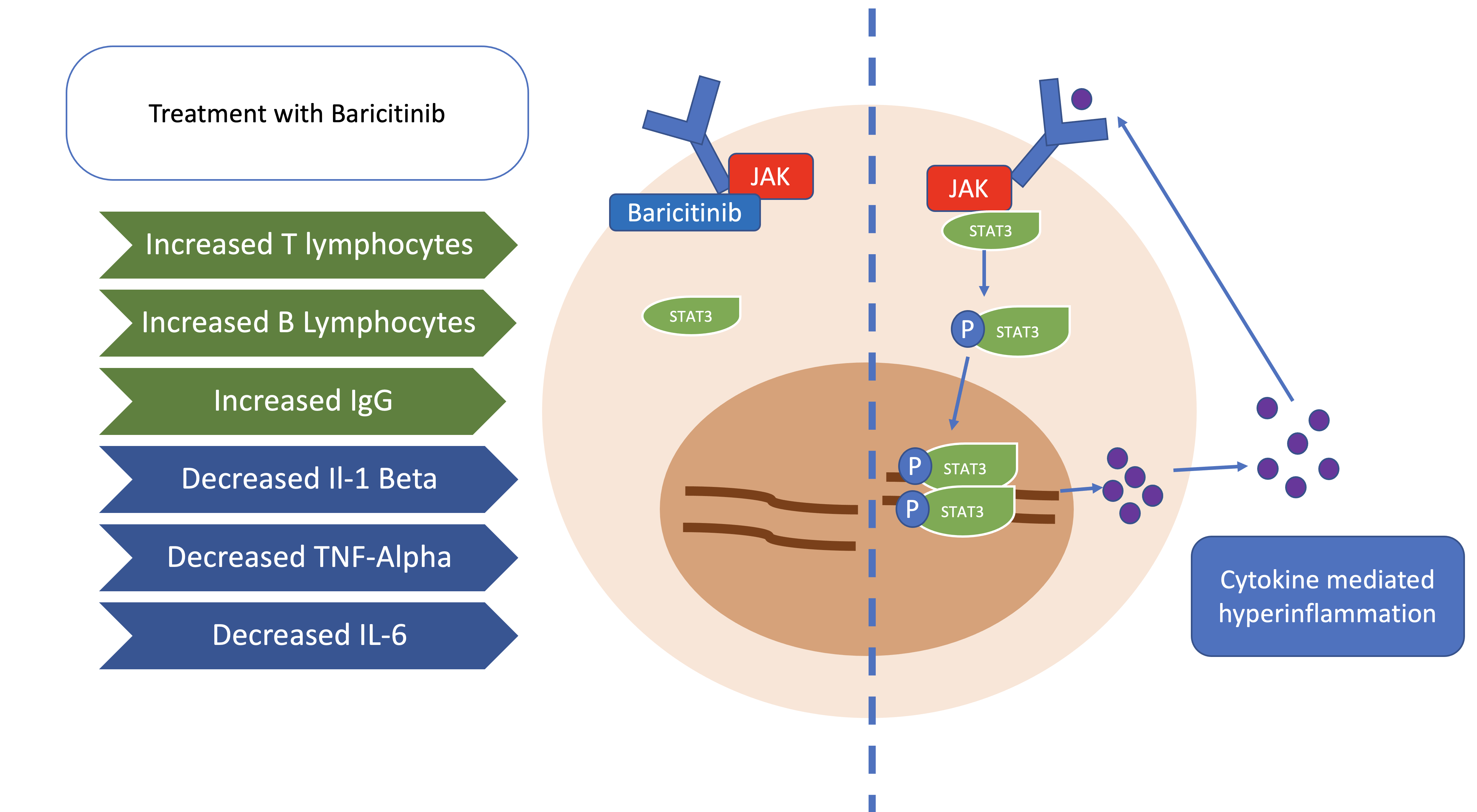
Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, Batani V, Trovato R, Fiore A, Petrova V, Hofer F, Barouni RM, Musiu C, Caligola S, Pinton L, Torroni L, Polati E, Donadello K, Friso S, Pizzolo F, Iezzi M, Facciotti F, Pelicci PG, Righetti D, Bazzoni P, Rampudda M, Comel A, Mosaner W, Lunardi C, Olivieri O. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. The Journal of clinical investigation. 2020 Dec 1:130(12):6409-6416. doi: 10.1172/JCI141772. Epub
[PubMed PMID: 32809969]
[6]
Jorgensen SCJ, Tse CLY, Burry L, Dresser LD. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy. 2020 Aug:40(8):843-856. doi: 10.1002/phar.2438. Epub 2020 Jul 27
[PubMed PMID: 32542785]
[7]
Liu KD, Gaffen SL, Goldsmith MA. JAK/STAT signaling by cytokine receptors. Current opinion in immunology. 1998 Jun:10(3):271-8
[PubMed PMID: 9638363]
Level 3 (low-level) evidence
[8]
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020 Mar 28:395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11
[PubMed PMID: 32171076]
