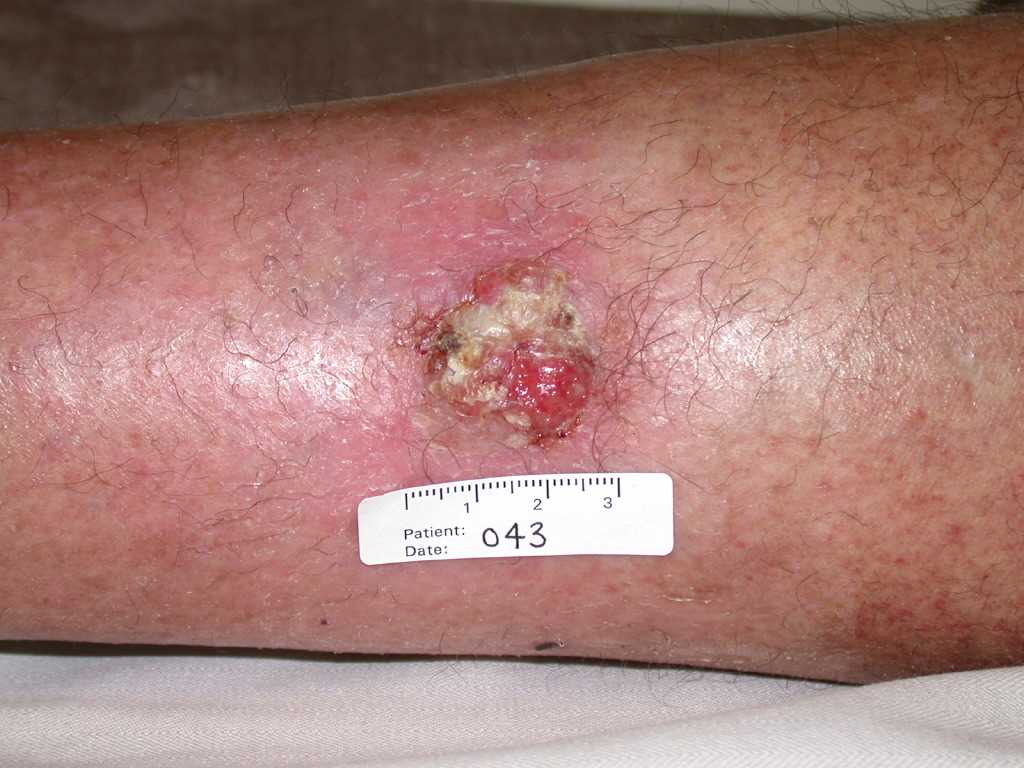
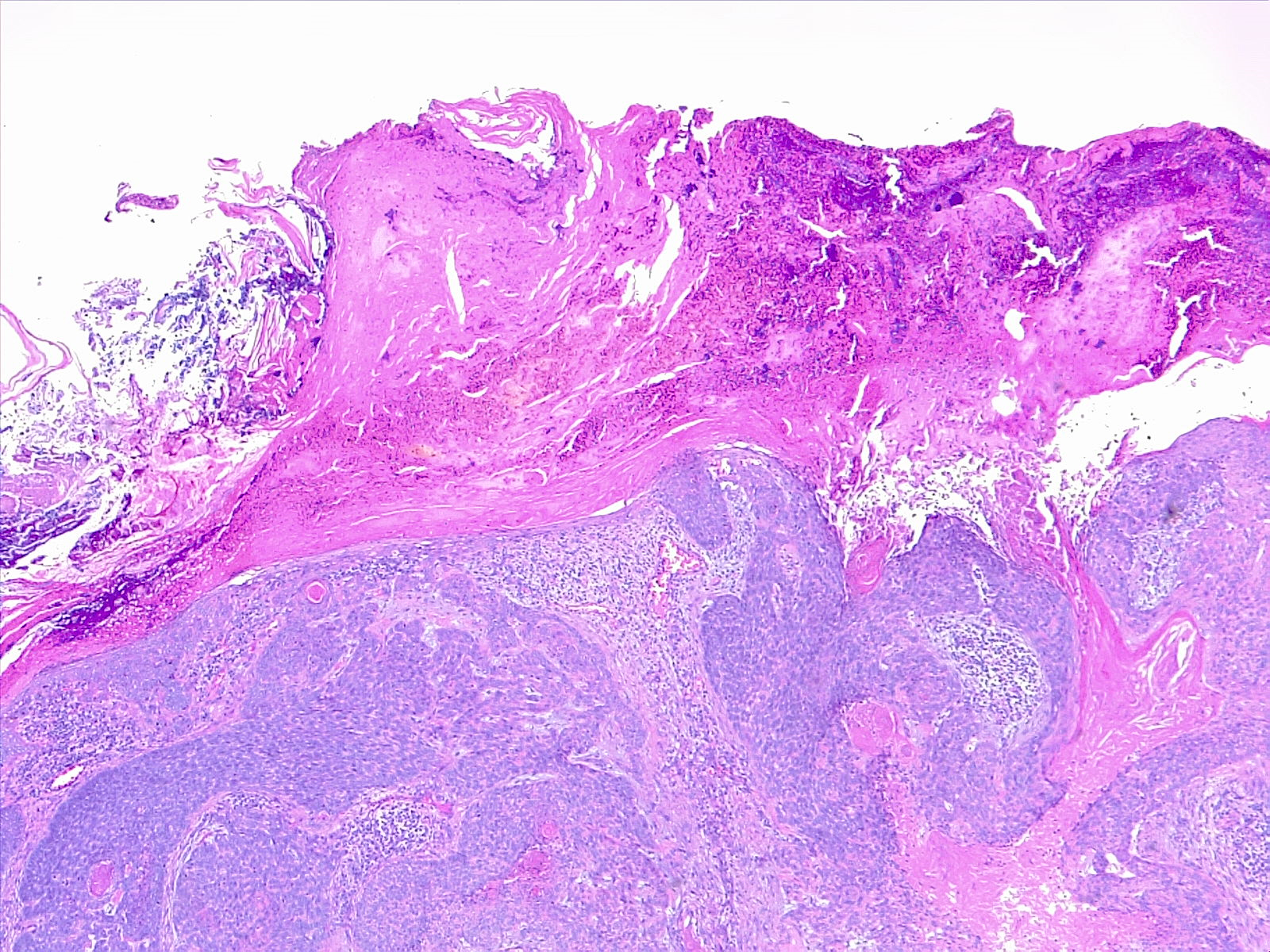
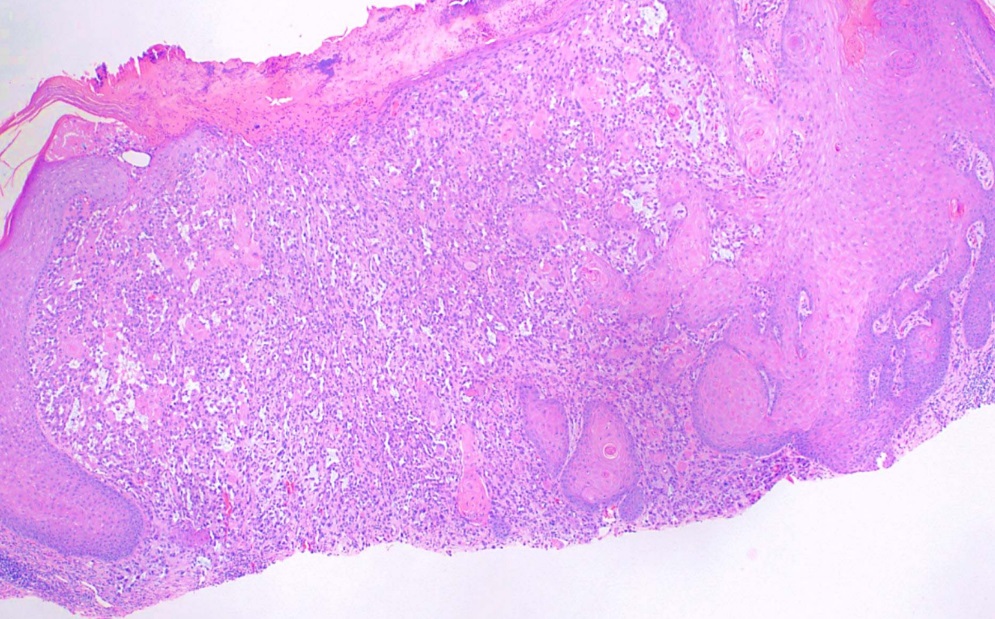
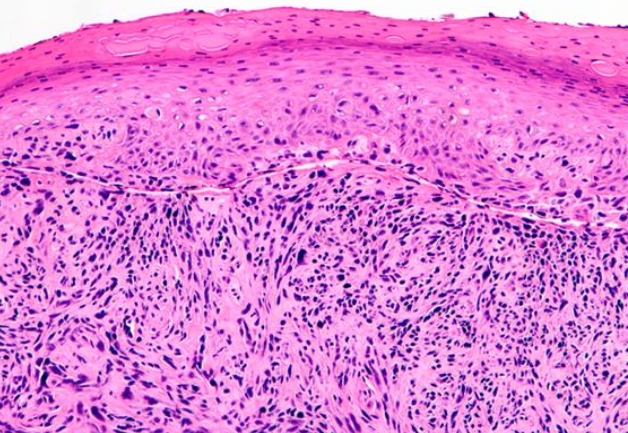
[1]
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. Journal of the American Academy of Dermatology. 2018 Feb:78(2):237-247. doi: 10.1016/j.jaad.2017.08.059. Epub
[PubMed PMID: 29332704]
[2]
Caudill J, Thomas JE, Burkhart CG. The risk of metastases from squamous cell carcinoma of the skin. International journal of dermatology. 2023 Apr:62(4):483-486. doi: 10.1111/ijd.16164. Epub 2022 Mar 24
[PubMed PMID: 35324009]
[3]
Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Management of advanced and high-stage tumors. Journal of the American Academy of Dermatology. 2018 Feb:78(2):249-261. doi: 10.1016/j.jaad.2017.08.058. Epub
[PubMed PMID: 29332705]
[4]
Omland SH, Ahlström MG, Gerstoft J, Pedersen G, Mohey R, Pedersen C, Kronborg G, Larsen CS, Kvinesdal B, Gniadecki R, Obel N, Omland LH. Risk of skin cancer in patients with HIV: A Danish nationwide cohort study. Journal of the American Academy of Dermatology. 2018 Oct:79(4):689-695. doi: 10.1016/j.jaad.2018.03.024. Epub 2018 Mar 26
[PubMed PMID: 29588249]
[5]
Jaju PD, Ransohoff KJ, Tang JY, Sarin KY. Familial skin cancer syndromes: Increased risk of nonmelanotic skin cancers and extracutaneous tumors. Journal of the American Academy of Dermatology. 2016 Mar:74(3):437-51; quiz 452-4. doi: 10.1016/j.jaad.2015.08.073. Epub
[PubMed PMID: 26892653]
[6]
Coggshall K, Farsani T, Ruben B, McCalmont TH, Berger TG, Fox LP, Shinkai K. Keratitis, ichthyosis, and deafness syndrome: a review of infectious and neoplastic complications. Journal of the American Academy of Dermatology. 2013 Jul:69(1):127-34. doi: 10.1016/j.jaad.2012.12.965. Epub 2013 Feb 4
[PubMed PMID: 23384797]
[8]
Bleeker MC, Visser PJ, Overbeek LI, van Beurden M, Berkhof J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016 Aug:25(8):1224-30. doi: 10.1158/1055-9965.EPI-16-0019. Epub 2016 Jun 2
