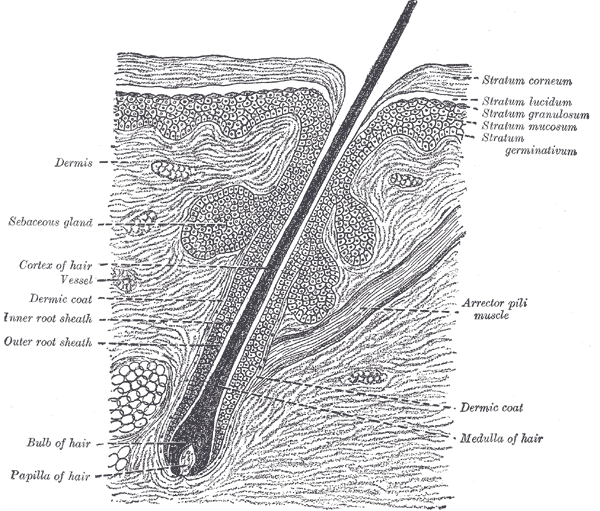
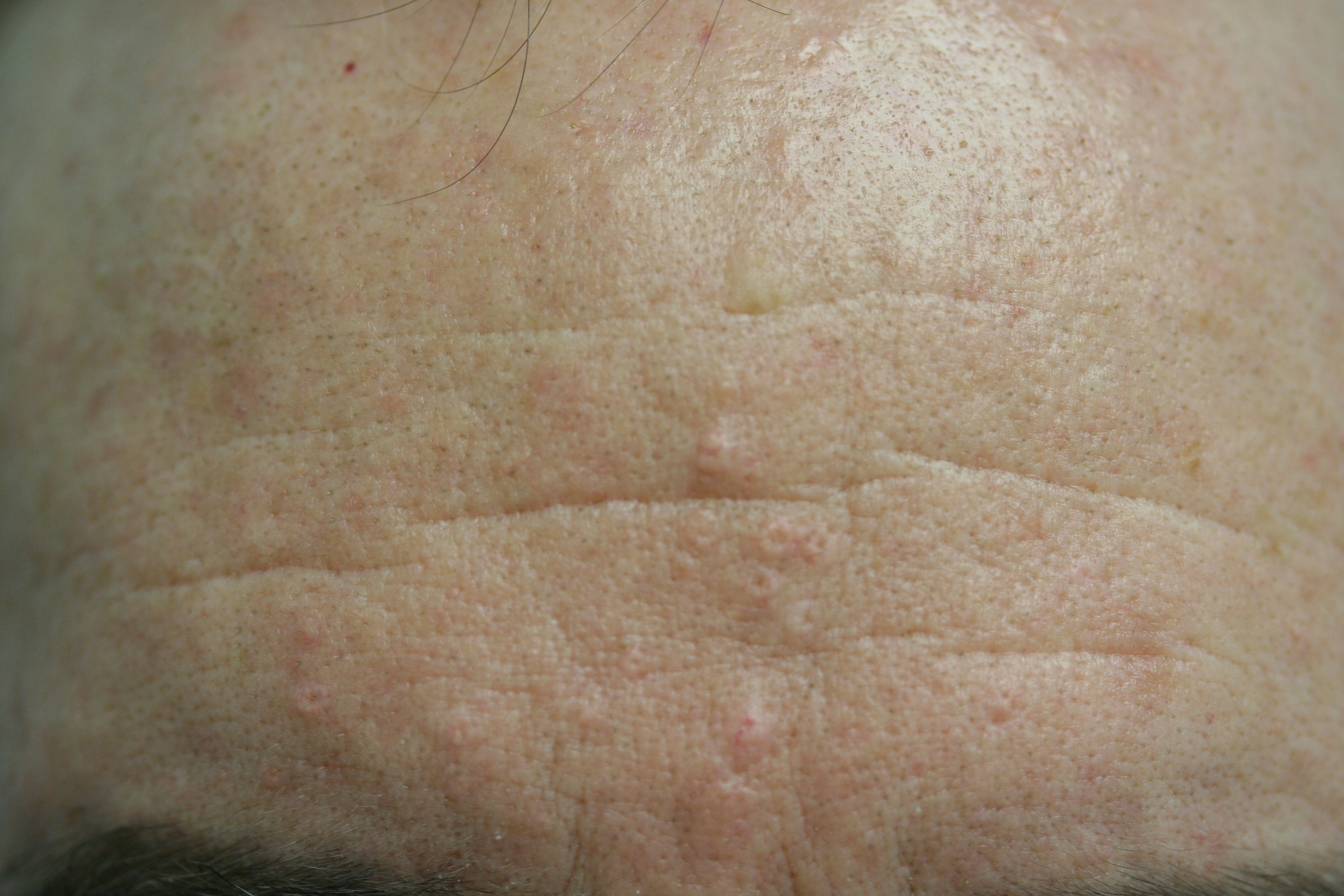
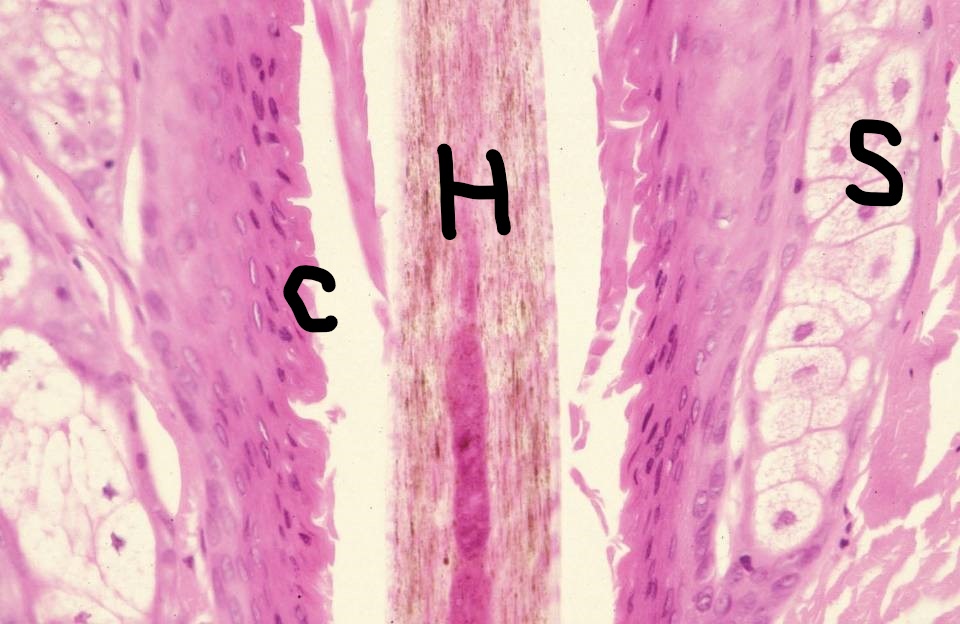
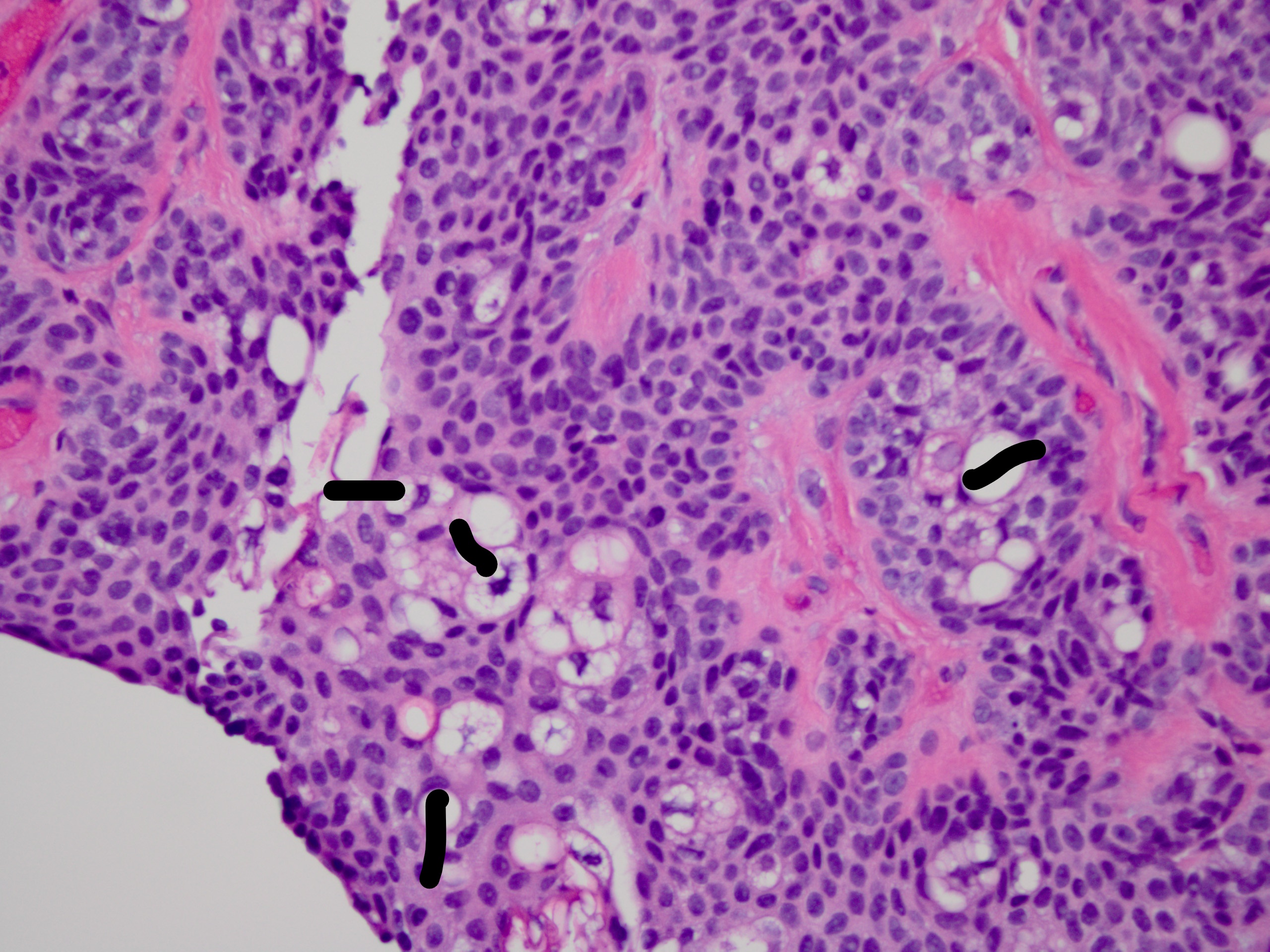
[1]
Kanada KN, Merin MR, Munden A, Friedlander SF. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. The Journal of pediatrics. 2012 Aug:161(2):240-5. doi: 10.1016/j.jpeds.2012.02.052. Epub 2012 Apr 11
[PubMed PMID: 22497908]
[2]
Moosavi Z, Hosseini T. One-year survey of cutaneous lesions in 1000 consecutive Iranian newborns. Pediatric dermatology. 2006 Jan-Feb:23(1):61-3
[PubMed PMID: 16445415]
Level 3 (low-level) evidence
[3]
Ireland AM, Harvey NT, Berry BD, Wood BA. Paediatric cutaneous adnexal tumours: a study of 559 cases. Pathology. 2017 Jan:49(1):50-54. doi: 10.1016/j.pathol.2016.10.003. Epub 2016 Nov 30
[PubMed PMID: 27914683]
Level 3 (low-level) evidence
[4]
Boschnakow A, May T, Assaf C, Tebbe B, Zouboulis ChC. Ciclosporin A-induced sebaceous gland hyperplasia. The British journal of dermatology. 2003 Jul:149(1):198-200
[PubMed PMID: 12890221]
[5]
Rosenfield RL, Deplewski D, Greene ME. Peroxisome proliferator-activated receptors and skin development. Hormone research. 2000:54(5-6):269-74
[PubMed PMID: 11595816]
[6]
Ataş H, Gönül M. Evaluation of the Efficacy of Cryosurgery in Patients With Sebaceous Hyperplasia of the Face. Journal of cutaneous medicine and surgery. 2017 May/Jun:21(3):202-206. doi: 10.1177/1203475416685076. Epub 2016 Dec 27
