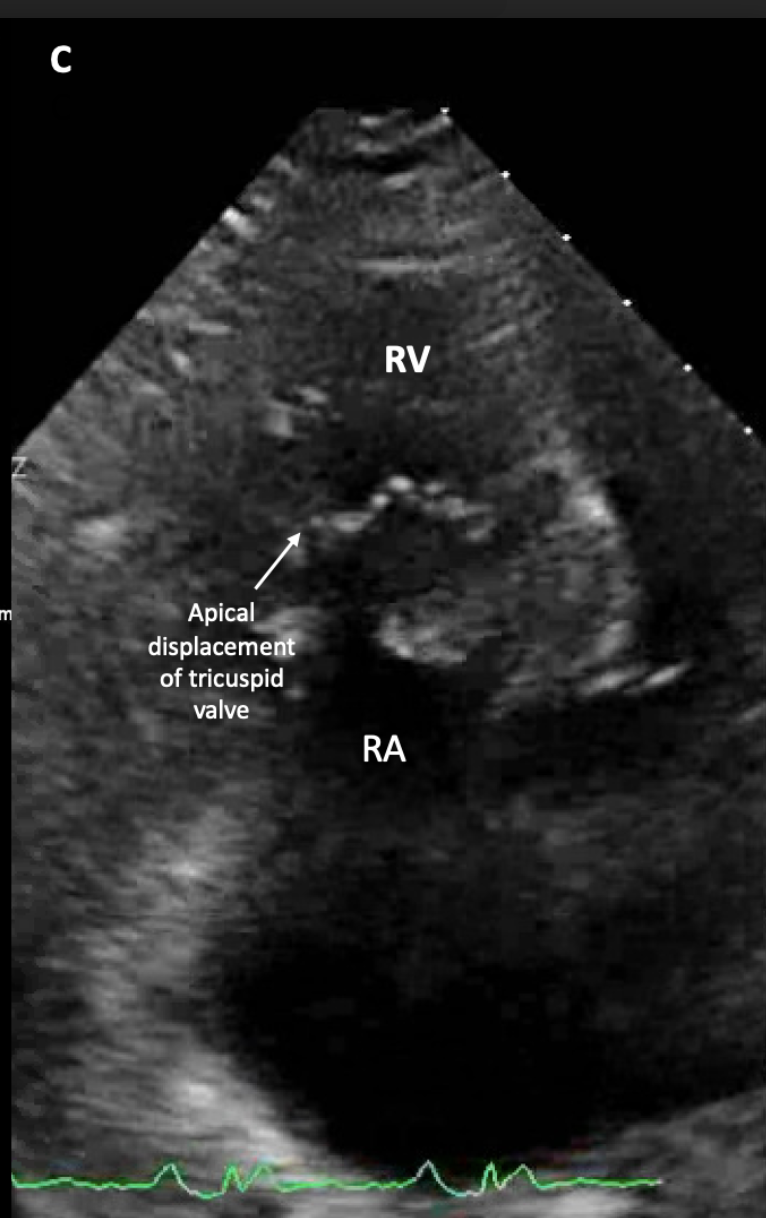
[2]
Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein's anomaly. Circulation. 2007 Jan 16:115(2):277-85
[PubMed PMID: 17228014]
[3]
Mazurak M, Kusa J. The Two Anomalies of Wilhelm Ebstein. Texas Heart Institute journal. 2017 Jun:44(3):198-201. doi: 10.14503/THIJ-16-6063. Epub 2017 Jun 1
[PubMed PMID: 28761400]
[4]
Holst KA, Connolly HM, Dearani JA. Ebstein's Anomaly. Methodist DeBakey cardiovascular journal. 2019 Apr-Jun:15(2):138-144. doi: 10.14797/mdcj-15-2-138. Epub
[PubMed PMID: 31384377]
[5]
Kumar TKS. Ebstein's anomaly in the neonate. Indian journal of thoracic and cardiovascular surgery. 2021 Jan:37(Suppl 1):17-25. doi: 10.1007/s12055-020-00942-z. Epub 2020 Mar 21
[PubMed PMID: 33603283]
[6]
Possner M, Gensini FJ, Mauchley DC, Krieger EV, Steinberg ZL. Ebstein's Anomaly of the Tricuspid Valve: an Overview of Pathology and Management. Current cardiology reports. 2020 Oct 9:22(12):157. doi: 10.1007/s11886-020-01412-z. Epub 2020 Oct 9
[PubMed PMID: 33037480]
Level 3 (low-level) evidence
[7]
Neumann S, Rüffer A, Sachweh J, Biermann D, Herrmann J, Jerosch-Herold M, Hazekamp M, Sinning C, Zengin E, Blankenberg S, Girdauskas E, Reichenspurner H, Kehl T, Müller G, Kozlik-Feldmann R, Rickers C. Narrative review of Ebstein's anomaly beyond childhood: Imaging, surgery, and future perspectives. Cardiovascular diagnosis and therapy. 2021 Dec:11(6):1310-1323. doi: 10.21037/cdt-20-771. Epub
[PubMed PMID: 35070800]
Level 3 (low-level) evidence
[8]
Yuan SM. Ebstein's Anomaly: Genetics, Clinical Manifestations, and Management. Pediatrics and neonatology. 2017 Jun:58(3):211-215. doi: 10.1016/j.pedneo.2016.08.004. Epub 2016 Nov 19
[PubMed PMID: 28017577]
[9]
Miranda-Fernández MC, Ramírez-Oyaga S, Restrepo CM, Huertas-Quiñones VM, Barrera-Castañeda M, Quero R, Hernández-Toro CJ, Tamar Silva C, Laissue P, Cabrera R. Identification of a New Candidate Locus for Ebstein Anomaly in 1p36.2. Molecular syndromology. 2018 May:9(3):164-169. doi: 10.1159/000488820. Epub 2018 Apr 28
[PubMed PMID: 29928183]
[10]
Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994 Jan 12:271(2):146-50
[PubMed PMID: 8031346]
[11]
Correa-Villaseñor A, Ferencz C, Neill CA, Wilson PD, Boughman JA. Ebstein's malformation of the tricuspid valve: genetic and environmental factors. The Baltimore-Washington Infant Study Group. Teratology. 1994 Aug:50(2):137-47
[PubMed PMID: 7801301]
[12]
Sharma N, Lalnunnem TJ, Nandwani M, Santa SA, Synrang BW. Ebstein Anomaly with Pregnancy: A Rare Case. Journal of reproduction & infertility. 2018 Apr-Jun:19(2):119-122
[PubMed PMID: 30009147]
Level 3 (low-level) evidence
[13]
Fuchs MM, Connolly HM. Ebstein Anomaly in the Adult Patient. Cardiology clinics. 2020 Aug:38(3):353-363. doi: 10.1016/j.ccl.2020.04.004. Epub 2020 Jun 6