[PubMed PMID: 27257093]
[9]
de Lanna CA, da Silva BNM, de Melo AC, Bonamino MH, Alves LDB, Pinto LFR, Cardoso AS, Antunes HS, Boroni M, Cohen Goldemberg D. Oral Lichen Planus and Oral Squamous Cell Carcinoma share key oncogenic signatures. Scientific reports. 2022 Nov 30:12(1):20645. doi: 10.1038/s41598-022-24801-6. Epub 2022 Nov 30
[PubMed PMID: 36450755]
[10]
Muzic JG, Schmitt AR, Wright AC, Alniemi DT, Zubair AS, Olazagasti Lourido JM, Sosa Seda IM, Weaver AL, Baum CL. Incidence and Trends of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma: A Population-Based Study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clinic proceedings. 2017 Jun:92(6):890-898. doi: 10.1016/j.mayocp.2017.02.015. Epub 2017 May 15
[PubMed PMID: 28522111]
[11]
Wehner MR, Cidre Serrano W, Nosrati A, Schoen PM, Chren MM, Boscardin J, Linos E. All-cause mortality in patients with basal and squamous cell carcinoma: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2018 Apr:78(4):663-672.e3. doi: 10.1016/j.jaad.2017.11.026. Epub 2017 Nov 13
[PubMed PMID: 29146125]
Level 1 (high-level) evidence
[12]
Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. Journal of the American Academy of Dermatology. 2013 Jun:68(6):957-66. doi: 10.1016/j.jaad.2012.11.037. Epub 2013 Feb 1
[PubMed PMID: 23375456]
[13]
Parekh V, Seykora JT. Cutaneous Squamous Cell Carcinoma. Clinics in laboratory medicine. 2017 Sep:37(3):503-525. doi: 10.1016/j.cll.2017.06.003. Epub
[PubMed PMID: 28802498]
[14]
Neagu TP, Ţigliş M, Botezatu D, Enache V, Cobilinschi CO, Vâlcea-Precup MS, GrinŢescu IM. Clinical, histological and therapeutic features of Bowen's disease. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2017:58(1):33-40
[PubMed PMID: 28523295]
[15]
Henderson SA, Torres-Cabala CA, Curry JL, Bassett RL, Ivan D, Prieto VG, Tetzlaff MT. p40 is more specific than p63 for the distinction of atypical fibroxanthoma from other cutaneous spindle cell malignancies. The American journal of surgical pathology. 2014 Aug:38(8):1102-10. doi: 10.1097/PAS.0000000000000245. Epub