[PubMed PMID: 28300439]
[7]
Horio T, Horio O, Miyauchi-Hashimoto H, Ohnuki M, Isei T. Photodynamic therapy of sebaceous hyperplasia with topical 5-aminolaevulinic acid and slide projector. The British journal of dermatology. 2003 Jun:148(6):1274-6
[PubMed PMID: 12828768]
[8]
Aghassi D, González E, Anderson RR, Rajadhyaksha M, González S. Elucidating the pulsed-dye laser treatment of sebaceous hyperplasia in vivo with real-time confocal scanning laser microscopy. Journal of the American Academy of Dermatology. 2000 Jul:43(1 Pt 1):49-53
[PubMed PMID: 10863223]
[9]
Bader RS, Scarborough DA. Surgical pearl: intralesional electrodesiccation of sebaceous hyperplasia. Journal of the American Academy of Dermatology. 2000 Jan:42(1 Pt 1):127-8
[PubMed PMID: 10607331]
[10]
Rosian R, Goslen JB, Brodell RT. The treatment of benign sebaceous hyperplasia with the topical application of bichloracetic acid. The Journal of dermatologic surgery and oncology. 1991 Nov:17(11):876-9
[PubMed PMID: 1757649]
[11]
de Berker DA, Taylor AE, Quinn AG, Simpson NB. Sebaceous hyperplasia in organ transplant recipients: shared aspects of hyperplastic and dysplastic processes? Journal of the American Academy of Dermatology. 1996 Nov:35(5 Pt 1):696-9
[PubMed PMID: 8912563]
[12]
Short KA, Williams A, Creamer D, Fuller LC. Sebaceous gland hyperplasia, human immunodeficiency virus and highly active anti-retroviral therapy. Clinical and experimental dermatology. 2008 May:33(3):354-5. doi: 10.1111/j.1365-2230.2007.02670.x. Epub 2008 Mar 16