[PubMed PMID: 32622490]
[14]
Connolly HM, Warnes CA. Ebstein's anomaly: outcome of pregnancy. Journal of the American College of Cardiology. 1994 Apr:23(5):1194-8
[PubMed PMID: 8144788]
[15]
Dearani JA, Mora BN, Nelson TJ, Haile DT, O'Leary PW. Ebstein anomaly review: what's now, what's next? Expert review of cardiovascular therapy. 2015 Oct:13(10):1101-9. doi: 10.1586/14779072.2015.1087849. Epub 2015 Sep 10
[PubMed PMID: 26357983]
[16]
Anderson KR, Zuberbuhler JR, Anderson RH, Becker AE, Lie JT. Morphologic spectrum of Ebstein's anomaly of the heart: a review. Mayo Clinic proceedings. 1979 Mar:54(3):174-80
[PubMed PMID: 431123]
[17]
Stephens EH, Dearani JA, Qureshi MY, Ammash N, Maleszewski JJ. The Congenital Tricuspid Valve Spectrum: From Ebstein to Dysplasia. World journal for pediatric & congenital heart surgery. 2020 Nov:11(6):783-791. doi: 10.1177/2150135120949235. Epub
[PubMed PMID: 33164686]
[18]
Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, Li Z, Hodge DO, Driscoll DJ, Mayo Clinic Congenital Heart Center. The outcomes of operations for 539 patients with Ebstein anomaly. The Journal of thoracic and cardiovascular surgery. 2008 May:135(5):1120-36, 1136.e1-7. doi: 10.1016/j.jtcvs.2008.02.034. Epub
[PubMed PMID: 18455593]
[19]
Khositseth A, Danielson GK, Dearani JA, Munger TM, Porter CJ. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. The Journal of thoracic and cardiovascular surgery. 2004 Dec:128(6):826-33
[PubMed PMID: 15573066]
[20]
Qureshi MY, O'Leary PW, Connolly HM. Cardiac imaging in Ebstein anomaly. Trends in cardiovascular medicine. 2018 Aug:28(6):403-409. doi: 10.1016/j.tcm.2018.01.002. Epub 2018 Jan 12
[PubMed PMID: 29409687]
[21]
Voges I, Al-Mallah MH, Scognamiglio G, Di Salvo G. Right Heart-Pulmonary Circulation Unit in Congenital Heart Diseases. Heart failure clinics. 2018 Jul:14(3):283-295. doi: 10.1016/j.hfc.2018.02.005. Epub
[PubMed PMID: 29966627]
[22]
Barbara DW, Edwards WD, Connolly HM, Dearani JA. Surgical pathology of 104 tricuspid valves (2000-2005) with classic right-sided Ebstein's malformation. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2008 May-Jun:17(3):166-71. doi: 10.1016/j.carpath.2007.07.005. Epub 2007 Oct 24
[PubMed PMID: 18402795]
[23]
Gerlis LM, Ho SY, Sweeney AE. Mitral valve anomalies associated with Ebstein's malformation of the tricuspid valve. The American journal of cardiovascular pathology. 1993:4(4):294-301
[PubMed PMID: 8305192]
[24]
Álvarez Macedo MR, Vázquez Antona CA. Uncommon mitral valve anomalies associated with Ebstein anomaly. Revista espanola de cardiologia (English ed.). 2021 Aug:74(8):717-719. doi: 10.1016/j.rec.2021.01.013. Epub 2021 Mar 27
[PubMed PMID: 33785267]
[25]