[PubMed PMID: 25029117]
[16]
Idriss MH, Barbosa N, Chang MB, Gibson L, Baum CL, Vidal NY. Concomitant hypertrophic lichen planus and squamous cell carcinoma: Clinical features and treatment outcomes. International journal of dermatology. 2022 Dec:61(12):1527-1531. doi: 10.1111/ijd.16321. Epub 2022 Jun 29
[PubMed PMID: 35766459]
[17]
Mufti A, Sachdeva M, Maliyar K, Sibbald RG. Squamous cell carcinoma arising within discoid lupus erythematous lesions: A systematic review. JAAD international. 2021 Mar:2():1-4. doi: 10.1016/j.jdin.2020.10.001. Epub 2020 Nov 30
[PubMed PMID: 34409345]
Level 1 (high-level) evidence
[18]
Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Smith Begolka W, Ratings Panel, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, McBurney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Journal of the American Academy of Dermatology. 2012 Oct:67(4):531-50. doi: 10.1016/j.jaad.2012.06.009. Epub 2012 Sep 5
[PubMed PMID: 22959232]
[19]
Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. Journal of the American Academy of Dermatology. 1992 Jun:26(6):976-90
[PubMed PMID: 1607418]
[20]
Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. The Journal of investigative dermatology. 2013 May:133(5):1188-96. doi: 10.1038/jid.2012.403. Epub 2012 Nov 29
[PubMed PMID: 23190903]
[21]
Stewart JR, Lang ME, Brewer JD. Efficacy of nonexcisional treatment modalities for superficially invasive and in situ squamous cell carcinoma: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2022 Jul:87(1):131-137. doi: 10.1016/j.jaad.2021.07.067. Epub 2021 Aug 8
[PubMed PMID: 34375669]
Level 1 (high-level) evidence
[22]
Nestor MS, Berman B, Goldberg D, Cognetta AB Jr, Gold M, Roth W, Cockerell CJ, Glick B. Consensus Guidelines on the Use of Superficial Radiation Therapy for Treating Nonmelanoma Skin Cancers and Keloids. The Journal of clinical and aesthetic dermatology. 2019 Feb:12(2):12-18
