Issues of Concern
Radiation Detectors
When radiation traverses through matter, it engages with it by imparting its energy through various processes, such as ionization or excitation of the atoms and molecules within that matter. This fundamental principle forms the basis of radiation detectors, where the measurement of ionization or excitation in matter enables the quantification of the energy deposited by the radiation.
Radiation detectors in nuclear medicine can be broadly categorized into the following 3 types:[4]
- Gas-filled detectors
- Solid-state detectors/semiconductor detectors
- Scintillation detectors
Gas-Filled Detectors
Gas-filled detectors utilize gas or air as the detection medium to monitor radiation events.[4] Signal production occurs when charged particles ionize the gas or air within the detection chamber. In these detectors, ionization produces a negative product, which is a free electron (e-), regardless of the gas used. The positive ions formed are dependent on the particular gas used. For instance, in Geiger-Müller (GM) detectors, common gases such as neon produce neon ions, whereas air generates nitrogen molecules.
The term "ion" can refer to either the positive or negative component of an ion pair. In a strong enough electric field, oppositely charged ions will separate and be collected at the device's electrodes. This separation is facilitated by applying a voltage across the electrodes.
Gas detectors are categorized into 3 main types: ionization chambers, proportional counters, and GM counters.
Ionization chambers are commonly used to measure gamma rays and x-ray radiation. However, they cannot distinguish between different types of radiation. These detectors are less effective at low exposure rates (<1 mR/h) due to their low operating voltages, making them better suited for measuring high exposure rates (from 1 to 1000 R/h). Typically, air is used as the chamber gas.
In contrast, proportional counters utilize a higher applied voltage and are particularly effective for detecting alpha and beta emitters. These detectors can differentiate the pulse length of alpha and beta emissions and are suitable for surface contamination measurements.[3][4] They offer a shorter dead time than GM detectors, making proportional counters superior for high count rates.
GM counters operate with the highest voltage among gas detectors, leading to a consistently high current.[5] These detectors are well suited for a range of exposure rates, typically from 0.1 mR/h to 1 R/h, and are effective for detecting surface contamination. They are often filled with neon gas but have a relatively long "dead" time. GM counters, however, cannot differentiate pulse lengths and thus cannot distinguish between different types of radiation.
Gas detectors are vital components in nuclear imaging and have common applications such as monitoring radiation exposure in pocket dosimeters and survey meters, as well as dose calibration of radiopharmaceuticals in nuclear pharmacies.[6][7]
In summary, gas-filled detectors are fundamental instruments that utilize gas or air for radiation detection, where incoming radiation ionizes gas molecules, producing electrons and positive ions. Electrons are gathered at the anode, whereas positive ions are collected at the cathode. The quantity of ionization is determined by measuring the current produced. The operational efficiency of these detectors is contingent upon factors such as their construction, the specific type and conditions of the gas used, and the voltage applied between the anode and cathode.
As mentioned below, the main types of gas-filled detectors are categorized by applied voltage.
Ionization chambers: Ionization chambers operate at voltages typically between 50 and 300 V, where they collect charges resulting from primary ionization.[5] However, these chambers generate a minimal current, making them unsuitable for counting radiation events. Furthermore, ionization chambers cannot differentiate between various radiation types and are less efficient for low-energy x-rays and gamma rays. However, they prove highly effective for high-energy gamma rays.
Proportional counters: Proportional counters operate at higher voltages compared to ionization chambers and induce secondary ionization through fast-moving electrons. They can amplify the current, which renders them suitable for counting radiation events. They can differentiate between radiation types based on their energy levels.[6][7]
Geiger-Müller counters: GM counters demand even higher voltage levels and ionize all gas molecules, which results in a high and constant current.[8] Although they can count radiation pulses, these counters cannot differentiate between radiation types. They may also exhibit cascade ionization, leading to repeated pulses, and to control this, GM counters use quenching methods, either electronically or with gas, to prevent continuous discharge.
The gas-filled detectors find utility in various applications. They are utilized for monitoring radiation through pocket dosimeters and survey meters.[9][10] They also serve as dose calibrators in nuclear medicine to measure radiopharmaceutical doses. However, these detectors also have limitations, as they face challenges when dealing with low-energy radiation due to their inability to penetrate effectively, and they may not detect high-energy radiation. In addition, they may require modifications to detect certain types of radiation effectively.
Solid-State Detectors/Semiconductor Detectors
A semiconductor detector gauges the interaction of charged particles with specific materials that are typically non-conductive but can transition into a conductive state under certain conditions, such as changes in structure or temperature. When ionizing radiation enters the semiconductor detector, it excites electrons, causing them to shift from their positions and generate electron-hole pairs. These pairs contain crucial information that constitutes the detection signal.
Semiconductor detectors exhibit higher efficiency than gas-filled detectors, primarily due to their increased density. Furthermore, they necessitate lower ionization energy, typically 3 to 5 eV, to generate a single ionization event. This is in contrast to gas-filled detectors, which require 34 eV to produce a single ionization and are well suited for these detectors. Examples of semiconductor detectors encompass, among others, lithium-drifted silicon and high-purity germanium.[8] These crystals are known for their high cost, demanding purity standards, and optimal performance at sub-freezing temperatures, often approximating the temperature of liquid nitrogen. However, recent advancements in detector technology have allowed for operation at room temperature, utilizing materials like telluride and cadmium zinc telluride (CZT), as opposed to silicon and germanium.[9][10] While offering advantages, these advanced detectors are constrained by their production costs and are primarily utilized in compact imaging probes and as detector elements in imaging devices.