[PubMed PMID: 18346183]
[13]
Liu YS, Cheng YP, Liu CI, Yang CY, Yang CY. Presenile diffuse familial sebaceous hyperplasia successfully treated with low-dose isotretinoin: A report of two cases and review of the published work. The Journal of dermatology. 2016 Oct:43(10):1205-1208. doi: 10.1111/1346-8138.13416. Epub
[PubMed PMID: 27130181]
Level 3 (low-level) evidence
[14]
Dupre A, Bonafe JL, Lamon P. Functional familial sebaceous hyperplasia of the face and premature sebaceous gland hyperplasia: a new and unique entity. Journal of the American Academy of Dermatology. 1983 Nov:9(5):768-9
[PubMed PMID: 6643776]
[15]
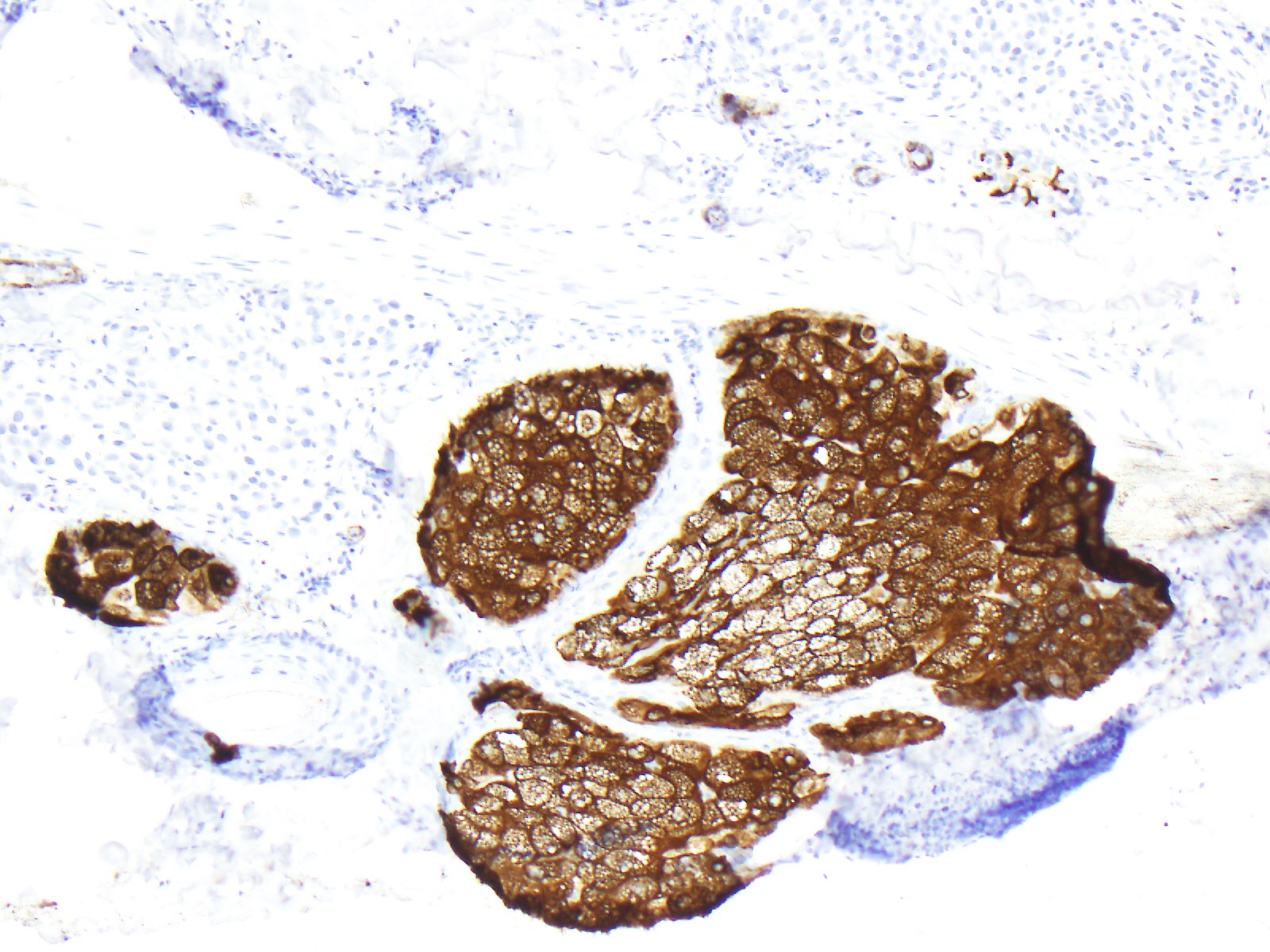
Iacobelli J, Harvey NT, Wood BA. Sebaceous lesions of the skin. Pathology. 2017 Dec:49(7):688-697. doi: 10.1016/j.pathol.2017.08.012. Epub 2017 Oct 25
[PubMed PMID: 29078997]
[16]
Coelho A, Luzar B. Superficial epithelioma with sebaceous differentiation: a case report with literature review. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2017 Sep:26(3):63-66
[PubMed PMID: 28941264]
Level 3 (low-level) evidence
[17]
Ito T, Yoshida Y, Furue M, Yamamoto O. Dermoscopic features of reticulated acanthoma (superficial epithelioma) with sebaceous differentiation. European journal of dermatology : EJD. 2012 Sep-Oct:22(5):704-6. doi: 10.1684/ejd.2012.1807. Epub