Level 2 (mid-level) evidence
[9]
McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmunity reviews. 2020 Jun:19(6):102537. doi: 10.1016/j.autrev.2020.102537. Epub 2020 Apr 3
[PubMed PMID: 32251717]
[10]
Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford, England). 2019 Feb 1:58(Suppl 1):i43-i54. doi: 10.1093/rheumatology/key276. Epub
[PubMed PMID: 30806709]
[11]
Stebbing J, Krishnan V, de Bono S, Ottaviani S, Casalini G, Richardson PJ, Monteil V, Lauschke VM, Mirazimi A, Youhanna S, Tan YJ, Baldanti F, Sarasini A, Terres JAR, Nickoloff BJ, Higgs RE, Rocha G, Byers NL, Schlichting DE, Nirula A, Cardoso A, Corbellino M, Sacco Baricitinib Study Group. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO molecular medicine. 2020 Aug 7:12(8):e12697. doi: 10.15252/emmm.202012697. Epub 2020 Jun 24
[PubMed PMID: 32473600]
[12]
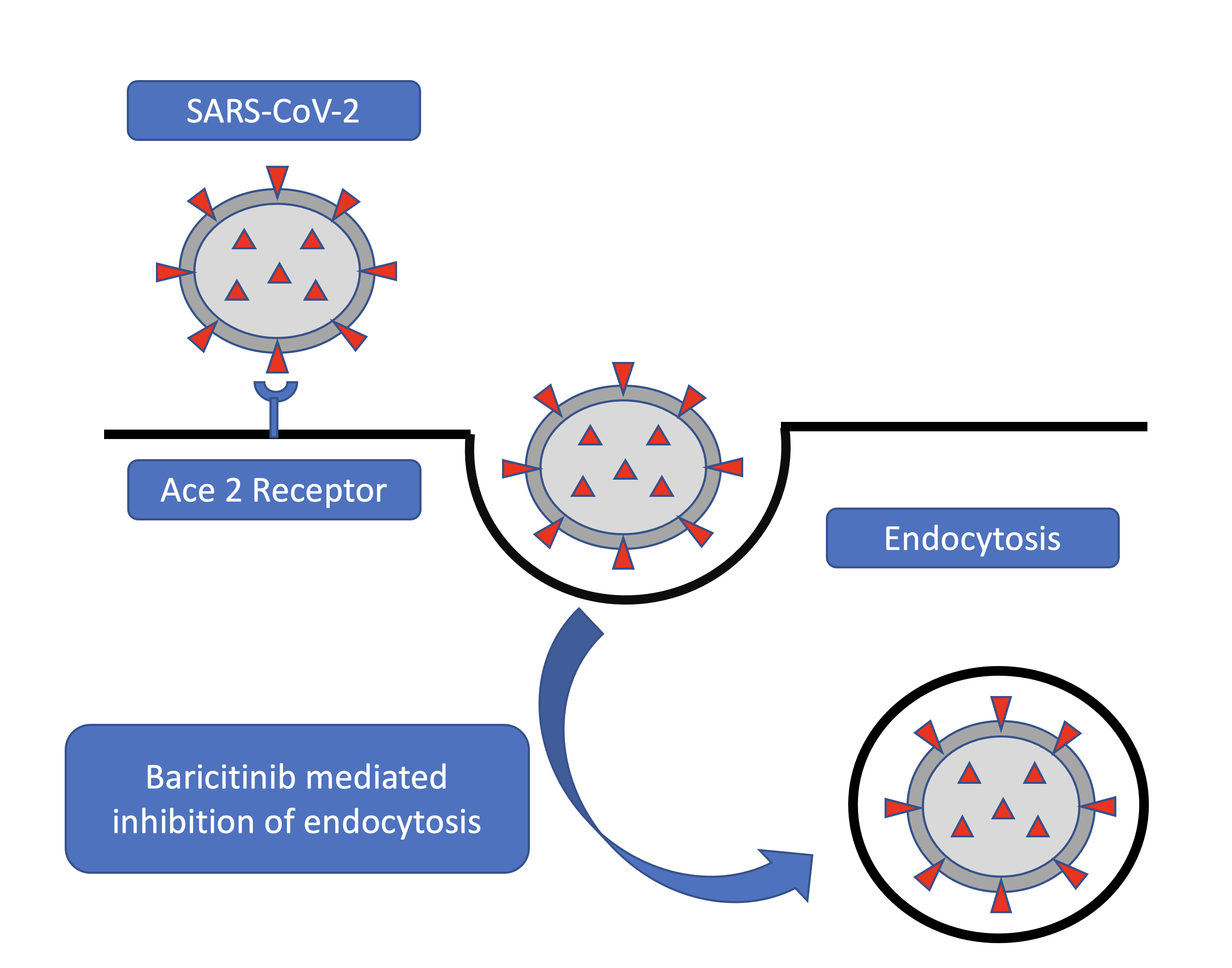
Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. Journal of virology. 2007 Aug:81(16):8722-9
[PubMed PMID: 17522231]
[13]
Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nature reviews. Molecular cell biology. 2018 May:19(5):313-326. doi: 10.1038/nrm.2017.132. Epub 2018 Feb 7
[PubMed PMID: 29410531]
[14]
Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. Journal of immunology (Baltimore, Md. : 1950). 2010 May 1:184(9):5298-307. doi: 10.4049/jimmunol.0902819. Epub 2010 Apr 2
[PubMed PMID: 20363976]
[15]
Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell research. 2008 Feb:18(2):290-301. doi: 10.1038/cr.2008.15. Epub
[PubMed PMID: 18227861]
[16]
Goletti D, Cantini F. Baricitinib Therapy in Covid-19 Pneumonia - An Unmet Need Fulfilled. The New England journal of medicine. 2021 Mar 4:384(9):867-869. doi: 10.1056/NEJMe2034982. Epub
[PubMed PMID: 33657299]
[17]
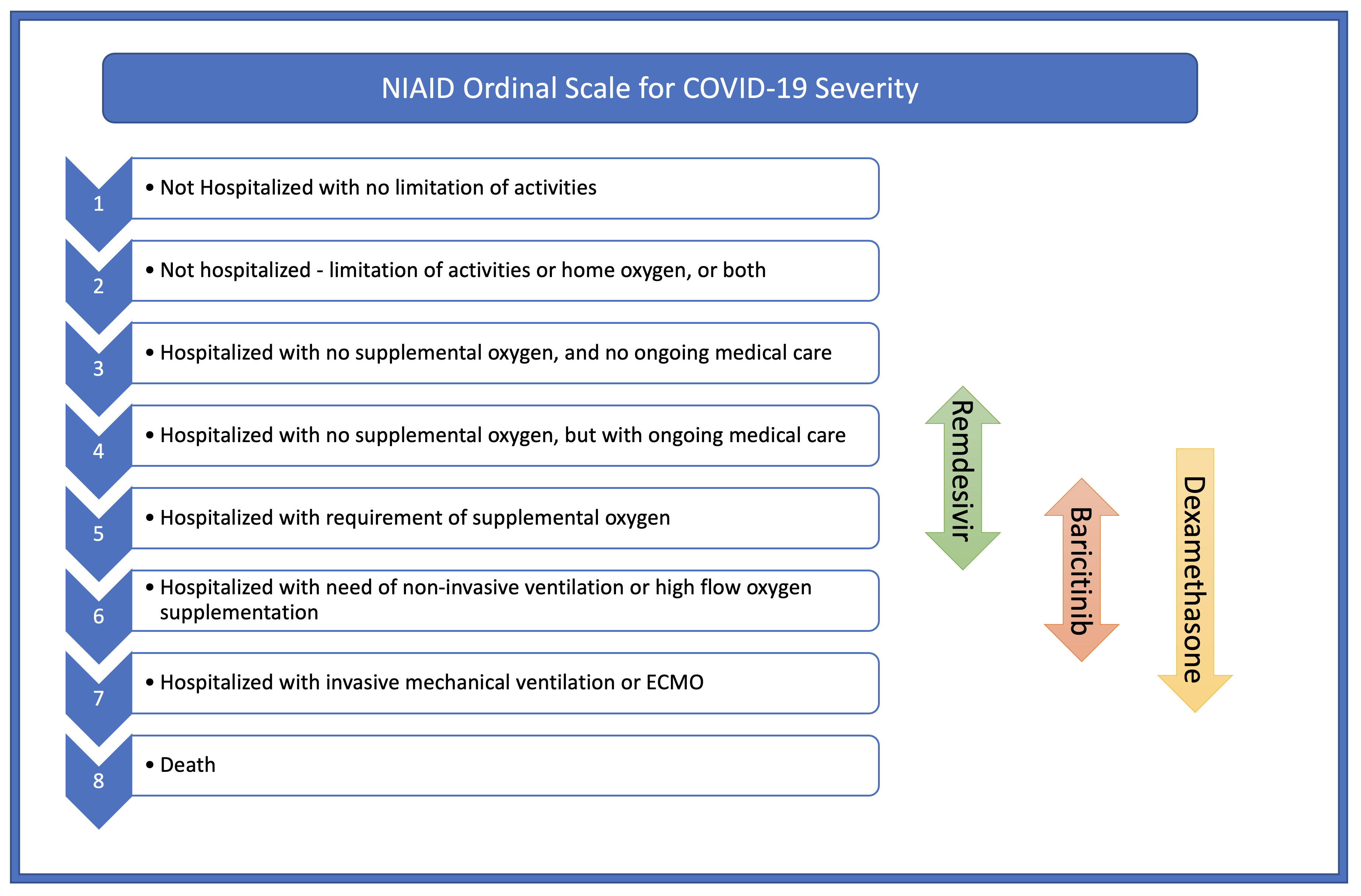
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. The New England journal of medicine. 2020 Nov 5:383(19):1813-1826. doi: 10.1056/NEJMoa2007764. Epub 2020 Oct 8
[PubMed PMID: 32445440]
[18]
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. The New England journal of medicine. 2021 Feb 25:384(8):693-704. doi: 10.1056/NEJMoa2021436. Epub 2020 Jul 17
[PubMed PMID: 32678530]
[19]
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH, ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. The New England journal of medicine. 2021 Mar 4:384(9):795-807. doi: 10.1056/NEJMoa2031994. Epub 2020 Dec 11
[PubMed PMID: 33306283]
[20]
Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford, England). 2021 Jan 5:60(1):399-407. doi: 10.1093/rheumatology/keaa587. Epub
[PubMed PMID: 33020836]
[21]
Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nature reviews. Rheumatology. 2017 Apr:13(4):234-243. doi: 10.1038/nrrheum.2017.23. Epub 2017 Mar 2
[PubMed PMID: 28250461]
[22]
Vyas D, O'Dell KM, Bandy JL, Boyce EG. Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis. The Annals of pharmacotherapy. 2013 Nov:47(11):1524-31. doi: 10.1177/1060028013512790. Epub
[PubMed PMID: 24285764]
[23]
Mogul A,Corsi K,McAuliffe L, Baricitinib: The Second FDA-Approved JAK Inhibitor for the Treatment of Rheumatoid Arthritis. The Annals of pharmacotherapy. 2019 Sep;
[PubMed PMID: 30907116]