In summary, semiconductor detectors remain non-conductive in their resting state but become conductive when subjected to ionization caused by radiation and voltage application. Semiconductor detectors find application in radiation detection, where the current they generate is directly related to the energy of the radiation, although they do not distinguish between radiation types. They are well-suited for energy-selective counting and do not necessitate a photomultiplier tube (PMT), as there is no light production within the detector's crystal. Due to their higher density, semiconductor detectors exhibit superior radiation detection capabilities compared to gas-filled detectors. In addition, they require only 3 to 5 eV to ionize, as opposed to the 34 eV required by gas-filled detectors, making them particularly effective for detecting low-energy radiations.
However, semiconductor detectors have certain limitations; for instance, there is a potential for thermal-induced current, which can introduce noise into the measurements. Moreover, impurities within the crystals can trap electrons, necessitating a requirement for high purity, and these detectors typically need storage at extremely low temperatures, around liquid nitrogen temperature (77 K or -196 ºC).[11] Although lithium-drifted detectors such as HPGe and Si(Li) offer solutions, they are challenged by storage and production complexities.
The modern alternatives to traditional semiconductor detectors include cadmium telluride (CdTe), CZT, and cesium iodide (CsI(Tl)). These advanced materials can operate at room temperature and possess higher atomic numbers than silicon and germanium.[12][13] They offer high detection efficiency, particularly for gamma rays, and maintain their efficiency even in compact sizes (up to 2 mm). Furthermore, they provide better discrimination among radiation energies, offering high energy resolution, approximately 6%, for gamma rays. However, production costs limit their widespread adoption, and they are primarily utilized in small imaging probes and as detector elements in imaging devices.
Scintillation Detectors
After being exposed to radiation, some materials can store energy. When this stored energy is released, it can create visible light. This process is known as scintillation.
Scintillation detectors harness the stored energy resulting from radiation exposure to emit photons, typically in the form of visible light. These detectors come in 2 main types—inorganic and organic.[14] Inorganic scintillators employ crystals, most commonly sodium iodide, to produce light signals upon interaction with gamma radiation. To enable room temperature operation, thallium-doped crystals are often used. Sodium iodide doped with thallium (NaI(Tl)) is the preferred scintillator for nuclear medicine imaging, particularly for detecting gamma rays in the 50- to 250-keV energy range. For applications involving higher energy ranges, such as PET imaging, scintillators with increased density are typically favored.
Although organic scintillators are less frequently used in imaging, they find applications in clinical laboratories for in vitro radioactivity measurements. Usually in liquid form, these scintillators involve adding radioactive material to the scintillator solution, enabling the detection of alpha, beta, and gamma emissions from the radioactive source.
The various types of scintillation detectors are mentioned below.
Inorganic scintillators: Inorganic scintillators are a fundamental component of radiation detection that operate in the form of crystals and scintillate when exposed to gamma rays, which can lead to photoelectric or Compton interactions. Inorganic scintillators have atoms that become ionized or excited, emitting light as they return to their resting state. The time taken to achieve this resting state is known as the scintillation decay time, a characteristic specific to each crystal. Sodium iodide, commonly used with a decay time of 250 nanoseconds, performs optimally at liquid nitrogen temperatures, although it produces minimal scintillations on its own. To address this, sodium iodide is often doped with thallium, resulting in the scintillator NaI(Tl). The light generated during the interaction of gamma rays with NaI(Tl) is converted into electric current using PMTs. Inorganic scintillators are primarily used for nuclear medicine imaging, particularly for detecting gamma rays in the energy range of 50 to 250 keV.[15] For higher energy ranges, alternative inorganic scintillators such as bismuth germanate (BGO), lutetium orthosilicate (LSO), and germanium orthosilicate (GSO) are preferred.[16]
Organic scintillators: Organic scintillators are less commonly employed in imaging and find greater utility in clinical laboratories for conducting in-vitro radioactivity measurements. These scintillators are typically in liquid form, and radioactive material is added to the solution to detect alpha, beta, and gamma emissions.[17] The process involved is known as liquid scintillation counting. The light generated by the interaction with the scintillation detector solution is converted into electric current through PMTs. However, as organic scintillators have a lower density, they result in lower detection efficiency compared to inorganic scintillators.
Photomultiplier tubes: PMTs can convert light signals from scintillators by utilizing photoemissive substances, such as cesium antimony, to transform incoming light into electrons. These electrons undergo a process of multiplication facilitated by metal electrodes known as dynodes, which multiply electrons when the voltage is incrementally increased. To operate effectively, PMTs demand a stable and substantial voltage supply.
Photodiodes: Photodiodes are constructed from semiconductors, such as silicon, and are proficient at converting light into electrons more efficiently than PMTs. They can be manufactured in smaller sizes; however, their inherent gain is limited to 1, making low-noise circuits a requirement for optimal performance.
Avalanche photodiodes represent an enhanced version, where a higher voltage difference results in increased electron energy and additional ionization. These photodiodes exhibit a substantially higher gain, typically ranging from 100 to 1000 times that of standard photodiodes.[18] As a result, they are utilized in PET/MRI hybrid systems.
Clinical Significance
The instruments commonly used in nuclear medicine are listed below.