[PubMed PMID: 22858895]
[18]
Friedman KJ, Boudreau S, Farmer ER. Superficial epithelioma with sebaceous differentiation. Journal of cutaneous pathology. 1987 Aug:14(4):193-7
[PubMed PMID: 3305610]
[19]
Ostler DA, Prieto VG, Reed JA, Deavers MT, Lazar AJ, Ivan D. Adipophilin expression in sebaceous tumors and other cutaneous lesions with clear cell histology: an immunohistochemical study of 117 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010 Apr:23(4):567-73. doi: 10.1038/modpathol.2010.1. Epub 2010 Jan 29
[PubMed PMID: 20118912]
Level 3 (low-level) evidence
[20]
Uhlenhake EE, Clark LN, Smoller BR, Shalin SC, Gardner JM. Nuclear factor XIIIa staining (clone AC-1A1 mouse monoclonal) is a sensitive and specific marker to discriminate sebaceous proliferations from other cutaneous clear cell neoplasms. Journal of cutaneous pathology. 2016 Aug:43(8):649-56. doi: 10.1111/cup.12726. Epub 2016 Jun 10
[PubMed PMID: 27153339]
[21]
Groesser L, Singer S, Peterhof E, Landthaler M, Heigl U, Schneider-Brachert W, Berneburg M, Hafner C. KRAS, HRAS and EGFR Mutations in Sporadic Sebaceous Gland Hyperplasia. Acta dermato-venereologica. 2016 Aug 23:96(6):737-41. doi: 10.2340/00015555-2351. Epub
[PubMed PMID: 26804118]
[22]
Daley TD. Intraoral sebaceous hyperplasia. Diagnostic criteria. Oral surgery, oral medicine, and oral pathology. 1993 Mar:75(3):343-7
[PubMed PMID: 8469546]
