Continuing Education Activity
Coronary artery disease is a common heart condition that involves atherosclerotic plaque formation in the vessel lumen. This leads to impairment in blood flow and thus oxygen delivery to the myocardium. It is a cause of major morbidity and mortality in the US and worldwide. To avoid the high morbidity and mortality associated with this condition, it must be promptly diagnosed and treated. This activity illustrates the evaluation, diagnosis, and management of coronary artery disease and highlights the role of the healthcare team in evaluating and treating patients with this condition.
Objectives:
- Identify the etiology of coronary artery disease.
- Outline the evaluation of coronary artery disease.
- Discuss the management options available for coronary artery disease.
Introduction
Coronary artery disease is a condition in which there is an inadequate supply of blood and oxygen to the myocardium. It results from occlusion of the coronary arteries and results in a demand-supply mismatch of oxygen. It typically involves the formation of plaques in the lumen of coronary arteries that impede blood flow. It is the major cause of death in the US and worldwide. At the beginning of the 20th century, it was an uncommon cause of death. Deaths due to CAD peaked in the mid-1960s and then decreased however, it still is the leading cause of death worldwide.[1]
Etiology
Coronary artery disease is a multifactorial phenomenon. Etiologic factors can be broadly categorized into non-modifiable and modifiable factors. Non-modifiable factors include gender, age, family history, and genetics. Modifiable risk factors include smoking, obesity, lipid levels, and psychosocial variables. In the Western world, a faster-paced lifestyle has led people to eat more fast foods and unhealthy meals which has led to an increased prevalence of ischemic heart diseases. In the US, better primary care in the middle and higher socioeconomic groups has pushed the incidence towards the later part of life. Smoking remains the number one cause of cardiovascular diseases. In 2016, the prevalence of smoking among the United States among adults was found to be at 15.5 %.[2]
The male gender is more predisposed than the female gender. Hypercholesterolemia remains an important modifiable risk factor for CAD. Increased low-density lipoproteins (LDL) increased the risk for CAD and elevated high-density lipoproteins (HDL) decrease the incidence of CAD. An individual's 10-year risk of atherosclerotic cardiovascular disease can be calculated using the ASCVD equation available online on the American Heart Association portal. Markers of inflammation are also strong risk factors for coronary artery disease. High sensitivity CRP (hsCRP) is thought to be the best predictor of coronary artery disease in some studies although uses for it in a practical setting are controversial.[3]
Epidemiology
Coronary artery disease is very common in both developed and developing worlds. In one study, it was estimated that CAD represented 2.2% of the overall global burden of disease and 32.7% of cardiovascular diseases. It costs over 200 billion dollars annually to the health care system in the United States. It is estimated that 7.6% of men and 5.0% of women in the US lived with coronary artery disease from 2009 to 2012 based on the national health survey done by the American Heart Association (AHA). This amount to 15.5 million Americans afflicted with the disease during this time.[4][5]
The incidence of CAD is observed to rise with age, regardless of gender. In the ONACI registry in France, the incidence of CAD was about 1% in the 45 to 65 age group, which increased to about 4% as the age group reached 75 to 84 years.[6]
Pathophysiology
The hallmark of the pathophysiology of CAD is the development of atherosclerotic plaque. Plaque is a build-up of fatty material that narrows the vessel lumen and impedes the blood flow. The first step in the process is the formation of a "fatty streak." Fatty streak is formed by subendothelial deposition of lipid-laden macrophages, also called foam cells. When a vascular insult occurs, the intima layer breaks, and monocytes migrate into the subendothelial space where they become macrophages. These macrophages take up oxidized low-density lipoprotein (LDL) particles, and foam cells are formed. T cells get activated, which releases cytokines only to aid in the pathologic process. Growth factors released activate smooth muscles, which also take up oxidized LDL particles and collagen and deposit along with activated macrophages and increase the population of foam cells. This process leads to the formation of subendothelial plaque.