Geiger-Müller Counters
Clinical use: GM counters find clinical application in monitoring low radiation levels, providing readings in counts per minute converted into milliRoentgen per hour (mR/h).[19]
Construction and operation: GM counters are typically constructed with an ionization chamber that operates at high voltage settings, ranging from 500 to 900 V. This chamber is made of metal, often aluminum or steel, and contains gases such as argon, alongside either halogen or methane. The detection window typically comprises mica and features an external mesh, occasionally accompanied by an aluminum cap. When radiation strikes this cap, it can lead to ionization, releasing electrons that subsequently ionize the gas molecules within the chamber. Increasing the pressure inside the chamber enhances the likelihood of ionization occurring.
Functionality limitations: GM counters have certain limitations, including the inability to differentiate between different types of radiation, as each type causes maximum ionization. Another limitation is the presence of a "dead time," typically ranging from 200 to 500 microseconds, during which the counter cannot process new incoming radiation due to continuous ionization pulses.
Quenching gases, such as organic solvent vapors or halogens, are employed to address these issues. These gases absorb electrons generated by ionizations, effectively ending the detector's "paralyzed" state. It's worth noting that organic solvents have a limited use-life, whereas halogen gases can be utilized indefinitely to mitigate these limitations.
Usage and calibration: GM counters are widely used for their portability, battery-operated design, durability, and cost-effectiveness. Some models come equipped with alarms that activate when they detect specific radiation levels, often called "area monitors," commonly used in nuclear medicine laboratories. To ensure their accuracy and optimal performance, these counters are typically calibrated using cesium-137, which emits high-energy photons at 662 keV. It is recommended to perform annual calibrations to maintain the reliability of GM counters.[20]
Detection efficiency: GM counters exhibit distinct characteristics in terms of detection efficiency. They effectively detect beta particles, achieving a 100% efficiency rate. However, their efficiency is notably lower when detecting gamma rays and x-rays, typically within the 1% to 2% range. Despite this lower efficiency, GM counters exhibit remarkable sensitivity, surpassing that of low-voltage ionization chambers by a factor of 10. This heightened sensitivity allows them to detect radiation levels up to approximately 100 mR/h.
Dose Calibrators
Clinical use: Dose calibrators serve a crucial clinical purpose, primarily used to measure radioactivity levels before administering radiopharmaceuticals to patients.
Function and construction: The dose calibrator functions as a specific type of ionization chamber, typically operating at low voltage settings of approximately 150 V. This calibrator specializes in measuring the current generated by radioactivity, notable for its absence of a dead time, thereby enabling continuous operation.
Within the dose calibrator, a sealed chamber contains argon and halogen gases maintained at high pressure ranging from 5 to 12 atm. Radiopharmaceuticals placed in a well surrounded by the ionization chamber generate varying currents dependent on the type and energy of radiation they emit.[21] Moreover, the dose calibrator features practical elements such as push buttons, settings to accommodate various radiopharmaceuticals, and an array of activity display units to facilitate accurate measurements.
Calibration and testing: Calibration and testing are essential to the dose calibrator's operation, ensuring accurate patient dosing.[22] The calibration process involves several critical steps for maintaining the precision and reliability of the dose calibrator's operation, including initial calibration performed at the time of installation. Furthermore, ongoing QC measures are implemented through constancy checks, which are carried out daily, accuracy checks performed annually, and linearity checks scheduled quarterly. In addition, geometric assessments are performed post-repairs or maintenance to confirm the device's continued accuracy and dependability. Long half-life radiotracers, such as cesium-137, are frequently utilized for constancy and accuracy checks. Notably, recalibration becomes necessary if a ±10% difference is detected between the measured and actual activity, ensuring the calibration remains precise and reliable.
The assessment of linearity in the dose calibrator involves the following 2 distinct methods:
- Decay method: This method involves measuring the maximum injected dose over a specified period, enabling the evaluation of linearity with activity decay.
- Shielding method: In this approach, lead sleeves or masks of increasing thickness are used to simulate the effects of decay, allowing for the examination of linearity under various shielding conditions.
In the context of dose calibrators, a deviation exceeding ±10% between measured and expected values indicates the need for instrument repairs.[23] Furthermore, it is essential to evaluate how sample volume and container shape impact measurements through geometric assessments. This analysis results in uniform measurement corrections to ensure the dose calibrator's accuracy and consistency.
Gamma Well Counters
Clinical use: Gamma well counters are primarily used in radioimmunoassays and for measuring radioactivity levels in various samples.[24]
Construction and components: Gamma well counters consist of a NaI(Tl) crystal with a central hole or well designed for the placement of radioactivity samples. The crystal's dimensions typically range from 5 to 23 cm in diameter and thickness. To ensure safety and accuracy, the counter is shielded with a lead ring attached to a PMT at the base. Additional electronic instruments integrated into the well counter include a preamplifier, amplifier, pulse height analyzer, and a counter, enhancing its functionality and measurement capabilities.
Benefits and challenges: Gamma well counters offer several advantages and face specific challenges in its operations. One of its notable benefits is the design of the well, which ensures that most of the radiation emitted by the sample is detected, resulting in a high geometric efficiency of approximately 99%. However, it is important to note that in cases where larger sample volumes are used, there is a potential for decreased efficiency due to radiation escaping from the well. To address this, correction factors are necessary for different sample volumes to maintain accurate measurements.
Calibration and efficiency: During the initial calibration following installation, long-lived radiotracers, such as cesium-137, are used to establish the baseline calibration. The calibration procedure includes adjusting voltage settings to identify the point at which maximum count levels are achieved, commonly referred to as high voltage calibration. Furthermore, regular calibration checks are imperative to maintain the accuracy of the counters and ensure precise measurements over time.