[23]
Malliah R, Gilhooly P, Lambert WC, Heller DS. Sebaceous hyperplasia of the vulva: case report and review of the literature. Journal of lower genital tract disease. 2006 Jan:10(1):55-7
[PubMed PMID: 16378033]
Level 3 (low-level) evidence
[24]
Chiriac A, Moldovan C, Coros MF, Podoleanu C, Moncea D, Stolnicu S. Bilateral areolar sebaceous hyperplasia in a post-menopausal woman. European journal of dermatology : EJD. 2016 Jun 1:26(3):299-300. doi: 10.1684/ejd.2016.2739. Epub
[PubMed PMID: 27032720]
[26]
Jakobiec FA, Cortes Barrantes P, Milman T, Lee NG, Fay A. Ocular Adnexal Adenomatoid Sebaceous Gland Hyperplasia: A Clinical and Immunopathologic Analysis in Relation to the Muir-Torre Syndrome. Ophthalmic plastic and reconstructive surgery. 2020 Jan/Feb:36(1):e6-e12. doi: 10.1097/IOP.0000000000001497. Epub
[PubMed PMID: 31593035]
[27]
Roma AA, Barry J, Pai RK, Billings SD. Sebaceous hyperplasia of the vulva: a series of cases reporting no association with the Muir-Torre syndrome. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2014 Jul:33(4):437-42. doi: 10.1097/PGP.0b013e31829ff21e. Epub
[PubMed PMID: 24901406]
Level 2 (mid-level) evidence
[28]
Kruse R, Rütten A, Schweiger N, Jakob E, Mathiak M, Propping P, Mangold E, Bisceglia M, Ruzicka T. Frequency of microsatellite instability in unselected sebaceous gland neoplasias and hyperplasias. The Journal of investigative dermatology. 2003 May:120(5):858-64