Over time, this plaque could grow in size or become stable if no further insult occurs to the endothelium. If it becomes stable, a fibrous cap will form, and the lesion will become calcified over time. As time passes, the lesion can become hemodynamically significant enough that not enough blood would reach the myocardial tissue at the time of increased demands, and angina symptoms would occur. However, symptoms would abate at rest as the oxygen requirement comes down. For a lesion to cause angina at rest, it must be at least 90% stenosed. Some plaques can rupture and lead to exposure of tissue factor, which culminates in thrombosis. This thrombosis could cause subtotal or total occlusion of the lumen and could result in the development of acute coronary syndrome (ACS) in the form of unstable angina, NSTEMI, or STEMI, depending on the level of insult.[7]
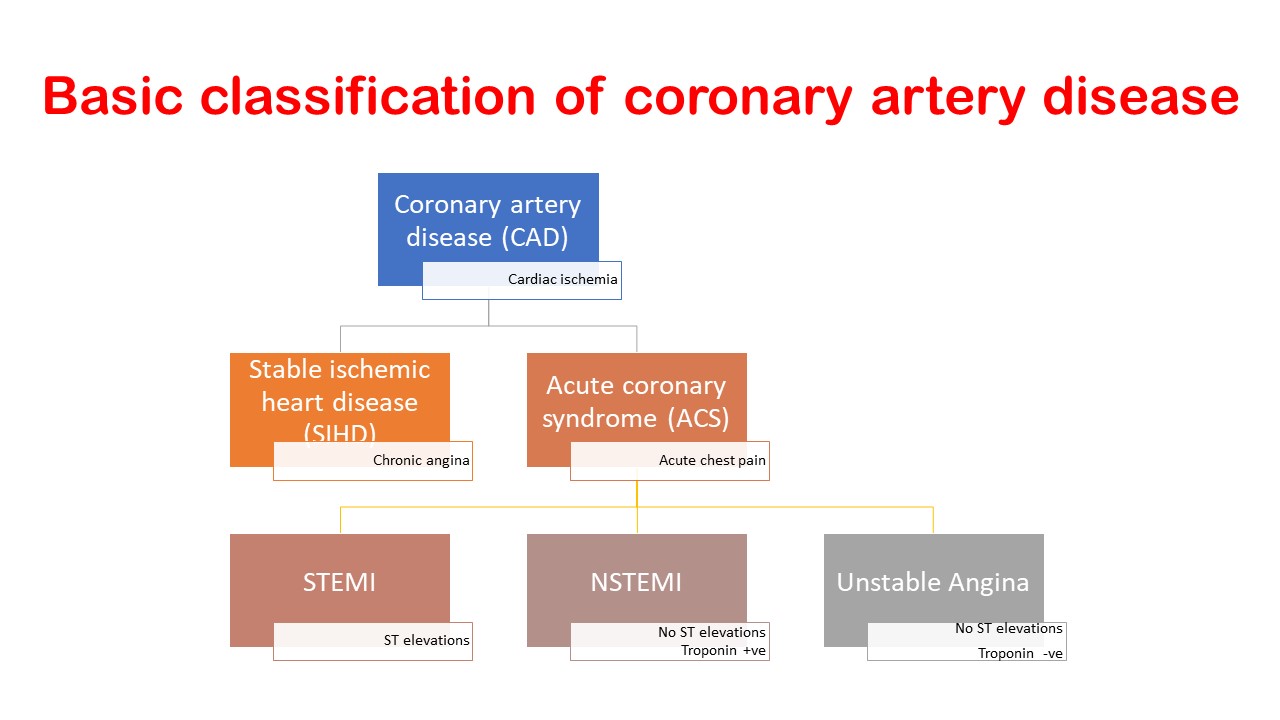
Classification of coronary artery disease is typically done as under:
- Stable ischemic heart disease (SIHD)
- Acute coronary syndrome (ACS)
- ST-elevation MI (STEMI)
- Non-ST elevation MI (NSTEMI)
- Unstable angina
History and Physical
It is very important to take a detailed history and physical examination before proceeding towards further workup. Coronary artery disease could manifest as stable ischemic heart disease (SIHD) or acute coronary syndrome (ACS). It can further progress into congestive heart failure (CHF) if not controlled. Patients should be asked about chest pain, its relation to physical activity, and radiation of the pain into the jaw, neck, left arm, or into the back. Dyspnea should be evaluated for rest and also on activity. The patient should also be asked about syncope, palpitations, tachypnea, lower extremity edema, orthopnea, and exercise capacity. A family history of ischemic heart diseases should be obtained along with dietary, smoking, and lifestyle habits.