Gamma well counters exhibit specific detection efficiencies for various radiotracers:
- Technetium-99m: For technetium-99m, the detection efficiency is at its maximum, reaching 100%.
- High-energy radiotracers: In the case of high-energy radiotracers, such as iodine-131, the detection efficiency typically ranges from 30% to 90%, which depends on the thickness of the crystal used for measurements.
Probe Counting Systems
Thyroid probe: The thyroid probe is specifically designed to precisely count radiation emitted by the thyroid gland, serving a distinct clinical purpose. The probe's construction and components include a 5-cm-thick NaI(Tl) crystal. The probe is equipped with a PMT, preamplifier, amplifier, pulse height analyzer, and scaler, similar to the components found in the well counter. The probe is typically mounted on a stand, allowing precise positioning over the patient's neck. To isolate and focus on counts originating from the thyroid gland specifically, the thyroid probe features a 25-cm-long cylindrical lead collimator. This specialized probe is often used in measuring the total thyroid uptake following the oral administration of radiotracers, including iodine-123 or iodine-131.[25]
Miniature gamma-ray probes: These probes are specialized tools primarily used in oncological applications, particularly for sentinel lymph node scintigraphy and radio-guided surgery. Given their role in surgical procedures, these probes must be highly efficient in detecting gamma radiation and easily maneuverable during surgery.[26] The probe comprises a 10-mm-thick CsI(Tl) scintillation crystal paired with a silicone photodiode. This choice of a silicon photodiode is made to replace the traditional PMT due to its lightweight properties and compatibility with the CsI(Tl) scintillation crystal, making the probe more convenient for surgical use.
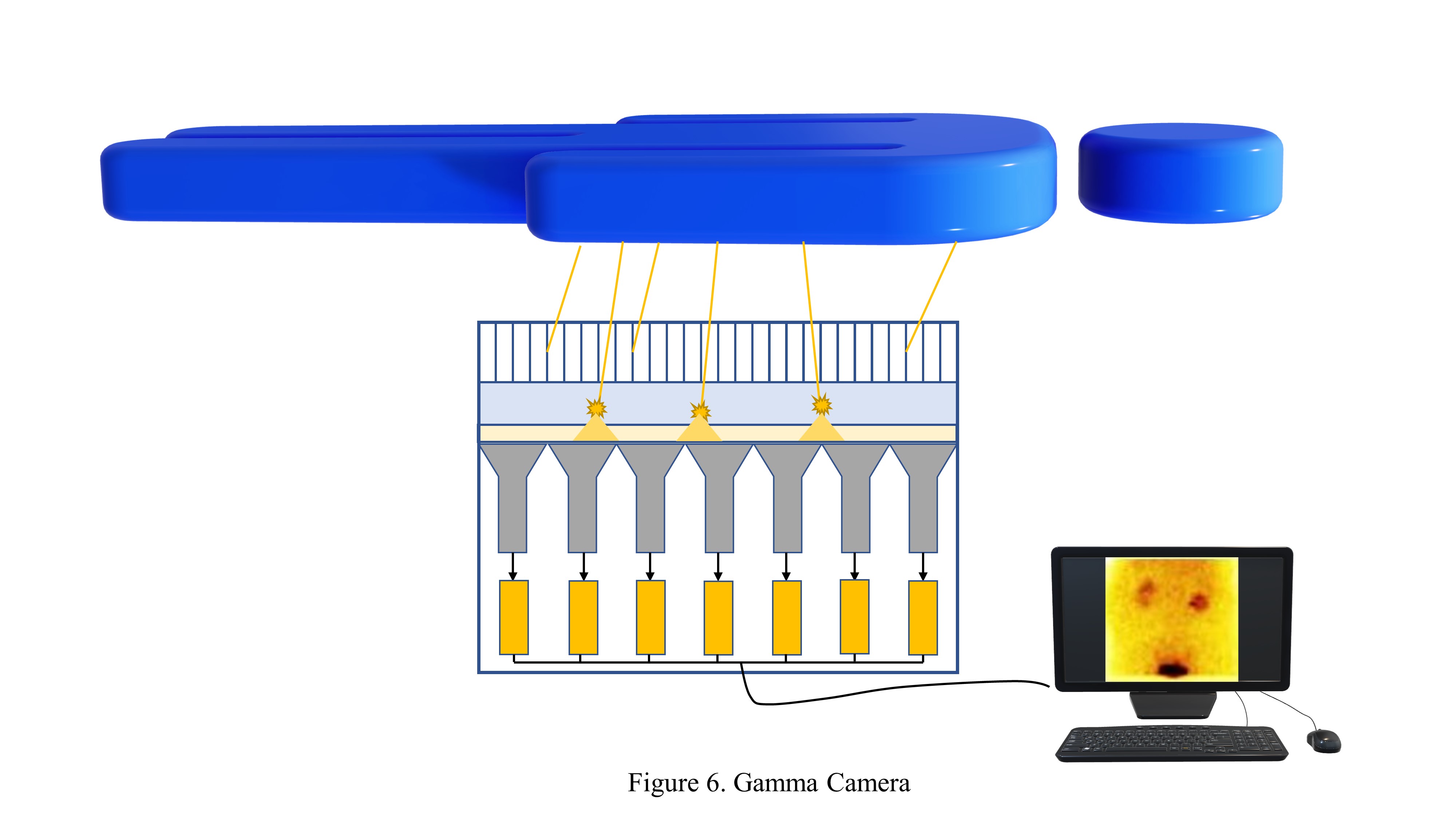
Gamma Cameras
Function and construction: The gamma camera is a critical tool in nuclear medicine, and its functionality relies on a combination of detector components, electronic instrumentation, and a computer system to deliver essential diagnostic imaging. Key performance characteristics, such as spatial and energy resolution, detection efficiency, image uniformity, and contrast, are crucial in determining the system's effectiveness in diagnosing various medical conditions.