Hernandez-Andrade E, Patwardhan M, Cruz-Lemini M, Luewan S. Early Evaluation of the Fetal Heart. Fetal diagnosis and therapy. 2017:42(3):161-173. doi: 10.1159/000477564. Epub 2017 Jul 5
[PubMed PMID: 28675906]
[26]
Freud LR, Escobar-Diaz MC, Kalish BT, Komarlu R, Puchalski MD, Jaeggi ET, Szwast AL, Freire G, Levasseur SM, Kavanaugh-McHugh A, Michelfelder EC, Moon-Grady AJ, Donofrio MT, Howley LW, Tierney ES, Cuneo BF, Morris SA, Pruetz JD, van der Velde ME, Kovalchin JP, Ikemba CM, Vernon MM, Samai C, Satou GM, Gotteiner NL, Phoon CK, Silverman NH, McElhinney DB, Tworetzky W. Outcomes and Predictors of Perinatal Mortality in Fetuses With Ebstein Anomaly or Tricuspid Valve Dysplasia in the Current Era: A Multicenter Study. Circulation. 2015 Aug 11:132(6):481-9. doi: 10.1161/CIRCULATIONAHA.115.015839. Epub 2015 Jun 9
[PubMed PMID: 26059011]
Level 2 (mid-level) evidence
[27]
Ciepłucha A, Trojnarska O, Kociemba A, Łanocha M, Barczynski M, Rozmiarek S, Kramer L, Pyda M. Clinical aspects of myocardial fibrosis in adults with Ebstein's anomaly. Heart and vessels. 2018 Sep:33(9):1076-1085. doi: 10.1007/s00380-018-1141-5. Epub 2018 Feb 21
[PubMed PMID: 29468473]
[28]
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008 Dec 2:118(23):e714-833. doi: 10.1161/CIRCULATIONAHA.108.190690. Epub 2008 Nov 7
[PubMed PMID: 18997169]
Level 1 (high-level) evidence
[29]
Shiina A, Seward JB, Edwards WD, Hagler DJ, Tajik AJ. Two-dimensional echocardiographic spectrum of Ebstein's anomaly: detailed anatomic assessment. Journal of the American College of Cardiology. 1984 Feb:3(2 Pt 1):356-70
[PubMed PMID: 6693624]
[30]
Kühn A, Meierhofer C, Rutz T, Rondak IC, Röhlig C, Schreiber C, Fratz S, Ewert P, Vogt M. Non-volumetric echocardiographic indices and qualitative assessment of right ventricular systolic function in Ebstein's anomaly: comparison with CMR-derived ejection fraction in 49 patients. European heart journal. Cardiovascular Imaging. 2016 Aug:17(8):930-5. doi: 10.1093/ehjci/jev243. Epub 2015 Oct 8
[PubMed PMID: 26453545]
Level 2 (mid-level) evidence
[31]
Atz AM, Munoz RA, Adatia I, Wessel DL. Diagnostic and therapeutic uses of inhaled nitric oxide in neonatal Ebstein's anomaly. The American journal of cardiology. 2003 Apr 1:91(7):906-8
[PubMed PMID: 12667588]
[32]
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr 2:139(14):e698-e800. doi: 10.1161/CIR.0000000000000603. Epub
[PubMed PMID: 30586767]
Level 1 (high-level) evidence
[33]
Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019 Apr 2:73(12):e81-e192. doi: 10.1016/j.jacc.2018.08.1029. Epub 2018 Aug 16
[PubMed PMID: 30121239]
Level 1 (high-level) evidence
[34]