Physical examination should include inspection, palpation, and auscultation. One should inspect for any acute distress, jugular venous distention, and peripheral edema. In palpation, one should palpate for fluid thrill and heave. The extent of peripheral edema if present should be evaluated. The distension of the jugular vein should be measured. In auscultation, the heart should be auscultated in all four locations and lungs should also be auscultated with a special focus on the lower zones.
Evaluation
There are several modalities to evaluate for coronary artery disease including EKG, Echo, CXR, Stress test, cardiac catheterization, and blood work to name the main ones. These tests are done depending on the context in which patients are presenting. The following are details on different diagnostic modalities we have available for the evaluation of coronary artery disease:
Electrocardiogram (EKG)
EKG is a very basic yet enormously helpful test in the evaluation of coronary artery disease. It measures electrical activity in the cardiac conduction system and is measured by 10 leads attached to the skin at standardized locations. It provides information about both the physiology and anatomy of the heart. It typically has 12 leads on the paper that is printed once the test is performed and each lead correlates with the specific location of the heart. Important information to notice on an EKG is a heart's rate, rhythm, and axis. After that, information regarding acute and chronic pathologic processes can be obtained. In acute coronary syndrome, one can see ST-segment changes and T wave changes. If an ACS has degenerated into arrhythmias, that can also be seen. In chronic settings, EKG can show information like axis deviation, bundle branch blocks, and ventricular hypertrophy. EKG is also a cost-effective and readily available testing modality that is not user-dependent.
Echocardiography
Echocardiography is an ultrasound of the heart. It is a useful and non-invasive mode of testing that is performed in both acute and chronic and inpatient and outpatient settings. In acute settings, it could tell about wall motion, valvular regurgitation and stenosis, infective or autoimmune lesions, and chamber sizes. It also is useful in the diagnosis of acute pulmonary pathologies like pulmonary embolism. It also evaluates the pericardial cavity. In chronic settings, it can be done to see the same information mentioned above and also a response to the therapy. It also is used in an outpatient setting as part of stress testing. In addition to diagnostics, it also has a role in therapeutics for example, pericardiocentesis could be performed with the needle-guided by echocardiography. This test is user-dependent and could be costly compared to EKG.[8]
Stress Test
The stress test is a relatively non-invasive test to evaluate for coronary artery disease. It is used in the setting of suspected angina or angina equivalent and is helpful in ruling in or out coronary pathology when interpreted in an appropriate setting. During the test, the heart is artificially exposed to stress and if the patient gets certain abnormal EKG changes in ST segments or gets symptoms of angina, the test is aborted at that point and coronary artery disease is diagnosed. EKGs are obtained before, during, and after the procedure, and the patient is continuously monitored for any symptoms. There are mainly two types of stress tests; exercise stress test and pharmacologic stress test. In exercise stress tests, the patient has to run on a treadmill until he achieves 85% of the age-predicted maximal heart rate. If a patient develops exertional hypotension, hypertension (>200/110 mmHg), ST-segment elevations or depression, or ventricular or supraventricular arrhythmias.[9]
Chest X-ray
Chest X-ray is an important component of the initial evaluation of cardiac disease. The standard imaging films include standing posteroanterior (PA) and left lateral decubitus. Sometimes, anteroposterior (AP) projection is obtained especially in inpatient settings with the patient lying down, however, this interpretation of AP films is significantly limited. Proper analysis of PA and AP views provides useful and cost-effective information about the heart, lungs, and vasculature. Interpretation should be done in a stepwise pattern so that important information is not overlooked.
Blood Work
Blood work aids in establishing the diagnosis and assessing therapeutic responses. In acute settings, cardiac enzymes and B-type natriuretic peptides are often done along with complete blood counts and metabolic panels. BNP provides information about volume overload of cardiogenic origin however it has its limitations. It can be falsely elevated in kidney diseases and falsely low in obesity. Cardiac enzymes like CK and troponin provide information about an acute ischemic event. In chronic settings, lipid panel provides important prognostic information. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) aid in assessing disease like acute pericarditis. Liver function tests (LFT) can be done to evaluate for an infiltrative process that can affect the liver and heart simultaneously like hemochromatosis. Liver tests are also done to assess increased right heart pressures, especially in chronic settings.