Advancements in technology persistently refine and improve the capabilities of gamma cameras, further expanding their utility in healthcare. The initial prototype of the gamma camera by Hal O. Anger in 1958 paved the way for ongoing modifications, culminating in the development of the modern gamma camera.[27]
The contemporary gamma camera comprises 3 fundamental components—detector elements, electronic instrumentation, and a computer system.
-
Detector components: The gamma camera comprises several critical detector components, as mentioned below.
- Collimators: Collimators are made primarily from materials such as lead or tungsten for absorptive collimation to isolate the desired radiation for imaging. Four common types of collimators are used in gamma cameras—parallel hole, converging, diverging, and pin-hole. Parallel hole collimators are the most frequently utilized type for gamma cameras.[28][29] Converging collimators can zoom in and non-invert images when the radiation source is placed in focus.[30][31] Diverging collimators provide minified, non-inverted images for specific applications. Pin-hole collimators create inverted but prominent images with a single hole.[32]
- Scintillation crystal: The gamma camera features a scintillation crystal, typically a 6- to 12.5-mm-thick rectangular NaI(Tl) crystal, and is enclosed in aluminum to safeguard it against moisture.[16][33]
- Photomultiplier tube: The gamma camera is equipped with 30 to 100 PMTs situated at the rear end of the crystal, and an optical guide aids them. PMTs convert the light signals generated within the crystal into electrons. They further amplify these signals through dynodes, producing electric pulses proportionate to the radiation interactions.[34] In addition, PMTs assist in pinpointing the origin of the light signals within the crystal. [35]
-
Electronic instrumentation: The electronic instrumentation within the gamma camera performs several crucial functions. The camera involves signal processing to convert raw detector data into interpretable images. This electronic system consists of essential components, including a preamplifier, pulse height analyzer, and an amplifier to accept valid signals from the detector. Pulse height analyzers come in 2 categories—single-channel, suitable for monoenergetic radiation, and multichannel, optimized for handling multiple energy gamma.[36] The system is programmed to discern and discard unwanted or scattered radiation events, only recording the accepted signals for image generation.
-
Computer system: The computer system integrated into the gamma camera specializes in nuclear medicine imaging and is adept at managing imaging protocols, data storage, image processing, and display.[37] The system operates with customized imaging protocols in adherence to established guidelines. Images can be acquired in grayscale or color scales, and software options enable image smoothing and background subtraction. Furthermore, the system supports region of interest (ROI) selection, which can be done automatically or manually, and facilitates the generation of time-activity curves.[38] Various image types, including planar, static, dynamic, and gated, can be produced by this versatile computer system.
Performance characteristics: The performance characteristics of the gamma camera include spatial and energy resolution, detection efficiency, and image uniformity and contrast.
-
Spatial resolution: Spatial resolution is an essential characteristic that indicates the gamma camera's capacity to distinguish closely spaced points. This comprises 2 primary components—collimator resolution and intrinsic resolution.[39] Spatial resolution can be evaluated both qualitatively and quantitatively with the help of bar phantoms and the full width at half maximum (FWHM) measurement.
-
Energy resolution: Energy resolution is a significant feature that characterizes the gamma camera's ability to differentiate between radiation energies. This aspect is notably affected by statistical fluctuations in photon production. The gamma camera generally provides better resolution for higher energy gamma rays.[40]
-
Detection efficiency: Detection efficiency measures how effectively the system converts incident gamma rays into valid signals. However, it can be influenced by factors such as detector size, crystal thickness, source distance, and gamma-ray energy. The type of collimators used can also impact detection efficiency, with high-sensitivity collimators improving the detection process.
-
Image uniformity: Image uniformity refers to the consistency in gamma camera responses. However, non-uniformities can occur due to photon production, transmission, and PMT output variations. To assess image uniformity, intrinsic and extrinsic uniformity tests are conducted. These tests help ensure that the camera provides consistent and reliable imaging results.[41]
-
Image contrast: Image contrast in gamma camera imaging depends on variations in radiopharmaceutical uptake in different body regions. Techniques such as background subtraction and contrast enhancement software can enhance the contrast in the resulting images, making them more diagnostically useful.
Single-Photon Emission Computed Tomography
SPECT involves the 3-dimensional (3D) acquisition of radiation sources that emit either monoenergetic (radiations with single energy) or polyenergetic (radiations with multiple energies) photons, such as technetium 99m and iodine 131, from all around the radioactive source (see Image. Technetium 99m MDP Scan).
SPECT cameras: SPECT cameras share a design resemblance with planar gamma cameras but come with additional features. They usually incorporate multiple camera heads, often in pairs, to improve efficiency. These camera heads rotate around the patient, capturing images from multiple angles. The camera heads may follow circular or elliptical orbits depending on the specific study. In advanced systems, the cameras can estimate body contours, enhancing imaging accuracy.[42]
Acquisition: The SPECT system captures a series of "projection views" as it rotates 360° around the patient.[43] Typically, 64 or 128 views are captured via the SPECT system, which is utilized to generate cross-sectional images. Sufficient counts are essential for achieving good resolution in the images. Managing patient motion during the scan is a critical challenge; the activity is monitored through cine loops or sinograms.[44] Two acquisition methods are commonly used—step-and-shoot and continuous scanning.[45] The images acquired during the scan are subsequently reconstructed using a specific software.