Oxenius A, Attenhofer Jost CH, Prêtre R, Dave H, Bauersfeld U, Kretschmar O, Seifert B, Balmer C, Valsangiacomo Buechel ER. Management and outcome of Ebstein's anomaly in children. Cardiology in the young. 2013 Feb:23(1):27-34. doi: 10.1017/S1047951112000224. Epub 2012 Mar 15
[PubMed PMID: 22417890]
[35]
Holst KA, Dearani JA, Said SM, Davies RR, Pizarro C, Knott-Craig C, Kumar TKS, Starnes VA, Kumar SR, Pasquali SK, Thibault DP, Meza JM, Hill KD, Chiswell K, Jacobs JP, Jacobs ML. Surgical Management and Outcomes of Ebstein Anomaly in Neonates and Infants: A Society of Thoracic Surgeons Congenital Heart Surgery Database Analysis. The Annals of thoracic surgery. 2018 Sep:106(3):785-791. doi: 10.1016/j.athoracsur.2018.04.049. Epub 2018 May 16
[PubMed PMID: 29777671]
[36]
Brown ML, Dearani JA, Danielson GK, Cetta F, Connolly HM, Warnes CA, Li Z, Hodge DO, Driscoll DJ. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. Journal of the American College of Cardiology. 2008 Aug 5:52(6):460-6. doi: 10.1016/j.jacc.2008.03.064. Epub
[PubMed PMID: 18672167]
[37]
Stulak JM, Sharma V, Cannon BC, Ammash N, Schaff HV, Dearani JA. Optimal surgical ablation of atrial tachyarrhythmias during correction of Ebstein anomaly. The Annals of thoracic surgery. 2015 May:99(5):1700-5; discussion 1705. doi: 10.1016/j.athoracsur.2015.01.037. Epub 2015 Mar 29
[PubMed PMID: 25825196]
[38]
Sainathan S, da Fonseca da Silva L, da Silva JP. Ebstein's anomaly: contemporary management strategies. Journal of thoracic disease. 2020 Mar:12(3):1161-1173. doi: 10.21037/jtd.2020.01.18. Epub
[PubMed PMID: 32274197]
[39]
Danielson GK, Driscoll DJ, Mair DD, Warnes CA, Oliver WC Jr. Operative treatment of Ebstein's anomaly. The Journal of thoracic and cardiovascular surgery. 1992 Nov:104(5):1195-202
[PubMed PMID: 1434695]
[40]
Carpentier A, Chauvaud S, Macé L, Relland J, Mihaileanu S, Marino JP, Abry B, Guibourt P. A new reconstructive operation for Ebstein's anomaly of the tricuspid valve. The Journal of thoracic and cardiovascular surgery. 1988 Jul:96(1):92-101
[PubMed PMID: 3386297]
[41]
Wackel P, Cannon B, Dearani J, Sessions K, Holst K, Johnson J, Cetta F. Arrhythmia after cone repair for Ebstein anomaly: The Mayo Clinic experience in 143 young patients. Congenital heart disease. 2018 Jan:13(1):26-30. doi: 10.1111/chd.12566. Epub 2018 Jan 8
[PubMed PMID: 29316261]
[42]
Wei W, Zhan X, Xue Y, Fang X, Liao H, Deng H, Liang Y, Wu S. Features of accessory pathways in adult Ebstein's anomaly. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2014 Nov:16(11):1619-25. doi: 10.1093/europace/euu028. Epub 2014 Mar 9
[PubMed PMID: 24614573]
[43]
Rajani R, Klein JL. Infective endocarditis: A contemporary update. Clinical medicine (London, England). 2020 Jan:20(1):31-35. doi: 10.7861/clinmed.cme.20.1.1. Epub
[PubMed PMID: 31941729]
[44]