Cardiac Catheterization
Cardiac catheterization is the gold standard and most accurate modality to evaluate ischemic coronary heart disease. It is however an invasive procedure with associated complications. Not everyone is a candidate for the procedure. In non ACS settings, patients with intermediate pretest probability for CAD are usually the right candidates for it. In the ACS setting, all STEMI patients and selected NSTEMI patients get an emergent cardiac catheterization. This procedure is done in a cardiac catheterization lab, is expertise dependent, and is done under moderate sedation. There is contrast exposure in the procedure which could cause serious allergic reactions and kidney injury.
Treatment / Management
Coronary artery disease could present either as stable ischemic heart disease (SIHD) or acute coronary syndrome (ACS). The former present in a chronic setting while the latter presents more in an acute setting. The management depends on the particular disease type. We will discuss the management of each subtype separately:
Stable Ischemic Heart Disease
Stable ischemic heart disease presents as stable angina. Stable angina typically presents as substernal chest pain or pressure that worsens with exertion or emotional stress and gets relieved with rest or nitroglycerin and is of 2 months duration. It is important to know that classic anginal symptoms could be absent and it could present differently with atypical symptoms and exertional dyspnea instead in certain demographic groups including women, elderly age, and diabetics. Management of SIHD includes both non-pharmacologic and pharmacologic interventions. Lifestyle modifications include smoking cessation, regular exercise, weight loss, good control of diabetes and hypertension, and a healthy diet. Pharmacologic interventions include cardioprotective and antianginal medications.
Every patient should get guideline-directed medical therapy (GDMT) which includes low dose aspirin, beta-blocker, as-needed nitroglycerin, and moderate to high-intensity statin. If symptoms are not controlled with this, beta-blocker therapy should be titrated up to heart rates 55-60, and the addition of calcium channel blocker and long-acting nitrates should be considered.[10] Ranolazine could also be added to relieve refractory anginal symptoms. If maximal GDMT has failed to relive angina, cardiac catheterization should be done to visualize the coronary anatomy and a decision should be made for percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) based on the patient profile.[11]
Acute Coronary Syndrome
The acute coronary syndrome presents as sudden onset substernal chest pain or pressure typically radiating to the neck and left arm and may be accompanied by dyspnea, palpitations, dizziness, syncope, cardiac arrest, or new-onset congestive heart failure. Prompt EKG is necessary for all patients with ACS to assess for STEMI and typically is done pre-hospital by an emergency medical services crew. STEMI is recognized by the presence of ST elevation in contiguous leads of 1 mm in limb leads or precordial leads excepting V2 and V3. In V2 and V3, men need to have 2 mm elevations and women 1.5 mm to qualify for STEMI diagnosis. New-onset left bundle branch block (LBBB) is also considered a STEMI equivalent. If STEMI is present, emergency PCI is warranted in a PCI capable facility or if a PCI facility is available within 2 hours distance. If the PCI capable facility is more than 2 hours away, intravenous thrombolytic therapy is indicated after making sure there are no contraindications to it.
It is important to differentiate a true STEMI from other conditions that mimic STEMI on EKG like acute pericarditis, Brugada syndrome, early repolarization changes, and LVH associated changes. All patients should get a full dose of sublingual aspirin (324 mg) upon presentation. Nitrates should be given for pain relief after making sure there are no contraindications to nitrates like hypotension, RV failure, and consumption of phosphodiesterase inhibitors in the past 24-48 hours. High-dose statin therapy and beta-blockers should also be initiated early. P2Y12 inhibitors (prasugrel, ticagrelor, or prasugrel) should be started based on the patient profile. Patients who have NSTE ACS should get anticoagulation, typically heparin or enoxaparin are used. For NSTEMI, early invasive therapy within 24 hours is advised for patients with intermediate to high TIMI scores (>2).[12][13]
Regular visits with cardiologists and family physicians are key to good long term management of coronary artery disease. Medication adherence and lifestyle modification are important.