Performance characteristics: The performance characteristics of a SPECT include spatial resolution, sensitivity, image uniformity and contrast, and attenuation.
- Spatial resolution: Spatial resolution is a critical performance characteristic of a SPECT system, and it quantifies the system's capacity to generate detailed images. This resolution is assessed in 2 distinct planes—axial and transaxial—allowing for a comprehensive evaluation of imaging quality.
- Sensitivity: Sensitivity is an essential performance metric in SPECT imaging, and it relies on several factors, including collimator efficiency, crystal thickness, and the energy of the gamma rays detected.
- Image uniformity: Image uniformity measures the camera's ability to provide consistent responses throughout the imaging process. Maintaining uniformity is vital for accurate diagnosis, with stringent standards allowing only a 1% level of non-uniformity, ensuring high-quality images.
- Image contrast: In SPECT imaging, the ability to enhance image contrast is a valuable feature, primarily attributed to using cross-sectional imaging techniques.
- Attenuation: Gamma rays attenuate differently depending on their depth of origin within the patient's body. To address this, corrections are often necessary to compensate for variations in gamma-ray attenuation. The advent of hybrid imaging, such as SPECT/CT, has greatly improved attenuation correction, resulting in enhanced image quality and diagnostic accuracy.
Image reconstruction: In SPECT imaging, one of the standard techniques for image reconstruction is filtered back projection (FBP).[46][47] Furthermore, iterative reconstruction methods are utilized to further enhance image quality by considering physical parameters.[48]
Specialized SPECT systems: In the realm of specialized SPECT systems, there are dedicated configurations designed for distinct clinical purposes—cardiac and brain SPECT techniques.
- Cardiac SPECT: Cardiac SPECT systems focus on cardiac imaging by utilizing specialized detector heads to enhance the accuracy of heart-related scans.[49]
- Brain SPECT: Brain SPECT systems are tailored to deliver higher sensitivity and resolution in neuroimaging, allowing for detailed exploration of brain functions and structures.[50] These specialized systems offer improved spatial resolution and contribute to studying various biological phenomena related to brain and cardiac health.
Positron Emission Tomography
Function and construction: PET imaging detects the annihilation of positrons and electrons, giving rise to 2 photons of 511 keV that travel in opposite directions. In clinical practice, positron-emitting radiopharmaceuticals, such as rubidium-82 and F-18 fluorodeoxyglucose, are administered to the patient. These radiopharmaceuticals emit positrons, which undergo annihilation with nearby electrons upon traveling a short distance (mere nanometers), producing the distinctive pair of 511 keV photons.
PET imaging utilizes photons generated during the annihilation events of radionuclides, including F-18, C-11, and O-15. PET uses coincidence imaging from the 2 photons of 511 keV to precisely triangulate and map radiotracer activity within the body. Radionuclides release positrons, which, upon annihilation with electrons, give rise to photons projecting in opposite directions. In PET imaging, the emitted photons interact with scintillation crystals, typically made of materials such as BGO, gadolinium silicate, or lutetium oxyorthosilicate. Here, whether both photons contribute to a "true" count or are too temporally distant to be recorded is determined. The photons possess sufficient kinetic energy to travel a short distance before annihilation, referred to as the mean positron range. The range of this mean positron distance varies depending on the density of the structure it interacts with. This contributes to the inherent limitation in spatial resolution, typically around 5 mm on current PET scanners.
PET cameras differ from gamma and SPECT cameras in several ways. First, they feature multiple detector rings comprising smaller units or blocks of scintillator detectors instead of the traditional camera heads. These detector rings are interspersed with movable lead septa and can be adjusted based on the study type being conducted. The detector elements comprise a block of large scintillation crystals, subdivided into smaller sub-segments or clusters of smaller crystals. Due to the high energy of 511 keV photons, these scintillation crystals need higher density than NaI(Tl) crystals. These denser crystals, such as BSO, GSO, and LSO, can detect photons more rapidly, enhancing their ability to capture coincidence events.
PET cameras are organized differently compared to typical SPECT cameras. They feature only a few PMTs that are distributed across multiple subdivisions of the detector elements.[31] These PMTs positioned behind the incident crystal receive the highest intensity of photon light, and their signals are subsequently analyzed in the pulse height analyzer to determine whether to accept or reject the signal.
Positron annihilation events are frequently positioned asymmetrically relative to the detector ring, resulting in a situation where 2 detectors may not simultaneously detect 2 coincident photons. Photons detected within a specific time window, typically around 5 to 15 nanoseconds, are considered "in coincidence."[32] Nearly 99% of the detected photons may fail to meet this criterion and are categorized as "isolated events," as they occur outside the required time frame and are consequently rejected. This process, known as "electronic collimation, " contributes to dead time.
Acquisition: Although there are efforts to isolate "true" events, image degradation can still occur due to noise from scattered photons and the simultaneous occurrence of multiple unrelated events. These random coincidence events decrease the image's resolution and can limit diagnostic image quality.[33]
PET cameras can acquire both 2D and 3D images. Typically, data obtained with the septa between the detector rings results in 2D images. However, the data can be processed to generate 3D views when the septa are removed. Septa can block the oblique photons antiparallel to the plane of the detector rings. Although this helps decrease scattered radiation, it also reduces the number of recorded events by the system, leading to lower sensitivity. This compromise is made to achieve improved image resolution.[34] When the septa are removed, the system records most of the photons, even those originating outside the plane of the detector ring, enabling data processing in a 3D format. However, this comes at the cost of reduced image resolution, as it also accepts many scattered photons.