Moe TG, Abrich VA, Rhee EK. Atrial Fibrillation in Patients with Congenital Heart Disease. Journal of atrial fibrillation. 2017 Jun-Jul:10(1):1612. doi: 10.4022/jafib.1612. Epub 2017 Jun 30
[PubMed PMID: 29250225]
[45]
Martín de Miguel I, Ávila P. Atrial Fibrillation in Congenital Heart Disease. European cardiology. 2021 Feb:16():e06. doi: 10.15420/ecr.2020.41. Epub 2021 Mar 9
[PubMed PMID: 33737960]
[46]
Deeg KH. Echocardiographic differential diagnosis of the cyanotic newborn. Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2015 Apr:36(2):104-18; quiz 119-20. doi: 10.1055/s-0034-1385493. Epub 2014 Dec 4
[PubMed PMID: 25474186]
[47]
Mihos CG, Larrauri-Reyes M, Yucel E, Santana O. Clinical presentation and echocardiographic characteristics of Uhl's anomaly. Echocardiography (Mount Kisco, N.Y.). 2017 Feb:34(2):299-302. doi: 10.1111/echo.13430. Epub 2016 Dec 29
[PubMed PMID: 28032368]
[48]
Kobza R, Kurz DJ, Oechslin EN, Prêtre R, Zuber M, Vogt P, Jenni R. Aberrant tendinous chords with tethering of the tricuspid leaflets: a congenital anomaly causing severe tricuspid regurgitation. Heart (British Cardiac Society). 2004 Mar:90(3):319-23
[PubMed PMID: 14966058]
[49]
Celermajer DS, Bull C, Till JA, Cullen S, Vassillikos VP, Sullivan ID, Allan L, Nihoyannopoulos P, Somerville J, Deanfield JE. Ebstein's anomaly: presentation and outcome from fetus to adult. Journal of the American College of Cardiology. 1994 Jan:23(1):170-6
[PubMed PMID: 8277076]
[50]
Attie F, Rosas M, Rijlaarsdam M, Buendia A, Zabal C, Kuri J, Granados N. The adult patient with Ebstein anomaly. Outcome in 72 unoperated patients. Medicine. 2000 Jan:79(1):27-36
[PubMed PMID: 10670407]
[51]
Attenhofer Jost CH, Tan NY, Hassan A, Vargas ER, Hodge DO, Dearani JA, Connolly H, Asirvatham SJ, McLeod CJ. Sudden death in patients with Ebstein anomaly. European heart journal. 2018 Jun 1:39(21):1970-1977a. doi: 10.1093/eurheartj/ehx794. Epub
[PubMed PMID: 29315367]
[52]
Holst KA, Dearani JA, Said S, Pike RB, Connolly HM, Cannon BC, Sessions KL, O'Byrne MM, O'Leary PW. Improving Results of Surgery for Ebstein Anomaly: Where Are We After 235 Cone Repairs? The Annals of thoracic surgery. 2018 Jan:105(1):160-168. doi: 10.1016/j.athoracsur.2017.09.058. Epub 2017 Nov 24
[PubMed PMID: 29174783]
[53]
Celermajer DS, Cullen S, Sullivan ID, Spiegelhalter DJ, Wyse RK, Deanfield JE. Outcome in neonates with Ebstein's anomaly. Journal of the American College of Cardiology. 1992 Apr:19(5):1041-6
[PubMed PMID: 1552092]
[54]
Andrews RE, Tibby SM, Sharland GK, Simpson JM. Prediction of outcome of tricuspid valve malformations diagnosed during fetal life. The American journal of cardiology. 2008 Apr 1:101(7):1046-50. doi: 10.1016/j.amjcard.2007.11.049. Epub 2008 Feb 6
[PubMed PMID: 18359329]