[PubMed PMID: 12713593]
[29]
Ponti G, Meschieri A, Pollio A, Ruini C, Manfredini M, Longo C, Mandel VD, Ciardo S, Tomasi A, Giannetti L, Pellacani G. Fordyce granules and hyperplastic mucosal sebaceous glands as distinctive stigmata in Muir-Torre syndrome patients: characterization with reflectance confocal microscopy. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2015 Aug:44(7):552-7. doi: 10.1111/jop.12256. Epub 2014 Sep 12
[PubMed PMID: 25213213]
[30]
Spraul CW, Jakobczyk-Zmija MJ, Lang GK. [Sebaceous hyperplasia of the lower eyelid]. Klinische Monatsblatter fur Augenheilkunde. 1999 Nov:215(5):319-20
[PubMed PMID: 10609249]
[31]
John AM, Schwartz RA. Muir-Torre syndrome (MTS): An update and approach to diagnosis and management. Journal of the American Academy of Dermatology. 2016 Mar:74(3):558-66. doi: 10.1016/j.jaad.2015.09.074. Epub
[PubMed PMID: 26892655]
[32]
Salim A, Reece SM, Smith AG, Harrison D, Ramsay HM, Harden PN, Fryer AA. Sebaceous hyperplasia and skin cancer in patients undergoing renal transplant. Journal of the American Academy of Dermatology. 2006 Nov:55(5):878-81
[PubMed PMID: 17052497]
[33]
Sato T, Tanaka M. Linear sebaceous hyperplasia on the chest. Dermatology practical & conceptual. 2014 Jan:4(1):93-5. doi: 10.5826/dpc.0401a16. Epub 2014 Jan 31
[PubMed PMID: 24520522]
[34]
Kato N, Yasuoka A. "Giant" senile sebaceous hyperplasia. The Journal of dermatology. 1992 Apr:19(4):238-41