Another crucial concept is "time of flight," which aims to compensate for differences in the distances traveled by photon pairs from the point of annihilation to the detectors. When one photon is relatively close to a detector, the other has a much longer distance to travel, and the precision of the point of annihilation is reduced. Fortunately, the resulting time difference between the incident photons can be manipulated. This time difference can be used to estimate the location of the positron annihilation event.
-
PET cameras: PET cameras are distinct from gamma and SPECT cameras, featuring multiple detector rings separated by lead septa. Within these rings, detector elements are presented, which are block detectors organized in circular or hexagonal configurations. Denser crystals such as BGO, LSO, and GSO are utilized to enhance detection efficiency and speed.[16] The arrangement of PMTs is different and coupled to block detectors.[51] PET cameras use annihilation coincidence detection to determine the origins of photons.[52]
-
Acquisition: PET acquisition focuses on capturing actual coincidence events resulting from positron annihilation, providing precise imaging.[53] The presence of scattered and random coincidence events can introduce noise, impacting image quality. PET can acquire 2D and 3D images, depending on whether the lead septa are present or removed. The time of flight measurement contributes to improved resolution, and the reconstruction process is similar to that used in SPECT imaging.[54]
-
Performance characteristics: The performance characteristics of a PET include spatial resolution, sensitivity, and attenuation.
- Spatial resolution: Spatial resolution in PET depends on detector size and is influenced by various factors that affect the accuracy of detecting annihilation events.
- Sensitivity: PET generally exhibits higher sensitivity compared to SPECT due to the absence of a collimator. However, sensitivity can vary based on camera design and the choice of scintillation crystals.
- Attenuation: Attenuation correction is crucial in PET imaging. This correction can be achieved through methods such as blank and transmission scans. The hybrid PET/CT approach provides improved attenuation correction details, although it may sometimes introduce artifacts.
Multimodality/Hybrid Imaging/Imaging Reconstruction
The data acquired from all the previously mentioned cameras undergoes processing to generate visual representations or images. In the case of conventional gamma cameras, these images are typically 2D and consist of a matrix divided into smaller pixels. The data or signals are stored in specific locations corresponding to the source of the signal. These signals are presented on the screen matrix, aligning with the respective signal locations. The matrix is divided into pixels, each assigned a distinct address or location.[35]. Each signal is associated with a specific address within the image matrix. A finer pixel size leads to improved signal localization and image resolution.
In SPECT and PET imaging, image reconstruction techniques are critical for producing high-quality 3D cross-sectional images based on the collected projection data. FBP, a commonly used technique, is an analytical reconstruction method in nuclear medicine imaging. Image reconstruction entails filtering the acquired projection data in the frequency domain and subsequently back-projecting the filtered data.[36] Although FBP is computationally efficient, it must consider physical phenomena such as attenuation and scatter. These components contribute to poor image quality.[37] However, iterative methods update the reconstructed image by continuously minimizing the difference between the acquired projection data and the forward projection of the current image estimate. These methods can integrate physical parameters, such as attenuation and scatter modeling, resulting in superior image quality compared to FBP.[37][38][39]
Multimodality imaging combines the functional and anatomical information provided by SPECT or PET with the high-resolution anatomical imaging capabilities of CT and MRI. SPECT/CT combines SPECT imaging with CT, providing functional and anatomical information in a single examination.[40] The benefits include accurate anatomical localization, improved attenuation correction, and better characterization of lesions by correlating functional and morphological information. However, SPECT/MRI holds an edge regarding superior soft tissue contrast, acquisition of functional images, and reduced radiation exposure compared to CT.[43][41][42]
Similarly, PET is combined with CT for better localization and attenuation correction of the acquired data. PET/CT is widely used in oncology for tumor detection, staging, and treatment response assessment because it combines metabolic information from PET with anatomical details from CT. [44] However, current research is dedicated to refining PET/MRI image reconstruction algorithms, enhancing motion correction methods, and creating innovative PET-compatible MRI hardware to expand clinical applications and improve image quality.[44][45] These hybrid imaging techniques represent a substantial advancement in diagnostic capabilities, promising continued enhancements in image quality, reduced radiation exposure, and expanded clinical uses.
In summary, multimodality imaging combines the functional and anatomical information provided by SPECT or PET with the high-resolution anatomical imaging capabilities of CT and MRI.
SPECT/CT: SPECT/CT is a hybrid imaging technique that combines SPECT and CT imaging, providing functional and anatomical details.[55] This integration offers several benefits, including accurate anatomical localization, improved attenuation correction, and enhanced lesion characterization by correlating functional and anatomical data. The CT scanner of SPECT/CT has an x-ray tube and a detector element arranged in a complete or partial ring. Low-dose CT scans are utilized for attenuation correction, whereas diagnostic CT scans help assess anatomical localization and lesion morphology. SPECT/MRI offers several advantages, including superior soft tissue contrast, functional imaging capabilities, and reduced radiation exposure compared to CT. This makes SPECT/MRI particularly valuable in clinical applications.[56]
PET/CT: PET/CT is a hybrid imaging modality that combines both PET and CT imaging techniques. This integration allows for enhanced localization and attenuation correction of acquired data, making it particularly valuable in the field of oncology for tumor detection, staging, and assessing treatment responses.[57] The technique also combines the metabolic data obtained from PET with the anatomical details provided by CT.