Differential Diagnosis
Coronary artery disease has a wide range of differential diagnoses because of the proximity of the heart with adjacent organs, including the lungs, stomach, big vessels, and musculoskeletal organs. Acute anginal chest pain could mimic acute pericarditis, myocarditis, prinzmetal angina, pericardial effusion, acute bronchitis, pneumonia, pleuritis, pleural effusion, aortic dissection, GERD, peptic ulcer disease, esophageal motility disorders, and costochondritis. Stable ischemic heart disease could also mimic GERD, Peptic ulcer disease, costochondritis, and pleuritis. History, physical examination, and diagnostic studies should be carefully carried out to narrow down the differential diagnosis and reach an accurate diagnosis.
Toxicity and Adverse Effect Management
Both medical and surgical management for ischemic heart disease is associated with their side effects and complications. These undesirable effects could be mitigated by careful selection, physician expertise, and patient education. Aspirin therapy is associated with bleeding, idiosyncratic, and allergic drug reactions.[14] Statin therapy can cause myalgias, diarrhea, and arthralgias among side effects.[15]
Beta-blockers could cause bradycardia and hypotension. ACEIs could result in hypotension, dizziness, creatinine elevation, cough, and allergic reactions including angioedema.[16] PCI can possibly cause coronary artery perforation, stent thrombosis in an acute setting, and in-stent restenosis on chronic basis.[17] CABG can have its own complications including but not limited to arrhythmias, cardiac tamponade, post-op bleeding, infection, renal impairment, and phrenic nerve injury.
Prognosis
The prognosis of the disease depends on multiple factors some of which could be modified while others are non-modifiable. Patient's age, gender, family history and genetics, ethnicity, dietary and smoking habits, medication compliance, availability of healthcare and financial status, and the number of arteries involved are some of the factors. Comorbid conditions including diabetes mellitus, hypertension, dyslipidemia, and chronic kidney disease also have a role in the overall outcome.[18]
Complications
Arrhythmias, acute coronary syndrome, congestive heart failure, mitral regurgitation, ventricular free wall rupture, pericarditis, aneurysm formation, and mural thrombi are the main complication associated with coronary artery disease.[19][20][10]
Deterrence and Patient Education
Coronary artery disease is caused by a combination of modifiable and nonmodifiable factors. Primary care providers should focus on the modifiable risk factor modification on each routine visit. Tight control of diabetes, hypertension and lipid levels in addition to smoking cessation, weight loss and exercise can make a huge difference. Since it is a global public health concern, in school curriculums and different avenues of media, more awareness needs to be created.
Pearls and Other Issues
Several landmark trials were done over the past few decades which have totally changed the way we care for coronary artery disease patients. It is beyond the scope of this article to discuss individual trial results, however, the following are the names of some important studies. ISIS-2, CURE, CLARITY-TIMI 28, TIRTON-TIMI 38, PLATO, and CURRENT-OASIS 7 trials were done regarding guidelines for antiplatelet medications.[21] SYNERGY, ACUITY, ExTRACT-TIMI 25, OASIS-5, and ATLAS ACS/ TIMI 52 were done about the use of anticoagulation. ADMIRAL, ACUITY, ISAR-REACT 3, and HORIZONS-AMI are famous trials regarding GpIIb/IIIa use, COMMIT for beta-blockers while SHOCK, DANAMI-2, BASKET-LATE, TIMACS, and BASKET-PROVE are about PCI and CABG. MIRACL trial was done about statin use.
Enhancing Healthcare Team Outcomes
Evaluation of ischemic heart disease can frequently present a diagnostic dilemma. Such patients can present non-specific symptoms like chest pain or shortness of breath. The cause of chest pain or shortness of breath could be due to a myriad of diseases including gastrointestinal, cardiac, musculoskeletal, psychological, and pulmonary causes. While a cardiologist is often involved as a central player, it is important to take other team members on board as indicated including a gastroenterologist, pulmonologist, and psychiatrist. Radiologists also are an important resource in the whole process. Nurses are a very important part of the diagnostic and therapeutic workup as well as they provide key bedside information not witnessed by the physicians in their short encounters.