[PubMed PMID: 1607487]
[35]
Mandal RK, Das A, Chakrabarti I, Agarwal P. Nevoid sebaceous hyperplasia mistaken as nevus sebaceous: Report of four cases. Indian journal of dermatology, venereology and leprology. 2017 Mar-Apr:83(2):213-216. doi: 10.4103/0378-6323.199424. Epub
[PubMed PMID: 28164886]
Level 3 (low-level) evidence
[36]
Zaballos P, Gómez-Martín I, Martin JM, Bañuls J. Dermoscopy of Adnexal Tumors. Dermatologic clinics. 2018 Oct:36(4):397-412. doi: 10.1016/j.det.2018.05.007. Epub 2018 Aug 16
[PubMed PMID: 30201149]
[37]
Kim NH, Zell DS, Kolm I, Oliviero M, Rabinovitz HS. The dermoscopic differential diagnosis of yellow lobularlike structures. Archives of dermatology. 2008 Jul:144(7):962. doi: 10.1001/archderm.144.7.962. Epub
[PubMed PMID: 18645159]
[38]
Kavoussi H, Rezaei M, Azimi M, Kavoussi R. Combination of CO2 laser therapy and curettage for sebaceous gland hyperplasia. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2019 Mar:28(1):11-14
[PubMed PMID: 30901063]
[39]
Simmons BJ, Griffith RD, Falto-Aizpurua LA, Bray FN, Nouri K, International League of Dermatological Societies, European Dermatology Forum. Light and laser therapies for the treatment of sebaceous gland hyperplasia a review of the literature. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015 Nov:29(11):2080-7. doi: 10.1111/jdv.13066. Epub 2015 Mar 2
[PubMed PMID: 25731611]