PET/MRI research: Research in the field of PET/MRI is primarily concentrated on enhancing image reconstruction algorithms and refining motion correction techniques. Furthermore, there is a focus on developing novel MRI hardware compatible with PET technology.[58] These efforts are directed toward improving clinical applications and overall image quality.
In conclusion, hybrid imaging techniques such as SPECT/CT, SPECT/MRI, and PET/CT represent significant advancements in diagnostic capabilities. They hold the potential to improve image quality, reduce radiation exposure, and expand the range of clinical applications available in the field of nuclear medicine.
Nuclear Medicine Imaging Instruments Quality Control
QC plays a crucial role in ensuring the accuracy and reliability of various instruments, including gamma cameras, SPECT/CT, and PET/CT systems.[59][60] QC protocols encompass regular tests and procedures to assess system performance, detect potential issues, and maintain optimal imaging quality. Although QC procedures may vary depending on production specifications, the below-mentioned techniques provide a concise summary of QC checks with which radiologists and nuclear medicine physicians should be acquainted.
Gamma cameras: In the realm of gamma cameras, QC procedures encompass a range of daily, weekly, and annual checks designed to uphold consistent and reliable imaging performance.
- Daily: In the daily QC routine, photopeak and uniformity tests are performed to ensure consistent image quality across the field of view.[61]
- Weekly: In the weekly QC procedures, spatial resolution and linearity tests are conducted to determine the system's resolution and identify distortions using bar phantoms.
- Annually: In the annual QC procedures, energy resolution tests are performed to differentiate between gamma photons of varied energy levels. In addition, camera sensitivity tests are conducted to measure the detection of low-level gamma emissions.
SPECT/CT: In the realm of SPECT/CT, QC procedures encompass a range of daily and weekly checks designed to uphold consistent and reliable imaging performance.
- Daily: In the daily QC routine, photopeak and uniformity tests are conducted similarly to gamma cameras.
- Weekly: Every week, operators assess the system's spatial resolution using phantoms and ensure the alignment of detectors with the axis of rotation through center of rotation (COR) assessments.
PET cameras: In the realm of PET cameras, QC procedures include daily and weekly checks to ensure consistent and reliable imaging performance.
- Daily: For PET cameras, daily QC measures involve the acquisition of a sinogram using a uniform radionuclide-filled cylindrical phantom to assess image uniformity.
- Weekly: Every week, normalization procedures are carried out by irradiating detector rings with germanium 68 to standardize counts, as per established protocols.[62]
CT scanner quality assurance: CT scanners are sophisticated medical devices used in diagnostic imaging; therefore, various quality assurance procedures are conducted regularly to uphold their accuracy and reliability.
- Daily: The daily assessments involve scrutinizing voltage or current settings, image noise, and CT number accuracy using phantoms.
- Monthly: Monthly evaluations examine spatial resolution, linearity, and mechanical factors.
Nursing, Allied Health, and Interprofessional Team Interventions
In conclusion, nuclear medicine, which traces its origins back to the late 19th century, has significantly evolved. Nuclear medicine relies on various radiation detectors, including gas-filled detectors, semiconductor detectors, and scintillation detectors. Key imaging instruments in nuclear medicine, such as the gamma camera, SPECT, and PET systems, play crucial roles in diagnosing diseases and providing valuable diagnostic information. These technologies remain on a trajectory of advancement, increasingly incorporating multimodality and hybrid instrumentation for more comprehensive and improved diagnostics.
Efficient and secure utilization of nuclear medicine equipment hinges on the collaborative efforts of an interprofessional healthcare team comprising physicians, medical physicists, nuclear medicine technologists, and nurses. In nuclear medicine, the medical team's collective expertise and shared decision-making responsibilities are pivotal in providing high-quality care, delivering precise diagnoses, and achieving positive patient outcomes.
The following multidisciplinary healthcare team members in the nuclear medicine department are expected to possess various competencies.
Nuclear Physicians
Nuclear physicians are expected to thoroughly understand nuclear medicine principles, procedures, and radiopharmaceuticals.[63] They should also possess expertise in interpreting nuclear medicine images and demonstrate proficiency in utilizing various techniques and protocols to enhance their decision-making skills.
Medical Physicists and Radiation Safety Officers
Medical physicists and radiation safety officers are entrusted with comprehensive knowledge of radiation physics, dosimetry, and safety principles. They are responsible for calibrating equipment and ensuring adherence to radiation safety guidelines.[64] Furthermore, they play a pivotal role in maintaining and assuring the precision and quality of nuclear medicine instruments.
Nuclear Medicine Technologists
Nuclear medicine technologists are expected to demonstrate a thorough understanding of nuclear medicine procedures, radiopharmaceuticals, and the operation of relevant instruments. They must proficiently operate nuclear medicine equipment, such as PET cameras and SPECT systems.[65] In addition, these technologists are responsible for equipment calibration and implementing QC measures to ensure precise and reliable results.
Nurses
Nurses are responsible for various critical tasks, including preparing patients for treatments and providing them with comprehensive information about procedures and necessary preparations. They may also occasionally assist in administering radiopharmaceuticals and continuously monitor patients for any adverse effects. Furthermore, nurses are dedicated to ensuring patients' overall care and well-being before, during, and after nuclear medicine procedures.