[PubMed PMID: 30881578]
Level 3 (low-level) evidence
[23]
Mager L, Gardeen S, Carr DR, Shahwan KT. Cemiplimab for the Treatment of Advanced Cutaneous Squamous Cell Carcinoma: Appropriate Patient Selection and Perspectives. Clinical, cosmetic and investigational dermatology. 2023:16():2135-2142. doi: 10.2147/CCID.S381471. Epub 2023 Aug 9
[PubMed PMID: 37581012]
Level 3 (low-level) evidence
[24]
Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Lin J, Gumuscu B, Swaby RF, Rischin D. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 Feb 1:41(4):790-802. doi: 10.1200/JCO.21.02508. Epub 2022 Oct 11
[PubMed PMID: 36219809]
[25]
Peng L, Wang Y, Hong Y, Ye X, Shi P, Zhang J, Zhao Q. Incidence and relative risk of cutaneous squamous cell carcinoma with single-agent BRAF inhibitor and dual BRAF/MEK inhibitors in cancer patients: a meta-analysis. Oncotarget. 2017 Oct 10:8(47):83280-83291. doi: 10.18632/oncotarget.21059. Epub 2017 Sep 19
[PubMed PMID: 29137342]
Level 1 (high-level) evidence
[26]
Mohan SV, Chang J, Li S, Henry AS, Wood DJ, Chang AL. Increased Risk of Cutaneous Squamous Cell Carcinoma After Vismodegib Therapy for Basal Cell Carcinoma. JAMA dermatology. 2016 May 1:152(5):527-32. doi: 10.1001/jamadermatol.2015.4330. Epub
[PubMed PMID: 26914338]
[27]
D'Arcy ME, Pfeiffer RM, Rivera DR, Hess GP, Cahoon EK, Arron ST, Brownell I, Cowen EW, Israni AK, Triplette MA, Yanik EL, Engels EA. Voriconazole and the Risk of Keratinocyte Carcinomas Among Lung Transplant Recipients in the United States. JAMA dermatology. 2020 Jul 1:156(7):772-779. doi: 10.1001/jamadermatol.2020.1141. Epub
[PubMed PMID: 32401271]
[28]
Waldman RA, Grant-Kels JM. The role of sunscreen in the prevention of cutaneous melanoma and nonmelanoma skin cancer. Journal of the American Academy of Dermatology. 2019 Feb:80(2):574-576.e1. doi: 10.1016/j.jaad.2018.06.069. Epub
[PubMed PMID: 30012373]